The Many Faces of Immune Thrombocytopenia: Mechanisms, Therapies, and Clinical Challenges in Oncological Patients
Abstract
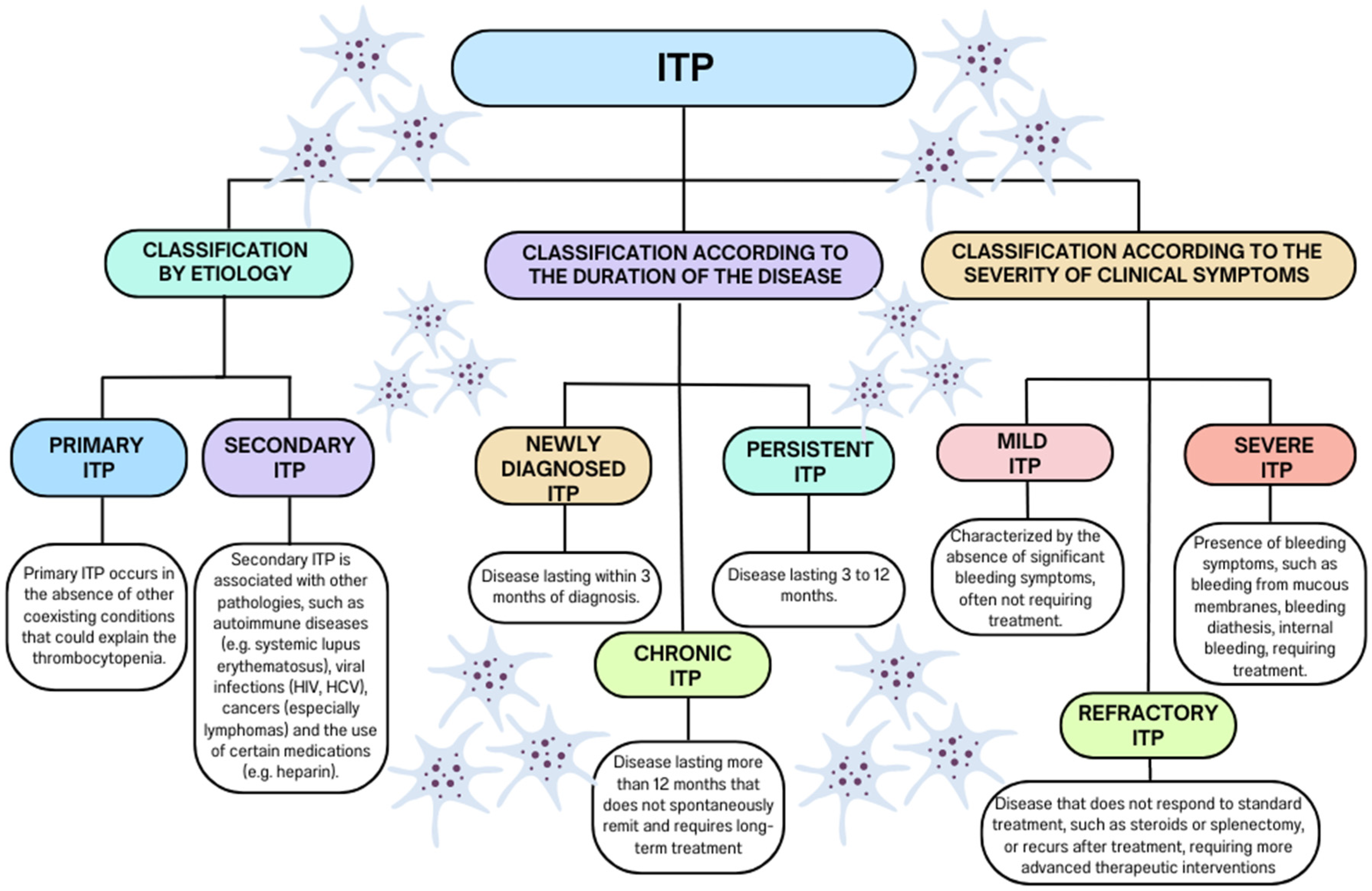
:1. Introduction
2. Pathogenesis of ITP
2.1. The Role of B and T Lymphocytes in the Pathogenesis of ITP
2.2. The Role of Genetic Polymorphisms and Epigenetic Modifications in the Pathogenesis of ITP
2.3. The Role of Viral and Bacterial Infections in the Pathogenesis of ITP
2.4. Nutrient Deficiencies in the Pathogenesis of ITP
2.5. Drugs Involved in the Development of ITP
3. ITP in Cancer
3.1. Primary ITP in Oncology Patients
3.2. Secondary ITP in Oncology Patients
4. Surgical Interventions and ITP
5. Treatment and Management of ITP in Oncology Patients
| Drug | Drug Category | Dosage | Effect | Target | Comments | References |
|---|---|---|---|---|---|---|
| Prednisone | Glucocorticosteroid | 1–2 mg/kg body weight daily | It suppresses the immune response by inhibiting the production of cytokines like IL-2 and reducing macrophage activation. | T cells, cytokines, and macrophages | Monitoring is necessary due to the impact on the course of cancer. Moreover, long-term use is associated with the risk of complications such as osteoporosis, infections, and diabetes | [20,219,220] |
| Prednisolone | Glucocorticosteroid | 1–2 mg/kg body weight daily. The initial dose may be maintained for 1–2 weeks and then gradually reduced depending on the patient’s clinical response. | It is a glucocorticosteroid that has anti-inflammatory and immunosuppressive effects, reducing the destruction of platelets by the immune system. It inhibits the production of autoantibodies and reduces the activation of macrophages, which are responsible for the phagocytosis of platelets. | Capillary endothelium. | Prednisolone is often used as a first-line treatment for ITP. In cancer patients, prednisolone can effectively quickly raise platelet counts, which is essential before surgery or during chemotherapy. However, its long-term use is associated with the risk of serious side effects, such as osteoporosis, hyperglycemia, hypertension, and increased susceptibility to infections. | [221] |
| Methylprednisolone | Glucocorticosteroid | 0.5–1 mg/kg body weight per day, but in severe cases of ITP, a higher dose, even 1–2 mg/kg body weight per day, may be used. In pulse therapy (especially in acute cases), doses of 500 mg to 1 g for 3 days are used, especially in severe cases such as internal bleeding or refractory thrombocytopenia. | It has anti-inflammatory and immunosuppressive effects. Methylprednisolone inhibits the activation of T and B lymphocytes, reducing the production of autoantibodies and inhibits macrophages responsible for the destruction of platelets. | T cells, cytokines, leukocytes, and phospholipase A2 | Methylprednisolone, mainly used in pulse form, is preferred in acute cases requiring rapid response. In situations of acute exacerbation of ITP, especially before surgery or in cases of bleeding, pulse doses of methylprednisolone can help to increase the platelet count quickly. In oncology patients, methylprednisolone may be an alternative to prednisolone, especially when high doses of corticosteroids are required. Pulse therapy with high doses of methylprednisolone may lead to fewer side effects than long-term low doses of prednisolone but requires intensive monitoring due to the risk of acute hyperglycemia, hypertension, and electrolyte disturbances. | [222] |
| Dexamethasone | Glucocorticosteroid | 40 mg daily for 4 days (pulse dosing), repeated every 2–4 weeks | Increases the number of platelets by inhibiting their destruction through immune modulation. | Platelets and immune cells | Preferred short-term treatment for oncology patients | [223,224] |
| IVIG | Intravenous immunoglobulin | 1 g/kg body weight daily for 1–2 days or 0.4 g/kg for 5 days; rapid but short-lasting effect | Binds to Fc receptors on macrophages, blocking their interaction with antibody-coated platelets, thus increasing circulating platelets | Fc receptors and macrophages | Used in emergencies. It has high costs and needs to be repeated. | [225] |
| Romiplostim | Peptide antibody | 1 μg/kg body weight once a week; the dose may be increased to 10 μg/kg depending on the platelet count | It is a TPO-RA agonist that acts on megakaryocytes in the bone marrow to stimulate platelet production. It mimics the action of endogenous thrombopoietin, promoting megakaryocyte maturation and increasing platelet production. | Thrombopoietin receptor on megakaryocytes | Individualize the dose depending on the response, and pay attention to interactions with cancer therapy. | [208] |
| Eltrombopag | Thrombopoietin receptor agonists | 50 mg once daily and 25 mg in patients with hepatic impairment or of Asian origin; avoid dietary calcium around administration | Binds to and activates the thrombopoietin receptor, promoting megakaryocyte differentiation and platelet production. | Thrombopoietin receptor on megakaryocytes | Regular monitoring of platelet counts, especially during chemotherapy. | [226] |
| Rituximab | Monoclonal anti-CD20 antibody | 375 mg/m2 body surface area once weekly for 4 weeks | Targets CD20 protein on B cells, leading to their depletion and reducing autoantibody production against platelets. | B cells (CD20) | Requires monitoring due to risk of immunosuppression; effective in 40–70% of patients, but lasting remission is rare; risk of complications | [212] |
| Avatrombopag | Thrombopoietin receptor agonists | 20 mg orally once daily. The dose may be increased depending on the patient’s response to treatment, with a maximum dose of 40 mg daily. | It is a TPO-RA agonist that stimulates platelet production by stimulating megakaryocytes. Its effects are similar to other TPO-RAs such as eltrombopag and romiplostim. | Bone marrow | Avatrombopag may be used in patients with ITP, especially those who require maintenance of adequate platelet levels during anticancer therapy. Avatrombopag has the advantage of not having significant interactions with food, which is an advantage over eltrombopag, which requires avoidance of dietary calcium. | [227] |
| Fostamatinib | Tyrosine kinase inhibitor | 100 mg orally twice daily. If response to treatment is inadequate, the dose may be increased to 150 mg twice daily after one month of treatment. | It is a Syk kinase (spleen tyrosine kinase) inhibitor that inhibits intracellular signaling pathways responsible for platelet phagocytosis by macrophages. By inhibiting platelet destruction, fostamatinib enables their survival and maintenance of appropriate levels in the bloodstream. | SYK in macrophages | Particularly useful in patients with refractory ITP where other forms of therapy have been ineffective. Its unique mechanism of action differs from other drugs used in ITP, making it an alternative for patients who do not respond to corticosteroids-, IVIG-, or TPO-RA-based therapies. | [228] |
| Drug | Primary Indication | Dosage | Effect | Target | Comments | References |
|---|---|---|---|---|---|---|
| Mycophenolate mofetil (MMF) | It is primarily indicated for the prevention of organ transplant rejection by acting as an immunosuppressant drug that suppresses the activity of the immune system to protect the transplanted organ. | 500 mg twice daily, which may be increased to 1 g twice daily depending on patient tolerance and response. | MMF acts as a purine synthesis inhibitor, which leads to a decrease in the proliferation of T and B lymphocytes, which play a key role in the pathogenesis of ITP. It inhibits the activity of immune cells responsible for the production of autoantibodies against platelets. | T cells, B cells | MMF is used as a second-line therapy in patients with chronic ITP, especially those who do not respond to other immunosuppressive drugs. MMF is well tolerated and has fewer side effects than some other immunosuppressive drugs. Regular monitoring of kidney function and white blood cell count is necessary, as the drug can lead to immunosuppression and an increased risk of infection. | [229] |
| Cyclosporine A | It is primarily indicated for the prevention of rejection of an organ transplant (e.g., kidney, heart, and liver). It acts as a powerful immunosuppressant drug that inhibits the activity of T lymphocytes, preventing the immune system from attacking the transplanted organ. | 3–5 mg/kg body weight daily, adjusted depending on patient response and blood cyclosporine levels. | It is a calcineurin inhibitor, which inhibits the activation and proliferation of T lymphocytes, reducing their ability to stimulate an immune response. In ITP, this drug reduces the production of autoantibodies against platelets. | T cells, cytokines | It requires monitoring of the drug concentration in the blood, as it can lead to nephrotoxicity and other side effects, such as hypertension and hypertrichosis. The use of this drug is also associated with the risk of immunosuppression. | [230] |
