Effect of Ultraviolet Radiation on the Enzymolytic and Biomechanical Profiles of Abdominal Aortic Adventitia Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Porcine Abdominal Aortic Adventitia Sample
2.1.2. Human Abdominal Aortic Adventitia Sample
2.1.3. Human Abdominal Aortic Aneurysmal Adventitia Sample
2.2. In-Vitro Elastolysis
2.3. In-Vitro Collagenolysis
2.4. Irradiation Procedure
2.5. Mechanical Testing
2.5.1. Protocol for Porcine Adventitia Samples
2.5.2. Protocol for Human Adventitia Samples
2.6. Statistical Analysis
3. Results
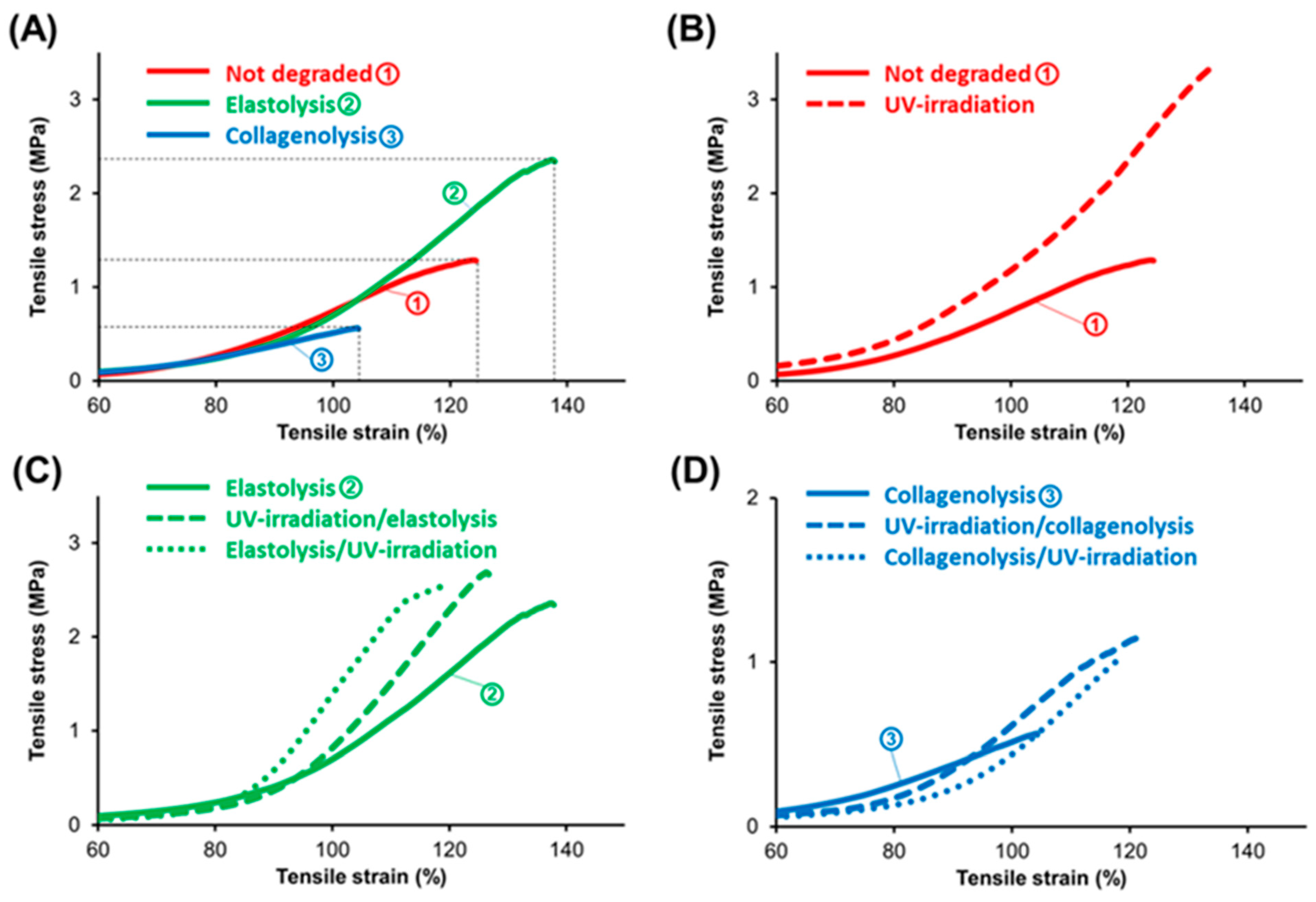
3.1. Experiment on Porcine Aortic Adventitia
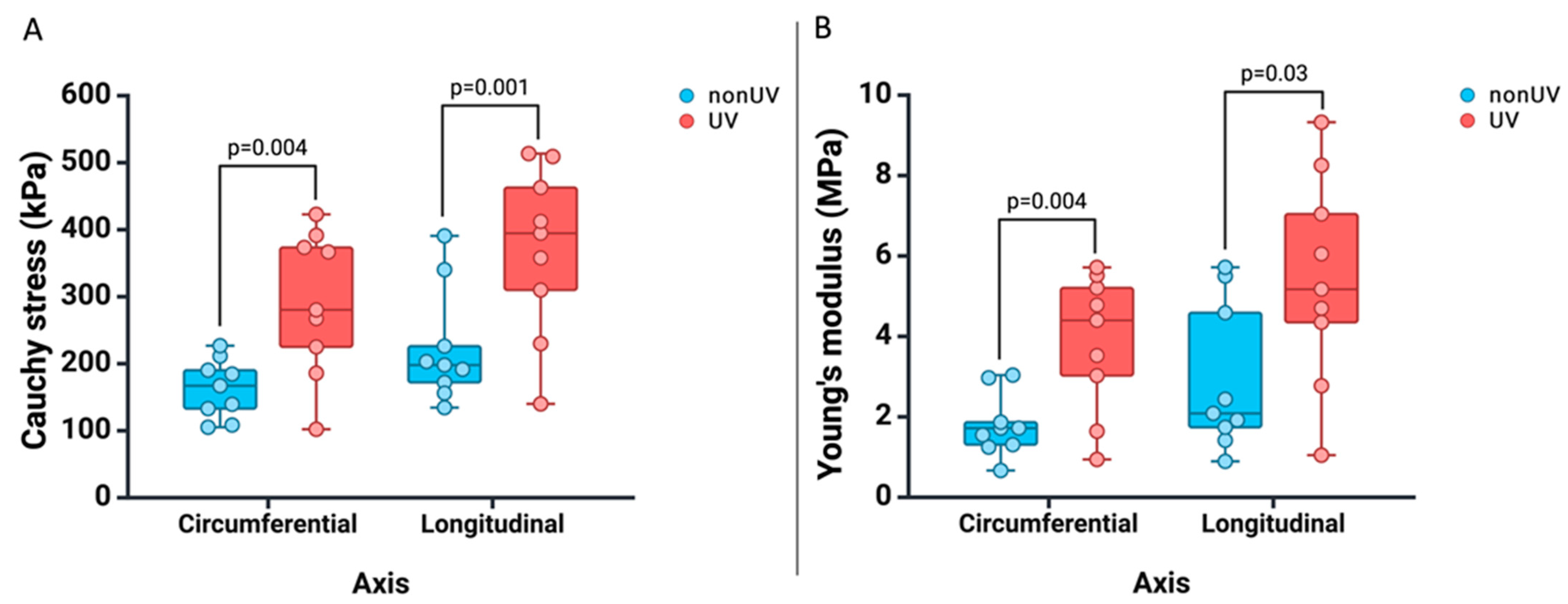
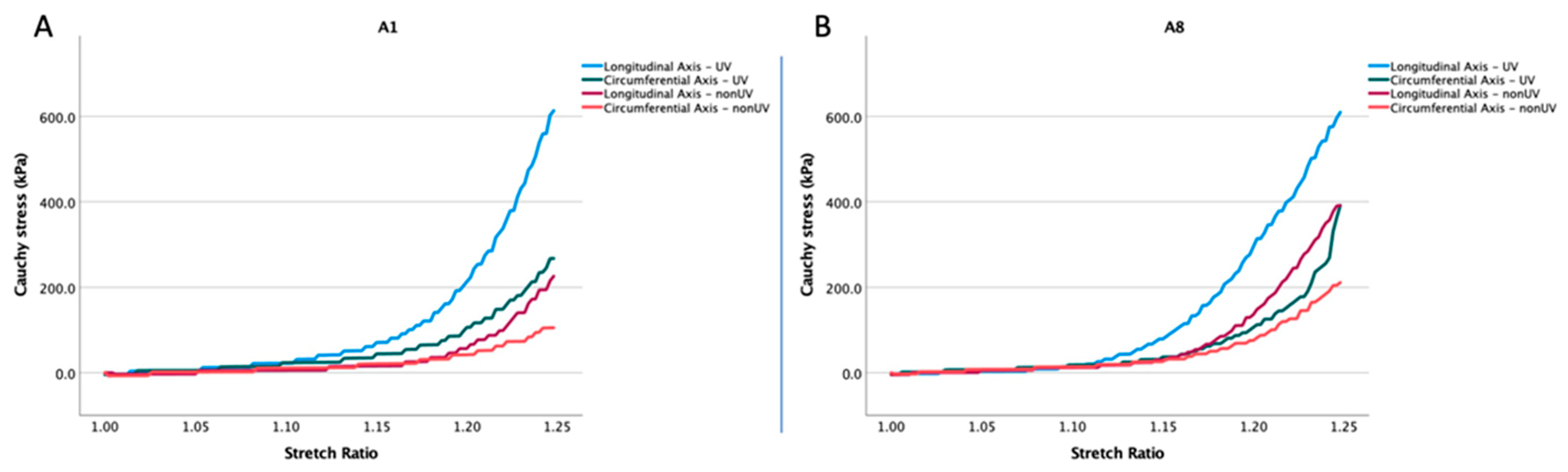
3.2. Experiment on the Human Aortic Adventitia
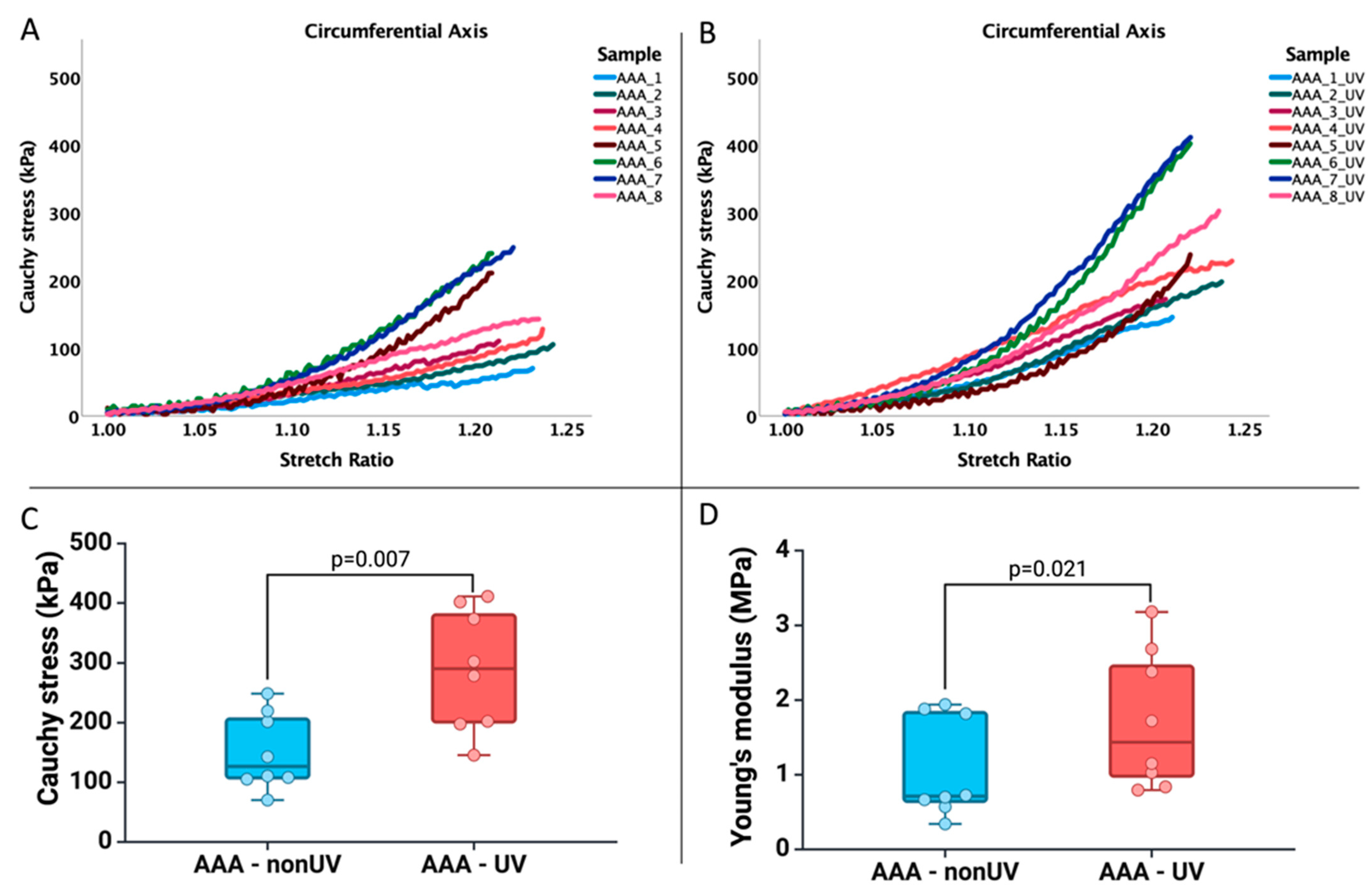
3.3. Experiment on the Human Abdominal Aortic Aneurysmal Adventitia Sample
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.W.; Geraghty, P.J.; Lee, J.K. Abdominal aortic aneurysms: Basic mechanisms and clinical implications. Curr. Probl. Surg. 2002, 39, 110–230. [Google Scholar] [CrossRef] [PubMed]
- Choke, E.; Cockerill, G.; Wilson, W.R.W.; Sayed, S.; Dawson, J.; Loftus, I.; Thompson, M.M. A review of biological factors implicated in abdominal aortic aneurysm rupture. Eur. J. Vasc. Endovasc. Surg. 2002, 30, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Keeling, W.B.; Armstrong, P.A.; Stone, P.A.; Bandyk, D.F.; Shames, M.L. An overview of matrix metalloproteinases in the pathogenesis and treatment of abdominal aortic aneurysms. Vasc. Endovasc. Surg. 2005, 39, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Didangelos, A.; Yin, X.; Mandal, K.; Saje, A.; Smith, A.; Xu, Q.; Jahangiri, M.; Mayr, M. Extracellular matrix composition and remodeling in human abdominal aortic aneurysms: A proteomics approach. Mol. Cell. Proteom. 2011, 10, M111.008128. [Google Scholar] [CrossRef] [PubMed]