| Azathioprine | It is primarily indicated for the prevention of organ transplant rejection and the treatment of autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, and Crohn’s disease. It acts as an immunosuppressant, inhibiting the activity of the immune system by blocking the proliferation of lymphocytes. | 1–2 mg/kg body weight per day. The dose may be adjusted depending on the tolerability and effectiveness of the treatment. | It is an immunosuppressive drug that works by inhibiting DNA synthesis in lymphocytes, which reduces their proliferation and activity. It limits the production of autoantibodies against platelets, which helps in the treatment of ITP. | T cells, B cells | Azathioprine therapy requires a long time to achieve a clinical effect. Blood counts must be monitored because azathioprine can cause myelosuppression. Regular liver function tests are also important because the drug can be hepatotoxic. | [231] |
| Danazol | It is used to treat endometriosis, hereditary angioedema, and ITP. It works by inhibiting hormone production and supporting the immune system, reducing the symptoms of these conditions. | 200–400 mg daily, depending on patient response. The dose may be gradually reduced once an adequate platelet count has been achieved. | Synthetic androgen acts immunomodulatory by reducing the production of autoantibodies and improving platelet count. It inhibits the production of IL-1 and TNF by monocytes, modulating the immune response. | IL-1, TNF, monocytes | It may lead to side effects related to sex hormones, such as virilization in women, voice changes, as well as hepatotoxicity, so liver function should be monitored. | [232] |
| Dapsone | It is primarily indicated for the treatment of leprosy (Hansen’s disease) and Pneumocystis jirovecii pneumonia in immunocompromised patients, often as an alternative to trimethoprim-sulfamethoxazole. It acts as an antibacterial and anti-inflammatory drug. Additionally, it is used in some autoimmune diseases, such as pemphigus and dermatitis herpetiformis, due to its anti-inflammatory and immunosuppressive properties | 50–100 mg daily. | It is an anti-inflammatory drug that has immunomodulatory effects, although its exact mechanism in ITP is not fully understood. It is known to reduce the production of autoantibodies and improve platelet count. Induces hemolysis, leading to erythrophagocytosis in the reticuloendothelial system and thus preventing platelet destruction. | Reticuloendothelial system | The drug may cause side effects such as hemolytic anemia and methemoglobinemia, so regular monitoring of blood counts is necessary. | [233] |
| Hydroxychloroquine | It is primarily indicated for the treatment of malaria and autoimmune diseases such as SLE and rheumatoid arthritis (RA). It acts as an anti-inflammatory and immunomodulatory drug, inhibiting the activity of the immune system and reducing the symptoms of inflammation in these diseases. | 200–400 mg daily, depending on the patient’s condition. | It is an immunomodulatory drug that inhibits the activity of immune cells, reducing the production of autoantibodies. It is mainly used in autoimmune diseases such as lupus erythematosus, but can be used in ITP to reduce the destruction of platelets. | Platelets, vascular system | Regular monitoring of eyesight is necessary, as long-term use of hydroxychloroquine may lead to retinopathy. | [234] |
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schifferli, A.; Cavalli, F.; Godeau, B.; Liebman, H.A.; Recher, M.; Imbach, P.; Kühne, T. Understanding Immune Thrombocytopenia: Looking Out of the Box. Front. Med. 2021, 8, 613192. [Google Scholar] [CrossRef] [PubMed]
- Platelet Disorders—Immune Thrombocytopenia (ITP)|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health/immune-thrombocytopenia (accessed on 10 September 2024).
- Johnsen, J. Pathogenesis in Immune Thrombocytopenia: New Insights. Hematol. Am. Soc. Hematol. Educ. Program 2012, 2012, 306–312. [Google Scholar] [CrossRef]
- Provan, D.; Semple, J.W. Recent Advances in the Mechanisms and Treatment of Immune Thrombocytopenia. eBioMedicine 2022, 76, 103820. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, P.F.; Segal, J.B. The Epidemiology of Immune Thrombocytopenic Purpura. Curr. Opin. Hematol. 2007, 14, 515–519. [Google Scholar] [CrossRef]
- Bussel, J.; Cooper, N.; Boccia, R.; Zaja, F.; Newland, A. Immune Thrombocytopenia. Expert Rev. Hematol. 2021, 14, 1013–1025. [Google Scholar] [CrossRef]
- Sotel, J.; Drabko, K. Primary Immune Thrombocytopaenia in Children—Analysis of Result of Treatment in a Single Centre. Pediatr. Pol. 2018, 93, 30–34. [Google Scholar] [CrossRef]
- Mititelu, A.; Onisâi, M.-C.; Roșca, A.; Vlădăreanu, A.M. Current Understanding of Immune Thrombocytopenia: A Review of Pathogenesis and Treatment Options. Int. J. Mol. Sci. 2024, 25, 2163. [Google Scholar] [CrossRef]
- Kohli, R.; Chaturvedi, S. Epidemiology and Clinical Manifestations of Immune Thrombocytopenia. Hamostaseologie 2019, 39, 238–249. [Google Scholar] [CrossRef]
- Abrahamson, P.E.; Hall, S.A.; Feudjo-Tepie, M.; Mitrani-Gold, F.S.; Logie, J. The Incidence of Idiopathic Thrombocytopenic Purpura among Adults: A Population-Based Study and Literature Review. Eur. J. Haematol. 2009, 83, 83–89. [Google Scholar] [CrossRef]
- Książek, A.; Szczepański, T. Primary Immune Thrombocytopenia in Children. Pediatr. Pol. 2021, 96, 53–59. [Google Scholar] [CrossRef]
- Guo, N.-H.; Fu, X.; Zi, F.-M.; Song, Y.; Wang, S.; Cheng, J. The Potential Therapeutic Benefit of Resveratrol on Th17/Treg Imbalance in Immune Thrombocytopenic Purpura. Int. Immunopharmacol. 2019, 73, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Barsam, S.J.; Psaila, B.; Forestier, M.; Page, L.K.; Sloane, P.A.; Geyer, J.T.; Villarica, G.O.; Ruisi, M.M.; Gernsheimer, T.B.; Beer, J.H.; et al. Platelet Production and Platelet Destruction: Assessing Mechanisms of Treatment Effect in Immune Thrombocytopenia. Blood 2011, 117, 5723–5732. [Google Scholar] [CrossRef] [PubMed]
- Audia, S.; Mahévas, M.; Nivet, M.; Ouandji, S.; Ciudad, M.; Bonnotte, B. Immune Thrombocytopenia: Recent Advances in Pathogenesis and Treatments. Hemasphere 2021, 5, e574. [Google Scholar] [CrossRef] [PubMed]
- Andreescu, M. The Link between Immune Thrombocytopenia and the Cytokine Profile: A Bridge to New Therapeutical Targets. Front. Hematol. 2023, 2, 1191178. [Google Scholar] [CrossRef]
- Grodzielski, M.; Goette, N.P.; Glembotsky, A.C.; Constanza Baroni Pietto, M.; Méndez-Huergo, S.P.; Pierdominici, M.S.; Montero, V.S.; Rabinovich, G.A.; Molinas, F.C.; Heller, P.G.; et al. Multiple Concomitant Mechanisms Contribute to Low Platelet Count in Patients with Immune Thrombocytopenia. Sci. Rep. 2019, 9, 2208. [Google Scholar] [CrossRef]
- Petito, E.; Gresele, P. Immune Attack on Megakaryocytes in Immune Thrombocytopenia. Res. Pract. Thromb. Haemost. 2024, 8, 102345. [Google Scholar] [CrossRef]
- Pietras, N.M.; Gupta, N.; Justiz Vaillant, A.A.; Pearson-Shaver, A.L. Immune Thrombocytopenia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Matzdorff, A.; Alesci, S.R.; Gebhart, J.; Holzhauer, S.; Hütter-Krönke, M.L.; Kühne, T.; Meyer, O.; Ostermann, H.; Pabinger, I.; Rummel, M.; et al. Expert Report on Immune Thrombocytopenia: Current Diagnostics and Treatment—Recommendations from an Expert Group from Austria, Germany, and Switzerland. Oncol. Res. Treat. 2023, 46, 5–44. [Google Scholar] [CrossRef]
- Gafter-Gvili, A. Current Approaches for the Diagnosis and Management of Immune Thrombocytopenia. Eur. J. Intern. Med. 2023, 108, 18–24. [Google Scholar] [CrossRef]
- Crickx, E.; Mahévas, M.; Michel, M.; Godeau, B. Older Adults and Immune Thrombocytopenia: Considerations for the Clinician. Clin. Interv. Aging 2023, 18, 115–130. [Google Scholar] [CrossRef]
- Kuter, D.J. The Treatment of Immune Thrombocytopenia (ITP)—Focus on Thrombopoietin Receptor Agonists. Ann. Blood 2021, 6, 7. [Google Scholar] [CrossRef]
- Kruse, C.; Kruse, A.; DiRaimo, J. Immune Thrombocytopenia: The Patient’s Perspective. Ann. Blood 2021, 6, 9. [Google Scholar] [CrossRef]
- Cines, D.B.; Liebman, H.; Stasi, R. Pathobiology of Secondary Immune Thrombocytopenia. Semin. Hematol. 2009, 46, S2–S14. [Google Scholar] [CrossRef] [PubMed]
- Eto, K.; Kunishima, S. Linkage between the Mechanisms of Thrombocytopenia and Thrombopoiesis. Blood 2016, 127, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Audia, S.; Mahévas, M.; Samson, M.; Godeau, B.; Bonnotte, B. Pathogenesis of Immune Thrombocytopenia. Autoimmun. Rev. 2017, 16, 620–632. [Google Scholar] [CrossRef]
- Swinkels, M.; Rijkers, M.; Voorberg, J.; Vidarsson, G.; Leebeek, F.W.G.; Jansen, A.J.G. Emerging Concepts in Immune Thrombocytopenia. Front. Immunol. 2018, 9, 880. [Google Scholar] [CrossRef]
- Vrbensky, J.R.; Nazy, I.; Clare, R.; Larché, M.; Arnold, D.M. T Cell–Mediated Autoimmunity in Immune Thrombocytopenia. Eur. J. Haematol. 2022, 108, 18–27. [Google Scholar] [CrossRef]
- Ye, Q.; Jiang, H.; Liao, X.; Chen, K.; Li, S. Identification and Validation of Gene Expression Pattern and Signature in Patients with Immune Thrombocytopenia. SLAS Discov. 2017, 22, 187–195. [Google Scholar] [CrossRef]
- Kerrigan, S.; Moran, N.; Kerrigan, S.; Moran, N. The Non-Thrombotic Role of Platelets in Health and Disease; InTech: Rijeka, Croatia, 2015; ISBN 978-953-51-2208-1. [Google Scholar]
- Ding, B.; Liu, L.; Li, M.; Song, X.; Zhang, Y.; Xia, A.; Liu, J.; Zhou, H. Anti-GPIb/IX Autoantibodies Are Associated with Poor Response to Dexamethasone Combined with Rituximab Therapy in Primary Immune Thrombocytopenia Patients. Platelets 2023, 34, 2258988. [Google Scholar] [CrossRef]