- Kuivaniemi, H.; Ryer, E.J.; Elmore, J.R.; Tromp, G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert. Rev. Cardiovasc. Ther. 2015, 13, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Hu, M.; Shen, M.; Kassiri, Z. Extracellular matrix, regional heterogeneity of the aorta, and aortic aneurysm. Exp. Mol. Med. 2019, 51, 160. [Google Scholar] [CrossRef]
- Golledge, J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 2019, 16, 225–242. [Google Scholar] [CrossRef]
- Liu, B.; Granville, D.J.; Golledge, J.; Kassiri, Z. Pathogenic mechanisms and the potential of drug therapies for aortic aneurysm. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H652–H670. [Google Scholar] [CrossRef]
- Adams, L.; Brangsch, J.; Hamm, B.; Makowski, M.R.; Keller, S. Targeting the extracellular matrix in abdominal aortic aneurysms using molecular imaging insights. Int. J. Mol. Sci. 2021, 22, 2685. [Google Scholar] [CrossRef]
- Swerdlow, N.J.; Wu, W.W.; Schermerhorn, M.L. Open and endovascular management of aortic aneurysms. Circ. Res. 2019, 124, 647–661. [Google Scholar] [CrossRef]
- Chirila, T.V.; Suzuki, S. Photocrosslinking of adventitial collagen in the porcine abdominal aorta: A preliminary approach to a strategy for prevention of aneurysmal rupture. Designs 2022, 6, 5. [Google Scholar] [CrossRef]
- Chirila, T.V.; Suzuki, S. Effects of ultraviolet-A radiation on enzymatically degraded tunica adventitia of the porcine abdominal aorta. Biomed. Mater. Dev. 2023, 1, 1000–1008. [Google Scholar] [CrossRef]
- Chirila, T.V.; Suzuki, S. Ultraviolet-induced mechanical augmentation of the degraded porcine aortic adventitia: Its significance for preventing aneurysmal rupture. Global Transl. Med. 2023, 2, 0897. [Google Scholar] [CrossRef]
- Arbanasi, E.-M.; Suzuki, S.; Ciucanu, C.C.; Muresan, A.V.; Cosarca, C.M.; Chirila, T.V.; Ion, A.P.; Arbanasi, E.-M.; Harpa, M.M.; Russu, E. Ex-vivo mechanical augmentation of human saphenous vein graft by UV-A irradiation in emergency vascular reconstruction—Preliminary results. J. Cardiovasc. Emerg. 2023, 9, 59–64. [Google Scholar]