- Peng, J.; Ma, S.-H.; Liu, J.; Hou, Y.; Liu, X.-M.; Niu, T.; Xu, R.-R.; Guo, C.-S.; Wang, X.-M.; Cheng, Y.-F.; et al. Association of Autoantibody Specificity and Response to Intravenous Immunoglobulin G Therapy in Immune Thrombocytopenia: A Multicenter Cohort Study. J. Thromb. Haemost. 2014, 12, 497–504. [Google Scholar] [CrossRef]
- Walter, O.; Ribes, A.; Germain, J.; Rieu, J.-B.; Comont, T.; Plat, A.; Rivière, B.; Deluche, L.; Delarue, L.; LeGoff, I.; et al. Association between Megakaryocyte Abnormalities on Bone Marrow Smear and Response to Thrombopoietin Receptor Agonists in Adult Patients with Primary Immune Thrombocytopenia. Platelets 2022, 33, 1153–1158. [Google Scholar] [CrossRef]
- Bussel, J.B.; Garcia, C.A. Diagnosis of Immune Thrombocytopenia, Including Secondary Forms, and Selection of Second-Line Treatment. Haematologica 2022, 107, 2018–2036. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carballeira, D.; Bernardo, Á.; Caro, A.; Soto, I.; Gutiérrez, L. Pathophysiology, Clinical Manifestations and Diagnosis of Immune Thrombocytopenia: Contextualization from a Historical Perspective. Hematol. Rep. 2024, 16, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Shan, N. Megakaryocytic Dysfunction in Immune Thrombocytopenia Is Linked to Autophagy. Cancer Cell Int. 2019, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Jurk, K.; Shiravand, Y. Platelet Phenotyping and Function Testing in Thrombocytopenia. J. Clin. Med. 2021, 10, 1114. [Google Scholar] [CrossRef]
- Quach, M.E.; Dragovich, M.A.; Chen, W.; Syed, A.K.; Cao, W.; Liang, X.; Deng, W.; De Meyer, S.F.; Zhu, G.; Peng, J.; et al. Fc-Independent Immune Thrombocytopenia via Mechanomolecular Signaling in Platelets. Blood 2018, 131, 787–796. [Google Scholar] [CrossRef]
- Hauswirth, A.W.; Skrabs, C.; Schützinger, C.; Raderer, M.; Chott, A.; Valent, P.; Lechner, K.; Jäger, U. Autoimmune Thrombocytopenia in Non-Hodgkin’s Lymphomas. Haematologica 2008, 93, 447–450. [Google Scholar] [CrossRef]
- Fattizzo, B.; Barcellini, W. Autoimmune Cytopenias in Chronic Lymphocytic Leukemia: Focus on Molecular Aspects. Front. Oncol. 2020, 9, 1435. [Google Scholar] [CrossRef]
- Woo, T.; Carter, M.; Follows, G.; Patten, P.E. Case Report: Successful Treatment of Refractory Immune Thrombocytopenia in Chronic Lymphocytic Leukaemia with Venetoclax Monotherapy. Front. Oncol. 2023, 13, 1260003. [Google Scholar] [CrossRef]
- Kurihara, Y.; Taoka, K.; Takagi, E.; Toyama, K.; Nakazaki, K.; Kurokawa, M. Treatment of Secondary Immune Thrombocytopenia with Non-Hodgkin Lymphoma: A Case Report and Literature Review. Intern. Med. 2021, 60, 1583–1588. [Google Scholar] [CrossRef]
- Cerreto, M.; Foà, R.; Natoni, A. The Role of the Microenvironment and Cell Adhesion Molecules in Chronic Lymphocytic Leukemia. Cancers 2023, 15, 5160. [Google Scholar] [CrossRef]
- Visco, C.; Ruggeri, M.; Laura Evangelista, M.; Stasi, R.; Zanotti, R.; Giaretta, I.; Ambrosetti, A.; Madeo, D.; Pizzolo, G.; Rodeghiero, F. Impact of Immune Thrombocytopenia on the Clinical Course of Chronic Lymphocytic Leukemia. Blood 2008, 111, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Maura, F.; Visco, C.; Novella, E.; Giaretta, I.; Tuana, G.; Agnelli, L.; Lionetti, M.; Fabris, S.; Guidotti, F.; Reda, G.; et al. Immune Thrombocytopenia in Patients with Chronic Lymphocytic Leukemia Is Associated with Stereotyped B-Cell Receptors. Blood 2011, 118, 2847. [Google Scholar] [CrossRef]
- Ghosh, K.; Shome, D.K.; Kulkarni, B.; Ghosh, M.K.; Ghosh, K. Fibrosis and Bone Marrow: Understanding Causation and Pathobiology. J. Transl. Med. 2023, 21, 703. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Kim, Y.K.; Min, S.H.; Kim, S.W.; Lee, Y.H.; Lee, J.M. Epidemiology and Viral Etiology of Pediatric Immune Thrombocytopenia through Korean Public Health Data Analysis. J. Clin. Med. 2021, 10, 1356. [Google Scholar] [CrossRef]
- Stasi, R.; Sarpatwari, A.; Segal, J.B.; Osborn, J.; Evangelista, M.L.; Cooper, N.; Provan, D.; Newland, A.; Amadori, S.; Bussel, J.B. Effects of Eradication of Helicobacter Pylori Infection in Patients with Immune Thrombocytopenic Purpura: A Systematic Review. Blood 2009, 113, 1231–1240. [Google Scholar] [CrossRef]
- Korzeniowska, K.; Cieślewicz, A.; Wietlicka, I.; Jabłecka, A. Secondary Immune Thrombocytopenia after Streptococcus Infection. Hematol. Clin. Pract. 2021, 12, 29–32. [Google Scholar] [CrossRef]
- Ayesh Haj Yousef, M.H.; Alawneh, K.M. Candida Albicans-Induced Chronic Thrombocytopenic Purpura. Acta Haematol. 2011, 126, 202–204. [Google Scholar] [CrossRef]
- Lacerda, M.V.G.; Mourão, M.P.G.; Coelho, H.C.C.; Santos, J.B. Thrombocytopenia in Malaria: Who Cares? Mem. Inst. Oswaldo Cruz 2011, 106 (Suppl. S1), 52–63. [Google Scholar] [CrossRef]
- Ji, L.; Cheng, Y. Treg Cell Abnormality and Its Potential Treatments in Patients with Primary Immune Thrombocytopenia. Clin. Transl. Discov. 2022, 2, e277. [Google Scholar] [CrossRef]
- Georgi, J.-A.; Middeke, J.M.; Bornhäuser, M.; Matzdorff, A.; Trautmann-Grill, K. Deciphering the Genetic Basis of Immune Thrombocytopenia: Current Evidence for Genetic Predisposition in Adult ITP. Blood Adv. 2023, 7, 3710–3724. [Google Scholar] [CrossRef]
- ITP and Cancer Risk: More Data and Some Questions—Chronic Immune Thrombocytopenia: Meeting the Challenge. Available online: https://www.medpagetoday.com/resource-centers/chronic-immune-thrombocytopenia-meeting-challenge/itp-and-cancer-risk-more-data-and-some-questions/3163 (accessed on 11 October 2024).
- Kuter, D.J. Managing Thrombocytopenia Associated with Cancer Chemotherapy. Oncology 2015, 29, 282. [Google Scholar] [PubMed]
- Tan, J.H.; Ahmad Azahari, A.H.S.; Ali, A.; Ismail, N.A.S. Scoping Review on Epigenetic Mechanisms in Primary Immune Thrombocytopenia. Genes 2023, 14, 555. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Sonar, S.; Nyahatkar, S.; Kalele, K.; Adhikari, M.D. Role of DNA Methylation in Cancer Development and Its Clinical Applications. Clin. Transl. Discov. 2024, 4, e279. [Google Scholar] [CrossRef]
- Lakshminarasimhan, R.; Liang, G. The Role of DNA Methylation in Cancer. Adv. Exp. Med. Biol. 2016, 945, 151–172. [Google Scholar] [CrossRef]
- Lu, Y.; Chan, Y.-T.; Tan, H.-Y.; Li, S.; Wang, N.; Feng, Y. Epigenetic Regulation in Human Cancer: The Potential Role of Epi-Drug in Cancer Therapy. Mol. Cancer 2020, 19, 79. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, H.; Wang, R.; Chen, Y.; Ouyang, X.; Li, W.; Sun, Y.; Peng, A. Cancer Epigenetics: From Laboratory Studies and Clinical Trials to Precision Medicine. Cell Death Discov. 2024, 10, 28. [Google Scholar] [CrossRef]
- Kanwal, R.; Gupta, S. Epigenetic Modifications in Cancer. Clin. Genet. 2012, 81, 303–311. [Google Scholar] [CrossRef]
- Hassler, M.R.; Egger, G. Epigenomics of Cancer—Emerging New Concepts. Biochimie 2012, 94, 2219–2230. [Google Scholar] [CrossRef]
- Lee, D.Y. Cancer Epigenomics and Beyond: Advancing the Precision Oncology Paradigm. J. Immunother. Precis. Oncol. 2020, 3, 147–156. [Google Scholar] [CrossRef]
- Fardi, M.; Solali, S.; Farshdousti Hagh, M. Epigenetic Mechanisms as a New Approach in Cancer Treatment: An Updated Review. Genes Dis. 2018, 5, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Gonçalves, V.; Lameirinhas, A.; Henrique, R.; Jerónimo, C. Metabolism and Epigenetic Interplay in Cancer: Regulation and Putative Therapeutic Targets. Front. Genet. 2018, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cui, S.; Wang, Y.; Xu, R. The Extensive Regulation of MicroRNA in Immune Thrombocytopenia. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221093595. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Pelosi, E.; Castelli, G.; Labbaye, C. miR-146 and miR-155: Two Key Modulators of Immune Response and Tumor Development. Non-Coding RNA 2017, 3, 22. [Google Scholar] [CrossRef]
- Stahlhut, C.; Slack, F.J. MicroRNAs and the Cancer Phenotype: Profiling, Signatures and Clinical Implications. Genome Med. 2013, 5, 111. [Google Scholar] [CrossRef]
- Li, M.; Gao, L.; Dang, Y.; Li, P.; Li, Z.; Chen, G.; Luo, D. Protective Potential of miR-146a-5p and Its Underlying Molecular Mechanism in Diverse Cancers: A Comprehensive Meta-Analysis and Bioinformatics Analysis. Cancer Cell Int. 2019, 19, 167. [Google Scholar] [CrossRef]
- Labbaye, C.; Testa, U. The Emerging Role of MIR-146A in the Control of Hematopoiesis, Immune Function and Cancer. J. Hematol. Oncol. 2012, 5, 13. [Google Scholar] [CrossRef]
- Zhang, L.; He, X.; Li, F.; Pan, H.; Huang, X.; Wen, X.; Zhang, H.; Li, B.; Ge, S.; Xu, X.; et al. The miR-181 Family Promotes Cell Cycle by Targeting CTDSPL, a Phosphatase-like Tumor Suppressor in Uveal Melanoma. J. Exp. Clin. Cancer Res. 2018, 37, 15. [Google Scholar] [CrossRef]
- Khodadi, E.; Asnafi, A.A.; Shahrabi, S.; Shahjahani, M.; Saki, N. Bone Marrow Niche in Immune Thrombocytopenia: A Focus on Megakaryopoiesis. Ann. Hematol. 2016, 95, 1765–1776. [Google Scholar] [CrossRef]
- Kashiwagi, H.; Tomiyama, Y. Pathophysiology and Management of Primary Immune Thrombocytopenia. Int. J. Hematol. 2013, 98, 24–33. [Google Scholar] [CrossRef]
- Immune Thrombocytopenia (ITP)—Diagnosis and Treatment—Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/idiopathic-thrombocytopenic-purpura/diagnosis-treatment/drc-20352330 (accessed on 11 September 2024).