- Dobrin, P.B.; Baker, W.H.; Gley, W.C. Elastolytic and collagenolytic studies of arteries. Arch. Surg. 1984, 119, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Dadgar, L.; Marois, Y.; Deng, X.; Guidoin, R. Arterial wall mechanical characteristics after treatment in collagenase: An in vitro aneurysm model. Clin. Investig. Med. 1997, 20, 25–34. [Google Scholar]
- Chow, M.-J.; Choi, M.; Yun, S.H.; Zhang, Y. The effect of static stretch on elastin degradation in arteries. PLoS ONE 2013, 8, e81951. [Google Scholar] [CrossRef]
- Chow, M.-J.; Mondonedo, J.R.; Johnson, V.M.; Zhang, Y. Progressive structural and biomechanical changes in elastin degraded aorta. Biomech. Model. Mechanobiol. 2013, 12, 361–372. [Google Scholar] [CrossRef]
- Wills, A.; Thompson, M.M.; Crowther, M.; Brindle, N.P.; Nasim, A.; Sayers, R.D.; Bell, P.R.F. Elastase-induced matrix degradation in arterial organ cultures: An in vitro model of aneurysmal disease. J. Vasc. Surg. 1996, 24, 667–679. [Google Scholar] [CrossRef][Green Version]
- Beenakker, J.-W.M.; Ashcroft, B.A.; Lindeman, J.H.N.; Oosterkamp, T.H. Mechanical properties of the extracellular matrix of the aorta studied by enzymatic treatments. Biophys. J. 2012, 102, 1731–1737. [Google Scholar] [CrossRef]
- Cheheltani, R.; McGoverin, C.M.; Rao, J.; Vorp, D.A.; Kiani, M.F.; Pleshko, N. Fourier transform infrared spectroscopy to quantify collagen and elastin in an in vitro model of extracellular matrix degradation in aorta. Analyst 2014, 139, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Kratzberg, J.A.; Walker, P.J.; Rikkers, E.; Raghavan, M.L. The effect of proteolytic treatment on plastic deformation of porcine aortic tissue. J. Mech. Behav. Biomed. Mater. 2009, 2, 65–72. [Google Scholar] [CrossRef] [PubMed]