- Immune Thrombocytopenia (ITP)—Symptoms and Causes. Available online: https://www.mayoclinic.org/diseases-conditions/idiopathic-thrombocytopenic-purpura/symptoms-causes/syc-20352325 (accessed on 11 September 2024).
- Nascimento, F.G.; Tanaka, P.Y. Thrombocytopenia in HIV-Infected Patients. Indian J. Hematol. Blood Transfus. 2012, 28, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Che, L.; Bi, H.; Fan, S.; Zhou, Z.; Min, H. Clinical Features and Treatment Effect of HIV-Associated Immune Thrombocytopenia—Single Center Ten-Years Data Summary. Platelets 2023, 34, 2200836. [Google Scholar] [CrossRef] [PubMed]
- Pishmisheva-Peleva, M.; Kotsev, S.; Emin, D.; Simonoski, N.; Shopova, M.; Argirova, R. Severe Thrombocytopenia in Primary EBV- Infection with No Signs of Infectious Mononucleosis. A Case Report. IDCases 2022, 30, e01643. [Google Scholar] [CrossRef] [PubMed]
- Páez-Guillán, E.-M.; Campos-Franco, J.; Alende, R.; Gonzalez-Quintela, A. Hematological Abnormalities Beyond Lymphocytosis During Infectious Mononucleosis: Epstein-Barr Virus-Induced Thrombocytopenia. Mediterr. J. Hematol. Infect. Dis. 2023, 15, e2023023. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.G.; Alanazi, N.; Yousef, A.; Alanazi, N.; Alotaibi, B.; Aljurf, M.; El Fakih, R. COVID-19 Associated with Immune Thrombocytopenia: A Systematic Review and Meta-Analysis. Expert Rev. Hematol. 2022, 15, 157–166. [Google Scholar] [CrossRef]
- Nguyen, H.; Nguyen, M.; Olenik, A. Immune Thrombocytopenic Purpura Following COVID-19 Infection: A Case Report and Literature Review. Cureus 2023, 15, e39342. [Google Scholar] [CrossRef]
- Shinno, K.; Banno, Y.; Kamimaki, I. Severe Immune Thrombocytopenia That Developed Immediately after COVID-19 in a School-Aged Patient: A Case Report. Front. Pediatr. 2023, 11, 1120093. [Google Scholar] [CrossRef]
- Seyedi, S.; Navid, S.; Saadatian, Z. Relapse of Immune Thrombocytopenia after Receiving AstraZeneca Coronavirus Disease-2019 Vaccine: A Case Report. Clin. Case Rep. 2023, 11, e7872. [Google Scholar] [CrossRef]
- Alaeddini, M.; Etemad-Moghadam, S. SARS-Cov-2 Infection in Cancer Patients, Susceptibility, Outcome and Care. Am. J. Med. Sci. 2022, 364, 511–520. [Google Scholar] [CrossRef]
- Lee, E.J.; Beltrami-Moreira, M.; Al-Samkari, H.; Cuker, A.; DiRaimo, J.; Gernsheimer, T.; Kruse, A.; Kessler, C.; Kruse, C.; Leavitt, A.D.; et al. SARS-CoV-2 Vaccination and ITP in Patients with de Novo or Preexisting ITP. Blood 2022, 139, 1564–1574. [Google Scholar] [CrossRef]
- Kuwana, M. Helicobacter Pylori-Associated Immune Thrombocytopenia: Clinical Features and Pathogenic Mechanisms. World J. Gastroenterol. 2014, 20, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Ihtesham, A.; Maqbool, S.; Nadeem, M.; Bilawal Abbas Janjua, M.; Sundus, O.; Bakht Naqqash, A.; Inayat Mohamed, W.; Turab Haider, S.; Ahmad, M.; Ahmad Talha Mustafa, M.; et al. Helicobacter Pylori Induced Immune Thrombocytopenic Purpura and Perspective Role of Helicobacter Pylori Eradication Therapy for Treating Immune Thrombocytopenic Purpura. AIMS Microbiol. 2021, 7, 284–303. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hong, J.; Chung, H.; Koh, Y.; Cho, S.-J.; Byun, J.M.; Kim, S.G.; Kim, I. Helicobacter Pylori Eradication Affects Platelet Count Recovery in Immune Thrombocytopenia. Sci. Rep. 2020, 10, 9370. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhou, H.; Holden, V.K.; Deepak, J.; Dhilipkannah, P.; Todd, N.W.; Stass, S.A.; Jiang, F. Streptococcus Pneumoniae Promotes Lung Cancer Development and Progression. iScience 2023, 26, 105923. [Google Scholar] [CrossRef] [PubMed]
- Sagar, A.E.S.; Evans, S.E. Pneumonia in the Cancer Patient. In Oncologic Critical Care; Nates, J.L., Price, K.J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 607–623. ISBN 978-3-319-74588-6. [Google Scholar]
- Yu, J.C.; Shliakhtsitsava, K.; Wang, Y.M.; Paul, M.; Farnaes, L.; Wong, V.; Kim, J.; Thornburg, C.D. Hematologic Manifestations of Nutritional Deficiencies: Early Recognition Is Essential to Prevent Serious Complications. J. Pediatr. Hematol. Oncol. 2019, 41, e182–e185. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.N.; Ghani, U.; Surani, S.; Aftab, A. Vitamin B12 Deficiency, a Rare Cause of Isolated Thrombocytopenia in Adults. Cureus 2023, 15, e44162. [Google Scholar] [CrossRef]
- Kaye, A.D.; Jeha, G.M.; Moll, V.; Ward, C.T.; Watson, M.R.; Wynn, J.T.; Hockstein, M.A.; Hall, K.M.; Viswanath, O.; Urits, I.; et al. Platelet Dysfunction Diseases and Conditions: Clinical Implications and Considerations. Adv. Ther. 2020, 37, 3707–3722. [Google Scholar] [CrossRef]
- Immune Thrombocytopenia (ITP)—Hematology and Oncology. Available online: https://www.merckmanuals.com/professional/hematology-and-oncology/thrombocytopenia-and-platelet-dysfunction/immune-thrombocytopenia-itp (accessed on 11 September 2024).
- Liu, X.; Hou, Y.; Hou, M. How We Treat Primary Immune Thrombocytopenia in Adults. J. Hematol. Oncol. 2023, 16, 4. [Google Scholar] [CrossRef]
- Bijaya, M.; Ansari, Z.; Koshy, B.; Sunder, A. Immune Thrombocytopenia Secondary to COVID-19 in a Vitamin B12-Deficient Patient: A Diagnostic Dilemma and Therapeutic Challenge. Cureus 2023, 15, e40199. [Google Scholar] [CrossRef]
- Sabry, W.; Elemary, M.; Burnouf, T.; Seghatchian, J.; Goubran, H. Vitamin B12 Deficiency and Metabolism-Mediated Thrombotic Microangiopathy (MM-TMA). Transfus. Apher. Sci. 2020, 59, 102717. [Google Scholar] [CrossRef]
- Schulz, E.; Holanda, R.A.R.; Filho, F.D.R.; de Lima Henn, G.A.; Oliveira, R.A. Bone Marrow Effects of High Dose Folic Acid Therapy in Chronic Idiopathic Thrombocytopenic Purpura. Blood 2005, 106, 4016. [Google Scholar] [CrossRef]
- DeLoughery, E.P.; Ravindran, A.; Ashrani, A.A.; Begna, K.H.; Hook, C.C.; Marshall, A.L.; Pruthi, R.K.; Wolanskyj-Spinner, A.P.; Go, R.S. Patterns and Utility of Vitamin B12 and Folate Testing in Patients with Isolated Thrombocytopenia. Ann. Hematol. 2019, 98, 1993–1994. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Rodionov, D.A.; Osterman, A.L.; Peterson, S.N. B Vitamins and Their Role in Immune Regulation and Cancer. Nutrients 2020, 12, 3380. [Google Scholar] [CrossRef] [PubMed]
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and Its Impact on Cancer Risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar] [CrossRef]
- Zwart, N.R.K.; Franken, M.D.; Tissing, W.J.E.; Lubberman, F.J.E.; McKay, J.A.; Kampman, E.; Kok, D.E. Folate, Folic Acid, and Chemotherapy-Induced Toxicities: A Systematic Literature Review. Crit. Rev. Oncol. Hematol. 2023, 188, 104061. [Google Scholar] [CrossRef]
- Alkan, A.; Mızrak, D.; Utkan, G. Lower Folate Levels in Gastric Cancer: Is It a Cause or a Result? World J. Gastroenterol. 2015, 21, 4101–4102. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Chiang, E.-P.; Shih, Y.-T.; Lane, H.-Y.; Lin, J.-T.; Wu, C.-Y. Lower Serum Folate Is Associated with Development and Invasiveness of Gastric Cancer. World J. Gastroenterol. 2014, 20, 11313–11320. [Google Scholar] [CrossRef]
- Kim, Y.-I. Role of Folate in Colon Cancer Development and Progression. J. Nutr. 2003, 133, 3731S–3739S. [Google Scholar] [CrossRef]
- Miranti, E.H.; Stolzenberg-Solomon, R.; Weinstein, S.J.; Selhub, J.; Männistö, S.; Taylor, P.R.; Freedman, N.D.; Albanes, D.; Abnet, C.C.; Murphy, G. Low Vitamin B12 Increases Risk of Gastric Cancer: A Prospective Study of One-Carbon Metabolism Nutrients and Risk of Upper Gastrointestinal Tract Cancer. Int. J. Cancer 2017, 141, 1120–1129. [Google Scholar] [CrossRef]
- Aoyama, T.; Hara, K.; Maezawa, Y.; Kazama, K.; Hashimoto, I.; Sawazaki, S.; Komori, K.; Tamagawa, H.; Tamagawa, A.; Kano, K.; et al. Clinical Course of Vitamin B12 Deficiency and Associated Risk Factors in Patients After Total Gastrectomy for Gastric Cancer. Anticancer Res. 2023, 43, 689–694. [Google Scholar] [CrossRef]
- Waheed, F.; Naseer, N.; Ahmed, T.; Nelson, J.C. Two Patients with Heparin-Induced Thrombocytopenia Followed by Idiopathic (Immune) Thrombocytopenic Purpura: Case Report. Am. J. Hematol. 2003, 73, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Al-Jafar, H.; Al-Yousef, A.; Al-Shatti, S.; Al-Banwan, K. Drug-Immune Thrombocytopenia with Thrombosis versus Heparin-Induced Thrombocytopenia: A Critical Clinical Controversy. Case Rep. Nephrol. Dial. 2015, 5, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Matzdorff, A.; Beer, J.-H. Immune Thrombocytopenia Patients Requiring Anticoagulation—Maneuvering Between Scylla and Charybdis. Semin. Hematol. 2013, 50, S83–S88. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E. Immunologic Effects of Heparin Associated with Hemodialysis: Focus on Heparin-Induced Thrombocytopenia. Semin. Nephrol. 2023, 43, 151479. [Google Scholar] [CrossRef]
- Chen, A.; Basit, M.; Monogue, L.M. A Large-Scale Retrospective Study on Thrombocytopenia Associated with Beta-Lactam Antibiotics. AMIA Annu. Symp. Proc. 2023, 2022, 359–367. [Google Scholar]
- Savage-Elliott, I.; Wu, V.J.; Sanchez, F.L. Drug-Induced Thrombocytopenia Secondary to Commonly Used Antibiotics in Total Joint Arthroplasty. Arthroplast. Today 2020, 6, 137–140. [Google Scholar] [CrossRef]
- Slaught, M.; Rasmussen, M.; Bougie, D.; Aster, R. Immune Thrombocytopenia Induced by Beta-Lactam Antibiotics: Cross-Reactions of Responsible Antibodies with Other Beta-Lactam Drugs. Transfusion 2021, 61, 1600–1608. [Google Scholar] [CrossRef]
- Drygalski, A.V.; Curtis, B.R.; Bougie, D.W.; McFarland, J.G.; Ahl, S.; Limbu, I.; Baker, K.R.; Aster, R.H. Vancomycin-Induced Immune Thrombocytopenia. N. Engl. J. Med. 2007, 356, 904–910. [Google Scholar] [CrossRef]
- MacDougall, K.N.; Parylo, S.; Sokoloff, A. A Case of Vancomycin-Induced Immune Thrombocytopenia. Cureus 2020, 12, e7940. [Google Scholar] [CrossRef]
- Yamanouchi, J.; Hato, T.; Shiraishi, S.; Takeuchi, K.; Yakushijin, Y.; Yasukawa, M. Vancomycin-Induced Immune Thrombocytopenia Proven by the Detection of Vancomycin-Dependent Anti-Platelet Antibody with Flow Cytometry. Intern. Med. 2016, 55, 3035–3038. [Google Scholar] [CrossRef]
- Dixit, R.; George, J.; Sharma, A.K. Thrombocytopenia Due to Rifampicin. Lung India 2012, 29, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Singh, A.; Chandra, A.; Kumar, S.; Gupta, R.K. Rifampicin-Induced Thrombocytopenia. Indian J. Pharmacol. 2010, 42, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Buoli, M.; Serati, M.; Botturi, A.; Altamura, A.C. The Risk of Thrombocytopenia During Valproic Acid Therapy: A Critical Summary of Available Clinical Data. Drugs R D 2018, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.; Shammout, A.; Asif, M.; Mullikin, A. Valproic Acid-Induced Thrombocytopenia in Treatment-Resistant GABRB3 Genetic Epilepsy: A Case Report. Cureus 2024, 16, e57030. [Google Scholar] [CrossRef]
- Kumar, R.; Chivukula, S.; Katukuri, G.R.; Chandrasekhar, U.K.; Shivashankar, K.N. Carbamazepine Induced Thrombocytopenia. J. Clin. Diagn. Res. 2017, 11, OD12–OD13. [Google Scholar] [CrossRef]
- Chen, W.; Lv, X.; Rong, R.; Wu, B. Carbamazepine-Induced Immune Thrombocytopenia Confirmed by Modified MASPAT Test. Transfus. Apher. Sci. 2021, 60, 103228. [Google Scholar] [CrossRef]
- Moulis, G.; Sommet, A.; Sailler, L.; Lapeyre-Mestre, M.; Montastruc, J.-L.; French Association of Regional Pharmacovigilance Centers. Drug-Induced Immune Thrombocytopenia: A Descriptive Survey in the French PharmacoVigilance Database. Platelets 2012, 23, 490–494. [Google Scholar] [CrossRef]
- Larson, J. A Rare Case of Immune Thrombocytopenia After Unintentional Acetaminophen Overdose. Grad. Med. Educ. Res. J. 2022, 4, 41. [Google Scholar] [CrossRef]
- Lara, J.P.; Santana, Y.; Gaddam, M.; Ali, A.; Malik, S.; Khaja, M. Diclofenac-Induced Thrombotic Thrombocytopenic Purpura with Concomitant Complement Dysregulation: A Case Report and Review of the Literature. J. Med. Case Rep. 2019, 13, 190. [Google Scholar] [CrossRef]
- Kuter, D.J. Treatment of Chemotherapy-Induced Thrombocytopenia in Patients with Non-Hematologic Malignancies. Haematologica 2022, 107, 1243–1263. [Google Scholar] [CrossRef]
- Efe, O.; Goyal, L.; Galway, A.; Zhu, A.X.; Niles, J.L.; Zonozi, R. Treatment of Gemcitabine-Induced Thrombotic Microangiopathy Followed by Gemcitabine Rechallenge with Eculizumab. Kidney Int. Rep. 2021, 6, 1464–1468. [Google Scholar] [CrossRef] [PubMed]
- Khaja, M.; Qureshi, Z.A.; Kandhi, S.; Altaf, F.; Yapor, L. Mitomycin-Induced Thrombotic Thrombocytopenic Purpura Treated Successfully with Plasmapheresis and Steroid: A Case Report. Cureus 2022, 14, e23525. [Google Scholar] [CrossRef] [PubMed]
- El-Ghazal, R.; Podoltsev, N.; Marks, P.; Chu, E.; Saif, M.W. Mitomycin–C-Induced Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome: Cumulative Toxicity of an Old Drug in a New Era. Clin. Color. Cancer 2011, 10, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Crosara, S.L.; Qumari, S.; Wall, G.C.; Belz, M.M. Mitomycin-C-Induced Thrombotic Thrombocytopenic Purpura. J. Pharm. Pract. Res. 2013, 43, 221–224. [Google Scholar] [CrossRef]
- Zhao, Z.-W.; Kang, W.-M.; Ma, Z.-Q.; Ye, X.; Yu, J.-C. Gastric Cancer with Severe Immune Thrombocytopenia: A Case Report. World J. Clin. Cases 2018, 6, 1024–1028. [Google Scholar] [CrossRef]
- Cockrell, D.C.; Kasthuri, R.S.; Altun, E.; Rose, T.L.; Milowsky, M.I. Secondary Immune Thrombocytopenia in Metastatic Renal Cell Carcinoma: A Case Report and Discussion of the Literature. Case Rep. Oncol. 2020, 13, 1349–1356. [Google Scholar] [CrossRef]
- Krauth, M.-T.; Puthenparambil, J.; Lechner, K. Paraneoplastic Autoimmune Thrombocytopenia in Solid Tumors. Crit. Rev. Oncol. Hematol. 2012, 81, 75–81. [Google Scholar] [CrossRef]
- Ghanavat, M.; Ebrahimi, M.; Rafieemehr, H.; Maniati, M.; Behzad, M.M.; Shahrabi, S. Thrombocytopenia in Solid Tumors: Prognostic Significance. Oncol. Rev. 2019, 13, 413. [Google Scholar] [CrossRef]
- Adelborg, K.; Veres, K.; Horváth-Puhó, E.; Clouser, M.; Saad, H.; Sørensen, H.T. Risk and Adverse Clinical Outcomes of Thrombocytopenia among Patients with Solid Tumors—A Danish Population-Based Cohort Study. Br. J. Cancer 2024, 130, 1485–1492. [Google Scholar] [CrossRef]
- Grinsztejn, E.; Perez, J.A.; DeSouza, S.I. Immunotherapy-Associated Immune Thrombocytopenia: Treatment Paradigms. Blood 2023, 142, 1210. [Google Scholar] [CrossRef]
- Vyas, P.; Wózniak, K.; Dudek, M.; Grzelka, E.; Cieslak, M.; Dryja, A.; Kraj, L.; Waszczuk-Gajda, A.; Vyas, S. Primary Immune Thrombocytopenia and Breast Cancer: Case Report and Review of Literature. J. Hematol. Blood Disord. 2017, 2, 1–5. [Google Scholar] [CrossRef]
- Ekstrand, C.; Bahmanyar, S.; Cherif, H.; Kieler, H.; Linder, M. Cancer Risk in Patients with Primary Immune Thrombocytopenia—A Swedish Nationwide Register Study. Cancer Epidemiol. 2020, 69, 101806. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, H.; Anand, K.; Liu, S.; Shah, S. Multiple Myeloma with Concurrent Immune Thrombocytopenic Purpura. Ecancermedicalscience 2020, 14, 1012. [Google Scholar] [CrossRef] [PubMed]
- Charalampous, C.; Goel, U.; Kapoor, P.; Binder, M.; Buadi, F.; Dingli, D.; Dispenzieri, A.; Fonder, A.; Gertz, M.; Gonsalves, W.; et al. Association of Thrombocytopenia with Disease Burden, High-Risk Cytogenetics, and Survival in Newly Diagnosed Multiple Myeloma Patients Treated with Novel Therapies. Clin. Lymphoma Myeloma Leuk. 2024, 24, e329–e335. [Google Scholar] [CrossRef]
- McMillan, R. Update on Chronic Immune Thrombocytopenic Purpura (ITP). J. Hematol. Oncol. 2009, 2, A5. [Google Scholar] [CrossRef]
- Cines, D.B.; Bussel, J.B.; Liebman, H.A.; Luning Prak, E.T. The ITP Syndrome: Pathogenic and Clinical Diversity. Blood 2009, 113, 6511–6521. [Google Scholar] [CrossRef]
- Haddad, T.C.; Zhao, S.; Li, M.; Patel, S.H.; Johns, A.; Grogan, M.; Lopez, G.; Miah, A.; Wei, L.; Tinoco, G.; et al. Immune Checkpoint Inhibitor-Related Thrombocytopenia: Incidence, Risk Factors and Effect on Survival. Cancer Immunol. Immunother. 2021, 71, 1157–1165. [Google Scholar] [CrossRef]
- Bagley, S.J.; Kosteva, J.A.; Evans, T.L.; Langer, C.J. Immune Thrombocytopenia Exacerbated by Nivolumab in a Patient with Non-Small-Cell Lung Cancer. Cancer Treat. Commun. 2016, 6, 20–23. [Google Scholar] [CrossRef]
- Liu, X.; Liang, X.; Liang, J.; Li, Y.; Wang, J. Immune Thrombocytopenia Induced by Immune Checkpoint Inhibitors in Solid Cancer: Case Report and Literature Review. Front. Oncol. 2020, 10, 530478. [Google Scholar] [CrossRef]
- Xie, W.; Hu, N.; Cao, L. Immune Thrombocytopenia Induced by Immune Checkpoint Inhibitrs in Lung Cancer: Case Report and Literature Review. Front. Immunol. 2021, 12, 790051. [Google Scholar] [CrossRef]
- Kaneko, Y.; Saito, S.; Takahashi, D.; Ui, T.; Haruta, H.; Kurashina, K.; Yamaguchi, H.; Hosoya, Y.; Kitayama, J.; Lefor, A.K.; et al. Combined Subtotal Gastrectomy and Splenectomy after Partial Splenic Embolization for a Patient with Gastric Cancer and Immune Thrombocytopenic Purpura: A Case Report. Int. J. Surg. Case Rep. 2019, 62, 140–143. [Google Scholar] [CrossRef]
- Zahra, F.T.; Ajmal, Z.; Ashraf, M.F.; Martin, T.C. S1824 A Rare Case of Immune Thrombocytopenic Purpura as Initial Presentation of Right-Sided Colon Cancer. Off. J. Am. Coll. Gastroenterol. ACG 2020, 115, S943. [Google Scholar] [CrossRef]