- White, J.V.; Mazzacco, S.L. Formation and growth of aortic aneurysms induced by adventitial elastolysis. Ann. N. Y. Acad. Sci. 1996, 800, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Gundiah, N.; Babu, A.R.; Pruitt, L.A. Effects of elastase and collagenase on the nonlinearity and anisotropy of porcine aorta. Physiol. Meas. 2013, 34, 1657–1673. [Google Scholar] [CrossRef] [PubMed]
- Schriefl, A.J.; Schmidt, T.; Balzani, D.; Sommer, G.; Holzapfel, G.A. Selective enzymatic removal of elastin and collagen from human abdominal aortas: Uniaxial mechanical response and constitutive modeling. Acta Biomater. 2015, 17, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Pukaluk, A.; Wolinski, H.; Viertler, C.; Regitnig, P.; Holzapfel, G.A.; Sommer, G. Changes in the microstructure of the human aortic adventitia under biaxial loading investigated by multi-photon microscopy. Acta Biomater. 2023, 161, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Gosline, J.; Lillie, M.; Carrington, E.; Guerette, P.; Ortlepp, C.; Savage, K. Elastic proteins: Biological and mechanical properties. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Montes, G.S. Structural biology of the fibres of the collagenous and elastic systems. Cell Biol. Int. 1996, 20, 15–27. [Google Scholar] [CrossRef]
- Banga, I. Thermal contraction of collagen and its dissolution with elastase. Nature 1953, 172, 1099. [Google Scholar] [CrossRef]
- Starkey, P.M. The effect of human neutrophil elastase and cathepsin G on the collagen of cartilage, tendon, and cornea. Acta Biol. Med. Ger. 1977, 36, 1549–1554. [Google Scholar]
- Kafienah, W.; Buttle, D.J.; Burnett, D.; Hollander, P.A. Cleavage of native Type I collagen by human neutrophil elastase. Biochem. J. 1998, 330, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Pukaluk, A.; Wolinski, H.; Viertler, C.; Regitnig, P.; Holzapfel, G.A.; Sommer, G. Changes in the microstructure of the human aortic medial layer under biaxial loading investigated by multi-photon microscopy. Acta Biomater. 