- Kilpatrick, K.; Shaw, J.L.; Jaramillo, R.; Toler, A.; Eisen, M.; Sangaré, L.; Soff, G.A. Occurrence and Management of Thrombocytopenia in Metastatic Colorectal Cancer Patients Receiving Chemotherapy: Secondary Analysis of Data from Prospective Clinical Trials. Clin. Color. Cancer 2021, 20, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Yacob, M.; Raju, R.; Vyas, F.; Joseph, P.; Sitaram, V. Management of Colorectal Cancer Liver Metastasis in a Patient with Immune Thrombocytopaenia. Ann. R. Coll. Surg. Engl. 2013, 95, e26–e27. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Gupta, S. Acute Drop of Platelets in Metastatic Colon Cancer. Clin. Case Rep. 2017, 5, 1862–1864. [Google Scholar] [CrossRef]
- Woo, H.S.; Lee, K.H.; Yoon, P.H.; Kim, S.J.; Park, I.; Kim, Y.S.; Ahn, H.K.; Hong, J.; Shin, D.B.; Sym, S.J. Oxaliplatin-Induced Immune-Mediated Thrombocytopenia: A Case Report. Cancer Res. Treat. 2015, 47, 949–953. [Google Scholar] [CrossRef]
- Tam, E.L.; Draksharam, P.L.; Park, J.A.; Sidhu, G.S. Acute Immune-Mediated Thrombocytopenia Due to Oxaliplatin and Irinotecan Therapy. Case Rep. Oncol. Med. 2019, 2019, 4314797. [Google Scholar] [CrossRef]
- Pang, Q.; Qu, K.; Bi, J.-B.; Liu, S.-S.; Zhang, J.-Y.; Song, S.-D.; Lin, T.; Xu, X.-S.; Wan, Y.; Tai, M.-H.; et al. Thrombocytopenia for Prediction of Hepatocellular Carcinoma Recurrence: Systematic Review and Meta-Analysis. World J. Gastroenterol. 2015, 21, 7895–7906. [Google Scholar] [CrossRef]
- Lim, H.I.; Cuker, A. Thrombocytopenia and Liver Disease: Pathophysiology and Periprocedural Management. Hematol. Am. Soc. Hematol. Educ. Program 2022, 2022, 296–302. [Google Scholar] [CrossRef]
- Schrecker, C.; Waidmann, O.; El Youzouri, H.; Trojan, J.; Schnitzbauer, A.A.; Bechstein, W.O.; Zeuzem, S.; Koch, C. Low Platelet Count Predicts Reduced Survival in Potentially Resectable Hepatocellular Carcinoma. Curr. Oncol. 2022, 29, 1475–1487. [Google Scholar] [CrossRef]
- Liver Cancer May Unexpectedly Be Influenced by Platelets|Center for Cancer Research. Available online: https://ccr.cancer.gov/news/article/liver-cancer-may-unexpectedly-be-influenced-by-platelets (accessed on 11 September 2024).
- Peng, W.; Li, C.; Zhang, X.; Wen, T.; Chen, Z. The Impact of Thrombocytopenia on Prognosis of HBV-Related Small Hepatocellular Carcinoma: A Propensity Score Matching Analysis. World J. Surg. Oncol. 2021, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Kodaz, H.; Bekir Hacioglu, M.; Elpen Kodaz, C.; Cinkaya, A.; Erdogan, B.; Cicin, I. Endometrial Carcinoma and Paraneoplastic Immune Thrombocytopenia. J. Oncol. Sci. 2016, 2, 25–26. [Google Scholar] [CrossRef]
- Chehal, A.; Taher, A.; Seoud, M.; Shamseddine, A. Idiopathic Thrombocytopenic Purpura and Ovarian Cancer. Eur. J. Gynaecol. Oncol. 2003, 24, 539–540. [Google Scholar] [PubMed]
- Shimada, T.; Saito, T.; Choi, I.; Yamaguchi, S.; Shimamoto, K.; Ariyoshi, K.; Okadome, M. Immune Thrombocytopenia Associated with Solid Cancer. J. Obstet. Gynaecol. Res. 2015, 41, 1495–1498. [Google Scholar] [CrossRef]
- Nagao, S.; Fujiwara, K.; Imafuku, N.; Kozuka, Y.; Kagawa, R.; Oda, T.; Maehata, K.; Ishikawa, H.; Koike, H.; Kohno, I. Relationship between Thrombocytopenia and Survival of Patients with Epithelial Ovarian Cancer (EOC) Who Received Paclitaxel and Carboplatin Chemotherapy. J. Clin. Oncol. 2005, 23, 5049. [Google Scholar] [CrossRef]
- Catoiu, C.A.; Tanase, I.C.; Stoica, B.G.; Paun, S.C. Severe Thrombocytopenia Cured After Ovarian Tumours Surgical Removal. Acta Sci. Med. Sci. 2021, 5, 21–25. [Google Scholar] [CrossRef]
- Bajorin, D.F.; McCaffrey, J.A.; Dodd, P.M.; Hilton, S.; Mazumdar, M.; Kelly, W.K.; Herr, H.; Scher, H.I.; Icasiano, E.; Higgins, G. Ifosfamide, Paclitaxel, and Cisplatin for Patients with Advanced Transitional Cell Carcinoma of the Urothelial Tract: Final Report of a Phase II Trial Evaluating Two Dosing Schedules. Cancer 2000, 88, 1671–1678. [Google Scholar] [CrossRef]
- Üyetürk, Ü.; Arslan, S.; Yüksel, M.; Altuntas, F. Paclitaxel Therapy and Immune Thrombocytopenic Purpura: Coincidence or Association? Turk. J. Hematol. 2011, 28, 151–152. [Google Scholar] [CrossRef]
- Wakana, K.; Yasugi, T.; Nako, Y.; Nei, T.; Ozaki, Y.; Mizutani, K. Successful Surgical Treatment and Chemotherapy for Ovarian Cancer in a Patient with Idiopathic Thrombocytopenic Purpura. Int. J. Clin. Oncol. 2011, 16, 447–449. [Google Scholar] [CrossRef]
- Nandigam, K. Severe Immune Thrombocytopenia in a Case of Cervical Carcinoma. Int. J. Cancer Res. 2006, 2, 159–160. [Google Scholar] [CrossRef]
- Liu, N.; Lv, D.; Schneider, R.R.; Yang, H.; Zhang, M.; Liu, Y.; Sun, M. Intracavitary Cardiac Metastasis of Cervical Squamous Cell Carcinoma with Immune Thrombocytopenia: A Rare Case Report. Front. Oncol. 2023, 13, 1239606. [Google Scholar] [CrossRef] [PubMed]
- Karateke, A.; Kaplanoglu, M.; Baloglu, A. Relations of Platelet Indices with Endometrial Hyperplasia and Endometrial Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 4905–4908. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wu, Z.; Xia, T.; Liu, D.; Yang, Y.; Tang, H. Pre-Treatment Thrombocytosis Predicts Prognosis of Endometrial Cancer: A Meta-Analysis of 11 Studies. Exp. Ther. Med. 2020, 19, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Vontela, N.R.; Lane, R.B.; Kovesdy, C.; Weir, A. The Incidence and Characterization of ITP in Prostate Cancer. Blood 2015, 126, 4646. [Google Scholar] [CrossRef]
- Qiu, G.; Li, S.; Li, B.; Yang, Q.; Deng, H.; Yang, Y.; Xie, X.; Lin, X.; Seki, N.; Miura, S.; et al. Immune Thrombocytopenia in a Small Cell Lung Cancer Patient Treated with Atezolizumab: A Case Report. Transl. Lung Cancer Res. 2022, 11, 2346–2355. [Google Scholar] [CrossRef]
- Ogawa, R.; Satoh, H.; Ishii, Y.; Ohtsuka, M. Chemotherapy for Small Cell Lung Cancer in a Patient with Idiopathic Thrombocytopenic Purpura. Respir. Med. Extra 2007, 3, 117–119. [Google Scholar] [CrossRef]
- Ohta, H.; Nagai, Y.; Shiihara, J.; Ohyanagi, F.; Hagiwara, K.; Koyama, S. Thrombocytopenia Due to Bone Marrow Metastasis of Small Cell Lung Cancer That Was Stabilized by Chemotherapy. Ann. Cancer Res. Ther. 2019, 27, 8–11. [Google Scholar] [CrossRef]
- Niu, J.; Goldin, T.; Markman, M.; Kundranda, M.N. Metastatic Breast Cancer with Extensive Osseous Metastasis Presenting with Symptomatic Immune Thrombocytopenic Purpura and Anemia: A Case Report and Review of the Literature. Case Rep. Oncol. 2015, 8, 256–263. [Google Scholar] [CrossRef]
- Yang, H.; He, F.; Yuan, T.; Xu, W.; Cao, Z. Clinical Features and Treatment of Bone Marrow Metastasis. Oncol. Lett. 2023, 26, 332. [Google Scholar] [CrossRef]
- Kołda, A.; Helbig, G.; Kopińska, A.; Wichary, R.; Pająk, J.; Kyrcz-Krzemień, S. Metastasis of Solid Tumors into Bone Marrow—Single Center Experience. Acta Haematol. Pol. 2017, 48, 130–134. [Google Scholar] [CrossRef]
- Betsch, D.M.; Gray, S.; Zed, S.E. A Case of Metastatic Prostate Cancer and Immune Thrombocytopenia. Curr. Oncol. 2017, 24, e434–e436. [Google Scholar] [CrossRef] [PubMed]
- Khasraw, M.; Baron-Hay, S. Immune Thrombocytopenic Purpura (ITP) and Breast Cancer. Does Adjuvant Therapy for Breast Cancer Improve Platelet Counts in ITP? Ann. Oncol. 2009, 20, 1282–1283. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Iasonos, A.; Mironov, S.; Scattergood, J.; Donat, S.M.; Bochner, B.H.; Herr, H.W.; Russo, P.; Boyle, M.G.; Bajorin, D.F. Prospective Trial of Ifosfamide, Paclitaxel, and Cisplatin (ITP) in Patients with Advanced Non-Transitional Cell (Non-TCC) Carcinomas of the Urothelial Tract. J. Clin. Oncol. 2006, 24, 4542. [Google Scholar] [CrossRef]
- Gao, A.; Zhang, L.; Zhong, D. Chemotherapy-Induced Thrombocytopenia: Literature Review. Discov. Oncol. 2023, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Albiol, S.; Ramírez De Olano, A.; Pujadas, J.; Maroto, P. Gemcitabine in the Treatment of Advanced Transitional Cell Carcinoma of the Urothelium. Ann. Oncol. 2006, 17, v113–v117. [Google Scholar] [CrossRef]
- Toyomasu, Y.; Shimabukuro, R.; Moriyama, H.; Eguchi, D.; Ishikawa, K.; Kishihara, F.; Fukuyama, Y.; Matsumata, T.; Mochiki, E.; Kuwano, H. Successful Perioperative Management of a Patient with Idiopathic Thrombocytopenic Purpura Undergoing Emergent Appendectomy: Report of a Case. Int. J. Surg. Case Rep. 2013, 4, 898–900. [Google Scholar] [CrossRef]
- George, J.N.; Buchanan, G.R. Surgery in the Patient with ITP. Available online: https://itpsupport.org.uk/wp-content/uploads/2024/04/11.-Surgery-in-the-Patient-with-ITP.pages.pdf (accessed on 11 September 2024).