2022, 151, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Niestrawska, J.A.; Viertler, C.; Regitnig, P.; Cohnert, T.U.; Sommer, G.; Holzapfel, G.A. Microstructure and mechanics of healthy and aneurysmatic abdominal aortas: Experimental analysis and modelling. J. R. Soc. Interface 2016, 13, 20160620. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, S.A.; Mulvihill, J.J.; Barrett, H.E.; Kavanagh, E.G.; Walsh, M.T.; McGloughlin, T.M.; Doyle, B.J. Determining the influence of calcification on the failure properties of abdominal aortic aneurysm (AAA) tissue. J. Mech. Behav. Biomed. Mater. 2015, 42, 154–167. [Google Scholar] [CrossRef] [PubMed]
- White, J.V.; Haas, K.; Phillips, S.; Comerota, A.J. Adventitial elastolysis is a primary event in aneurysm formation. J. Vasc. Surg. 1993, 17, 371–381. [Google Scholar] [CrossRef]
- White, J.V. Aneurysm formation in vivo by the topical degradation of adventitial elastin. J. Vasc. Surg. 1994, 20, 153–155. [Google Scholar] [CrossRef]
- Raghavan, M.L.; da Silva, E.S. Mechanical properties of AAA tissue. Stud. Mechanobiol. Tissue Eng. Biomater. 2011, 7, 139–162. [Google Scholar]
- Roy, D.; Holzapfel, G.A.; Kauffmann, C.; Soulez, G. Finite element analysis of abdominal aortic aneurysms: Geometrical and structural reconstruction with application of an anisotropic material model. IMA J. Appl. Math. 2014, 79, 1011–1026. [Google Scholar] [CrossRef]
- Pierce, D.M.; Maier, F.; Weisbecker, H.; Viertler, C.; Verbrugghe, P.; Famaey, N.; Fourneau, I.; Herijgers, P.; Holzapfel, G.A. Human thoracic and abdominal aortic aneurysmal tissues: Damage experiments, statistical analysis and constitutive modeling. J. Mech. Behav. Biomed. Mater. 2015, 41, 92–107. [Google Scholar] [CrossRef]
- Tong, J.; Holzapfel, G.A. Structure, mechanics, and histology of intraluminal thrombi in abdominal aortic aneurysms. Ann. Biomed. Eng. 2015, 43, 1488–1501. [Google Scholar] [CrossRef]
- Niestrawska, J.A.; Regitnig, P.; Viertler, C.; Regitnig, P.; Cohnert, T.U.; Babu, A.R.; Holzapfel, G.A. The role of tissue remodeling in mechanics and pathogenesis of abdominal aortic aneurysms. Acta Biomater 2019, 88, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.W.; Curci, J.A.; Ennis, T.L.; Mao, D.; Pagano, M.B.; Pham, C.T. Pathophysiology of abdominal aortic aneurysms: Insights from the elastase-induced model in mice with different genetic backgrounds. Ann. N. Y. Acad. Sci. 2006, 1085, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Yin, L.; Shen, J.; Liu, Z. A modified murine abdominal aortic aneurysm rupture model using elastase perfusion and angiotensin II infusion. Ann. Vasc. Surg. 2020, 67, 474–481. [Google Scholar] [CrossRef] [PubMed]



| Aorta Sample | Sex | Age | Weight (kg) | Height (cm) | BMI | Aorta Diameter (cm) | Aorta Length (cm) |
|---|---|---|---|---|---|---|---|
| A1 | F | 86 | 55 | 155 | 22.8 | 1.96 | 10.2 |
| A2 | F | 70 | 40 | 139 | 20.7 | 1.7 | 8.1 |
| A3 | M | 47 | 82 | 176 | 26.4 | 1.78 | 9.5 |
| A4 | M | 80 | 90 | 170 | 31.1 | 2.26 | 12.6 |
| A5 | F | 79 | 64 | 158 | 25.6 | 1.84 | 10.5 |
| A6 | M | 86 | 64 | 161 | 24.6 | 1.88 | 12.7 |
| A7 | F | 87 | 63 | 147 | 29.1 | 1.76 | 9.5 |
| A8 | M | 62 | 61 | 163 | 22.9 | 1.54 | 10 |
| A9 | M | 42 | 91 | 174 | 30.2 | 1.94 | 10.4 |
| Mean value | 71 | 67.7 | 160.3 | 25.9 | 1.85 | 10.38 | |
| Axis | Biomechanical Properties | Treatment | Mean | Minimum | Maximum | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Longitudinal (Ox) | Cauchy stress (kPa) | Non-UV | 223.6 | 134.5 | 390.8 | 157.7 | 289.5 | 0.001 |
| UV | 370.1 | 140.1 | 513.6 | 273.1 | 467.01 | |||
| Young’s modulus (kPa) | Non-UV | 2992.2 | 893.5 | 5718.4 | 1511.1 | 4333.3 | 0.03 | |
| UV | 5412.8 | 1047.6 | 9325 | 3412.8 | 7412.7 | |||
| Circumferential (Oy) | Cauchy stress (kPa) | Non-UV | 162.9 | 102.2 | 226.9 | 129.2 | 196.6 | 0.004 |
| UV | 290.6 | 105.2 | 422.9 | 201.8 | 372.9 | |||
| Young’s modulus (kPa) | Non-UV | 1787.3 | 666.1 | 3038 | 1190.1 | 2383.6 | 0.004 | |