- Immune Thrombocytopenia Surgical Management. Available online: https://www.rarediseaseadvisor.com/hcp-resource/immune-thrombocytopenia-surgical-management/ (accessed on 11 September 2024).
- Immune Thrombocytopenia (ITP) Treatment & Management: Approach Considerations, Thrombopoietin Receptor Agonists, Treatment in Children. 2023. Available online: https://emedicine.medscape.com/article/202158-treatment?form=fpf (accessed on 11 September 2024).
- Zitek, T.; Weber, L.; Pinzon, D.; Warren, N. Assessment and Management of Immune Thrombocytopenia (ITP) in the Emergency Department: Current Perspectives. Open Access Emerg. Med. 2022, 14, 25–34. [Google Scholar] [CrossRef]
- Madkhali, M.A. Recent Advances in the Management of Immune Thrombocytopenic Purpura (ITP): A Comprehensive Review. Medicine 2024, 103, e36936. [Google Scholar] [CrossRef]
- Falanga, A.; Russo, L.; Milesi, V. The Coagulopathy of Cancer. Curr. Opin. Hematol. 2014, 21, 423–429. [Google Scholar] [CrossRef]
- Rho, S.; Wang, C.; Hosseini Dehkordi, S.H.; Sears, J.J.; Hu, Z.I. Bleeding and Thrombotic Events in Bevacizumab-Treated Patients with Colorectal Cancer on Novel Oral Anticoagulants and Antiplatelet Medications. Am. Heart J. Plus Cardiol. Res. Pract. 2023, 27, 100283. [Google Scholar] [CrossRef]
- Deptuła, M.; Zieliński, J.; Wardowska, A.; Pikuła, M. Wound Healing Complications in Oncological Patients: Perspectives for Cellular Therapy. Postepy Dermatol. Alergol. 2019, 36, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kenig, J. Wound Healing in Older Oncologic Patients. Nowotwory. J. Oncol. 2021, 71, 49–51. [Google Scholar] [CrossRef]
- Immunotherapy and Organ-Related Inflammation—Side Effects—NCI. Available online: https://www.cancer.gov/about-cancer/treatment/side-effects/organ-inflammation (accessed on 11 September 2024).
- Dougan, M. Understanding and Overcoming the Inflammatory Toxicities of Immunotherapy. Cancer Immunol. Res. 2020, 8, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Song, Q.; Zhang, P. Metabolic Modifications, Inflammation, and Cancer Immunotherapy. Front. Oncol. 2021, 11, 703681. [Google Scholar] [CrossRef]
- Biolato, M.; Vitale, F.; Galasso, T.; Gasbarrini, A.; Grieco, A. Minimum Platelet Count Threshold before Invasive Procedures in Cirrhosis: Evolution of the Guidelines. World J. Gastrointest. Surg. 2023, 15, 127–141. [Google Scholar] [CrossRef]
- Platelet Transfusion Before Surgery for People with Low Platelet Counts. Available online: https://www.cochrane.org/CD012779/HAEMATOL_platelet-transfusion-surgery-people-low-platelet-counts (accessed on 11 September 2024).
- Estcourt, L.J.; Malouf, R.; Doree, C.; Trivella, M.; Hopewell, S.; Birchall, J. Prophylactic Platelet Transfusions Prior to Surgery for People with a Low Platelet Count. Cochrane Database Syst. Rev. 2017, 2017, CD012779. [Google Scholar] [CrossRef]
- Perioperative Blood Management: Strategies to Minimize Transfusions—UpToDate. Available online: https://www.uptodate.com/contents/perioperative-blood-management-strategies-to-minimize-transfusions#H1920440186 (accessed on 11 September 2024).
- ISBT Platelet Transfusion. Available online: https://www.isbtweb.org/resources/educational-modules-on-clinical-use-of-blood/platelet-transfusion.html (accessed on 11 September 2024).
- Yuan, S.; Otrock, Z.K. Platelet Transfusion. Clin. Lab. Med. 2021, 41, 621–634. [Google Scholar] [CrossRef]
- Wei, Y.; Ji, X.; Wang, Y.; Wang, J.; Yang, E.; Wang, Z.; Sang, Y.; Bi, Z.; Ren, C.; Zhou, F.; et al. High-Dose Dexamethasone vs Prednisone for Treatment of Adult Immune Thrombocytopenia: A Prospective Multicenter Randomized Trial. Blood 2016, 127, 296–302. [Google Scholar] [CrossRef]
- Neunert, C.E. Management of Newly Diagnosed Immune Thrombocytopenia: Can We Change Outcomes? Blood Adv. 2017, 1, 2295–2301. [Google Scholar] [CrossRef]
- Mazzucconi, M.G.; Rodeghiero, F.; Avvisati, G.; De Stefano, V.; Gugliotta, L.; Ruggeri, M.; Vianelli, N.; Fazi, P.; Paoloni, F.; Sargentini, V.; et al. Prednisone vs High-Dose Dexamethasone in Newly Diagnosed Adult Primary Immune Thrombocytopenia: A Randomized Trial. Blood Adv. 2024, 8, 1529–1540. [Google Scholar] [CrossRef]
- Cuker, A.; Liebman, H.A. Corticosteroid Overuse in Adults with Immune Thrombocytopenia: Cause for Concern. Res. Pract. Thromb. Haemost. 2021, 5, e12592. [Google Scholar] [CrossRef] [PubMed]
- Almizraq, R.J.; Branch, D.R. Efficacy and Mechanism of Intravenous Immunoglobulin Treatment for Immune Thrombocytopenia in Adults. Ann. Blood 2021, 6, 2. [Google Scholar] [CrossRef]
- Hansen, R.J.; Balthasar, J.P. Mechanisms of IVIG Action in Immune Thrombocytopenic Purpura. Clin. Lab. 2004, 50, 133–140. [Google Scholar] [PubMed]
- Rassouli, S. A Guide to IVIG Treatment for ITP|AmeriPharma™ Specialty. Available online: https://ameripharmaspecialty.com/a-guide-to-ivig-treatment-for-itp/ (accessed on 11 September 2024).
- Shen, N.; Qiao, J.; Jiang, Y.; Yan, J.; Wu, R.; Yin, H.; Zhu, S.; Li, J. Thrombopoietin Receptor Agonists Use and Risk of Thrombotic Events in Patients with Immune Thrombocytopenic Purpura: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Biomed. Rep. 2024, 20, 44. [Google Scholar] [CrossRef]
- Sanfilippo, K.M.; Cuker, A. TPO-RAs and ITP Remission: Cause or Coincidence? Blood 2023, 141, 2790–2791. [Google Scholar] [CrossRef]
- Pulanić, D.; Bátorová, A.; Bodó, I.; Červinek, L.; Ionita, I.; Lissitchkov, T.; Melikyan, A.; Podolak-Dawidziak, M. Use of Thrombopoietin Receptor Agonists in Adults with Immune Thrombocytopenia: A Systematic Review and Central European Expert Consensus. Ann. Hematol. 2023, 102, 715–727. [Google Scholar] [CrossRef]
- Yassin, M.A.; Al-Rasheed, M.; Al-Khaboori, M.; Marashi, M.; Osman, H.; Wali, Y.; Al Kindi, S.; Alsayegh, F.; Provan, D. Thrombopoietin-Receptor Agonists for Adult Patients with Immune Thrombocytopenia: A Narrative Review and an Approach for Managing Patients Fasting Intermittently. Front. Cardiovasc. Med. 2023, 10, 1260487. [Google Scholar] [CrossRef]
- Zaja, F.; Carpenedo, M.; Baratè, C.; Borchiellini, A.; Chiurazzi, F.; Finazzi, G.; Lucchesi, A.; Palandri, F.; Ricco, A.; Santoro, C.; et al. Tapering and Discontinuation of Thrombopoietin Receptor Agonists in Immune Thrombocytopenia: Real-World Recommendations. Blood Rev. 2020, 41, 100647. [Google Scholar] [CrossRef]
- Neunert, C.; Terrell, D.R.; Arnold, D.M.; Buchanan, G.; Cines, D.B.; Cooper, N.; Cuker, A.; Despotovic, J.M.; George, J.N.; Grace, R.F.; et al. American Society of Hematology 2019 Guidelines for Immune Thrombocytopenia. Blood Adv. 2019, 3, 3829–3866. [Google Scholar] [CrossRef]
- Scott, M. Not So BenignImmune Thrombocytopenia: New and Emerging Therapies for a Challenging “Benign” Hematologic Disease. Hematologist 2024, 21. [Google Scholar] [CrossRef]
- Bussel, J.B.; Kuter, D. Preparing Patients with Immune Thrombocytopenia for Surgery: What Are the Options? Lancet Haematol. 2020, 7, e626–e627. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-J.; Shan, N.-N. Immunomodulatory Cytokine Interleukin-35 and Immune Thrombocytopaenia. J. Int. Med. Res. 2020, 48, 300060520976477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, K.; Li, T.; He, H.; Hou, L.; Wu, X.; Sun, Y.; Zheng, L.; Chen, Z.; Qin, B.; et al. Prednison Provokes Serum and Vasoactive Substances in a Mice Model of Immune Thrombocytopenia. Iran. J. Basic Med. Sci. 2016, 19, 1010–1015. [Google Scholar] [PubMed]
- Li, T.; He, H.; Hou, L.; Xu, Y.; Wu, X.; Sun, Y.; Zheng, L.; Chen, Z.; Chen, X.; Qin, B. Regulation of Non-Classical Immune Parameters in Immune Thrombocytopenic Purpura Mice by a Spleen-Invigorating, Qi-Replenishing and Blood-Containing Formula. J. Tradit. Chin. Med. Sci. 2015, 2, 91–98. [Google Scholar] [CrossRef]
- Thachil, J. Alternate Considerations for Current Concepts in ITP. Hematology 2014, 19, 163–168. [Google Scholar] [CrossRef]
- Yasir, M.; Goyal, A.; Sonthalia, S. Corticosteroid Adverse Effects. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Mithoowani, S.; Gregory-Miller, K.; Goy, J.; Miller, M.C.; Wang, G.; Noroozi, N.; Kelton, J.G.; Arnold, D.M. High-Dose Dexamethasone Compared with Prednisone for Previously Untreated Primary Immune Thrombocytopenia: A Systematic Review and Meta-Analysis. Lancet Haematol. 2016, 3, e489–e496. [Google Scholar] [CrossRef]
- Arai, Y.; Matsui, H.; Jo, T.; Kondo, T.; Takaori-Kondo, A. Efficacy of Dexamethasone for Acute Primary Immune Thrombocytopenia Compared to Prednisolone: A Systematic Review and Meta-Analysis. TH Open 2017, 1, e73–e81. [Google Scholar] [CrossRef]
- Xiao, Z.; Murakhovskaya, I. Rituximab Resistance in ITP and Beyond. Front. Immunol. 2023, 14, 1215216. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Lin, N.; Song, X.; Dai, Q. Eltrombopag Improves Refractory Thrombocytopenia in Patients with Sjögren’s Syndrome. Sci. Prog. 2022, 105, 368504221102786. [Google Scholar] [CrossRef]
- Jain, S.; Gernsheimer, T.; Kolodny, S.; Bernheisel, C.; Vredenburg, M.; Panch, S.R. Additional Efficacy Analysis of Avatrombopag Phase III Data for the Treatment of Adults with Immune Thrombocytopenia. Platelets 2023, 34, 2195016. [Google Scholar] [CrossRef]
- Gonzalez-Lopez, T.J.; Perez Segura, G.; Domingo, A.; Lopez Ansoar, E.; Diaz Galvez, F.J.; Jimenez Barcenas, R.; Martinez Carballeira, D.; De Miguel Llorente, D.; Perona Blazquez, A.; Aguilar-Monserrate, G.; et al. Efficacy and Safety of Fostamatinib for Immune Thrombocytopenia in Clinical Practice in Spain: Interim Results of Fostames, Our National Fostamatinib Registry. Blood 2023, 142, 2591. [Google Scholar] [CrossRef]