| UV | 3861.2 | 941.1 | 5715.8 | 2547 | 5175.4 | |||
| Sample | Sex | Age (years) | AAA Diameter (mm) | BMI | Hypertension Yes/No | Diabetes Yes/No | Active Smoking Yes/No | Ruptured Yes/No |
|---|---|---|---|---|---|---|---|---|
| AAA_1 | M | 63 | 66 | 22.95 | No | No | Yes | No |
| AAA_2 | M | 74 | 75 | 25.39 | Yes | Yes | No | No |
| AAA_3 | M | 78 | 89 | 20.5 | No | Yes | No | Yes |
| AAA_4 | F | 72 | 77 | 28.4 | Yes | Yes | Yes | No |
| AAA_5 | M | 80 | 91 | 31.4 | Yes | No | Yes | Yes |
| AAA_6 | F | 79 | 83 | 32.05 | Yes | No | Yes | Yes |
| AAA_7 | M | 81 | 115 | 27.99 | Yes | No | Yes | Yes |
| AAA_8 | M | 75 | 65 | 27.05 | Yes | Yes | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbănaşi, E.-M.; Russu, E.; Arbănaşi, E.-M.; Ciucanu, C.C.; Mureșan, A.V.; Suzuki, S.; Chirilă, T.V. Effect of Ultraviolet Radiation on the Enzymolytic and Biomechanical Profiles of Abdominal Aortic Adventitia Tissue. J. Clin. Med. 2024, 13, 633. https://doi.org/10.3390/jcm13020633
Arbănaşi E-M, Russu E, Arbănaşi E-M, Ciucanu CC, Mureșan AV, Suzuki S, Chirilă TV. Effect of Ultraviolet Radiation on the Enzymolytic and Biomechanical Profiles of Abdominal Aortic Adventitia Tissue. Journal of Clinical Medicine. 2024; 13(2):633. https://doi.org/10.3390/jcm13020633
Chicago/Turabian StyleArbănaşi, Emil-Marian, Eliza Russu, Eliza-Mihaela Arbănaşi, Constantin Claudiu Ciucanu, Adrian Vasile Mureșan, Shuko Suzuki, and Traian V. Chirilă. 2024. "Effect of Ultraviolet Radiation on the Enzymolytic and Biomechanical Profiles of Abdominal Aortic Adventitia Tissue" Journal of Clinical Medicine 13, no. 2: 633. https://doi.org/10.3390/jcm13020633
APA StyleArbănaşi, E.-M., Russu, E., Arbănaşi, E.-M., Ciucanu, C. C., Mureșan, A. V., Suzuki, S., & Chirilă, T. V. (2024). Effect of Ultraviolet Radiation on the Enzymolytic and Biomechanical Profiles of Abdominal Aortic Adventitia Tissue. Journal of Clinical Medicine, 13(2), 633. https://doi.org/10.3390/jcm13020633