- Abdelwahab, O.A.; Mechi, A.; Gahlan, S.; Hamadein, F.-E.; Kadhim, H.; Ismail, D.; Soliman, Y.; El-Samahy, M. Efficacy and Safety of Mycophenolate Mofetil in Patients with Immune Thrombocytopenic Purpura: A Systematic Review and Meta-Analysis. Clin. Rheumatol. 2024, 43, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Hlusi, A.; Szotkowski, T.; Indrak, K. Refractory Immune Thrombocytopenia. Successful Treatment with Repeated Cyclosporine A: Two Case Reports. Clin. Case Rep. 2015, 3, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Pramanik, S.; Sandal, R.; Jandial, A.; Sahu, K.K.; Singh, K.; Khera, S.; Meshram, A.; Khurana, H.; Somasundaram, V.; et al. Safety and Efficacy of Azathioprine in Immune Thrombocytopenia. Am. J. Blood Res. 2021, 11, 217–226. [Google Scholar] [PubMed]
- Maloisel, F.; Andrès, E.; Zimmer, J.; Noel, E.; Zamfir, A.; Koumarianou, A.; Dufour, P. Danazol Therapy in Patients with Chronic Idiopathic Thrombocytopenic Purpura: Long-Term Results. Am. J. Med. 2004, 116, 590–594. [Google Scholar] [CrossRef]
- Hill, Q.A. How Does Dapsone Work in Immune Thrombocytopenia? Implications for Dosing. Blood 2015, 125, 3666–3668. [Google Scholar] [CrossRef]
- Faggiano, A.; Mazzilli, R.; Natalicchio, A.; Adinolfi, V.; Argentiero, A.; Danesi, R.; D’Oronzo, S.; Fogli, S.; Gallo, M.; Giuffrida, D.; et al. Corticosteroids in Oncology: Use, Overuse, Indications, Contraindications. An Italian Association of Medical Oncology (AIOM)/Italian Association of Medical Diabetologists (AMD)/Italian Society of Endocrinology (SIE)/Italian Society of Pharmacology (SIF) Multidisciplinary Consensus Position Paper. Crit. Rev. Oncol. Hematol. 2022, 180, 103826. [Google Scholar] [CrossRef]
- Aldea, M.; Orillard, E.; Mansi, L.; Marabelle, A.; Scotte, F.; Lambotte, O.; Michot, J.-M. How to Manage Patients with Corticosteroids in Oncology in the Era of Immunotherapy? Eur. J. Cancer 2020, 141, 239–251. [Google Scholar] [CrossRef]
- Steroids (Dexamethasone, Prednisolone, Methylprednisolone and Hydrocortisone). Available online: https://www.cancerresearchuk.org/about-cancer/treatment/drugs/steroids (accessed on 11 October 2024).
- Ueno, M.; Takabatake, H.; Hata, A.; Kayahara, T.; Morimoto, Y.; Notohara, K.; Mizuno, M. Mycophenolate Mofetil for Immune Checkpoint Inhibitor-Related Hepatotoxicity Relapsing during Dose Reduction of Corticosteroid: A Report of Two Cases and Literature Review. Cancer Rep. 2022, 5, e1624. [Google Scholar] [CrossRef]
- Leckel, K.; Beecken, W.-D.; Jonas, D.; Oppermann, E.; Coman, M.C.; Beck, K.-F.; Cinatl, J.; Hailer, N.P.; Auth, M.K.H.; Bechstein, W.O.; et al. The Immunosuppressive Drug Mycophenolate Mofetil Impairs the Adhesion Capacity of Gastrointestinal Tumour Cells. Clin. Exp. Immunol. 2003, 134, 238–245. [Google Scholar] [CrossRef]
- Kadokawa, Y.; Inoue, S.; Tatsumi, A.; Uchida, M.; Fujita, K.; Takagi, M.; Inoue, T.; Ohe, S.; Nakai, Y.; Otsuka, T.; et al. Efficacy and Safety of Mycophenolate Mofetil in Treating Immune-related Hepatitis Induced by Immune Checkpoint Inhibitor Use: A Retrospective Study. JGH Open 2023, 7, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Durnian, J.M.; Stewart, R.M.; Tatham, R.; Batterbury, M.; Kaye, S.B. Cyclosporin-A Associated Malignancy. Clin. Ophthalmol. 2007, 1, 421–430. [Google Scholar] [PubMed]
- Cockburn, I.T.R.; Krupp, P. The Risk of Neoplasms in Patients Treated with Cyclosporine A. J. Autoimmun. 1989, 2, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Hojo, M.; Morimoto, T.; Maluccio, M.; Asano, T.; Morimoto, K.; Lagman, M.; Shimbo, T.; Suthanthiran, M. Cyclosporine Induces Cancer Progression by a Cell-Autonomous Mechanism. Nature 1999, 397, 530–534. [Google Scholar] [CrossRef]
- Mohammadi, O.; Kassim, T.A. Azathioprine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Pasternak, B.; Svanström, H.; Schmiegelow, K.; Jess, T.; Hviid, A. Use of Azathioprine and the Risk of Cancer in Inflammatory Bowel Disease. Am. J. Epidemiol. 2013, 177, 1296–1305. [Google Scholar] [CrossRef]
- Ashfaq, S.; Pellegrini, M.V.; Can, A.S. Danazol. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Danazol: Uses, Dosage, Side Effects. Available online: https://www.drugs.com/danazol.html (accessed on 11 October 2024).
- Urbancic, K.F.; Pisasale, D.; Wight, J.; Trubiano, J.A. Dapsone Safety in Hematology Patients: Pathways to Optimizing Pneumocystis Jirovecii Pneumonia Prophylaxis in Hematology Malignancy and Transplant Recipients. Transpl. Infect. Dis. 2018, 20, e12968. [Google Scholar] [CrossRef]
- Subramaniam, A.; Corallo, C.; Nagappan, R. Dapsone-Associated Methaemoglobinaemia in Patients with a Haematologic Malignancy. Anaesth. Intensive Care 2010, 38, 1070–1076. [Google Scholar] [CrossRef]
- Hanif, N.; Anwer, F. Rituximab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Rituximab: Principles of Use and Adverse Effects in Rheumatologic Disease—UpToDate. Available online: https://www.uptodate.com/contents/rituximab-principles-of-use-and-adverse-effects-in-rheumatologic-disease (accessed on 11 October 2024).
- Rituximab. Available online: https://go.drugbank.com/drugs/DB00073 (accessed on 11 October 2024).

| Primary ITP | Secondary ITP | |
|---|---|---|
| Definition | This is a form of ITP in which no clear cause can be identified. It is a diagnosis of exclusion, meaning that other causes of thrombocytopenia must be ruled out before it can be diagnosed. | In this case, thrombocytopenia develops as a result of known predisposing factors, such as other diseases, infections, medications, or tumors. Secondary ITP is a reaction to these factors and is not a primary autonomic disorder. |
| Etiology | Pathogenesis is associated with an abnormal immune response against one’s own platelets. This leads to the formation of autoantibodies against platelet surface antigens, leading to their destruction, mainly in the spleen. The exact cause of this immune response is unknown, making this type idiopathic. | It has well-defined causes, such as follows:
|
| Immunological mechanism | Autoantibodies, mainly IgG, bind to platelet surface antigens such as glycoproteins GPIIb/IIIa and GPIb/IX. Coating platelets with these antibodies leads to their recognition by Fcγ receptors on the surface of macrophages, which facilitates their phagocytosis, mainly in the spleen and liver. This mechanism shortens the lifespan of platelets from the normal 7–10 days to only a few hours, leading to a significant reduction in platelet counts in peripheral blood and, consequently, to thrombocytopenia. In addition to destroying circulating platelets, ITP can also affect megakaryocytes, the precursor cells responsible for platelet production in the bone marrow. Autoantibodies can bind to megakaryocytes, inhibiting their maturation and production of new platelets. As a result, ITP is associated with excessive platelet destruction and reduced production of new platelets, which further worsens thrombocytopenia | The immunological mechanism is similar but results from immune activation as a result of another disease. These include the destruction of megakaryocyte precursors by the immune system, exposure to certain drugs, toxins, infections, and other disease states such as aplastic anemia. The resulting reduction in megakaryocyte precursors and ineffective regulation of thrombopoiesis may lead to secondary ITP. Factors that may reduce the number of megakaryocyte precursors include cancer, such as myelodysplasia, acute leukemia, or metastatic bone marrow disease, which can disrupt the average production and function of these cells. In these cases, the bone marrow environment is altered, leading to impaired megakaryopoiesis. In addition, the destruction of megakaryocyte precursors by the immune system, exposure to certain toxins such as benzene, and the use of certain drugs such as quinine can further reduce their numbers. |
| Clinical course | It can be acute or chronic. In children, it is often acute and transient, and in adults, it is more often chronic. In patients with chronic primary ITP, treatment can be long-term, and the disease is characterized by recurrent episodes of thrombocytopenia. | It usually has a more chronic course than primary ITP. Prognosis depends on the underlying disease, and ITP often resolves with successful treatment of the secondary cause (e.g., eradication of infection, control of autoimmune disease, and discontinuation of medication). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kos, M.; Tomaka, P.; Mertowska, P.; Mertowski, S.; Wojnicka, J.; Błażewicz, A.; Grywalska, E.; Bojarski, K. The Many Faces of Immune Thrombocytopenia: Mechanisms, Therapies, and Clinical Challenges in Oncological Patients. J. Clin. Med. 2024, 13, 6738. https://doi.org/10.3390/jcm13226738
Kos M, Tomaka P, Mertowska P, Mertowski S, Wojnicka J, Błażewicz A, Grywalska E, Bojarski K. The Many Faces of Immune Thrombocytopenia: Mechanisms, Therapies, and Clinical Challenges in Oncological Patients. Journal of Clinical Medicine. 2024; 13(22):6738. https://doi.org/10.3390/jcm13226738
Chicago/Turabian StyleKos, Marek, Piotr Tomaka, Paulina Mertowska, Sebastian Mertowski, Julia Wojnicka, Anna Błażewicz, Ewelina Grywalska, and Krzysztof Bojarski. 2024. "The Many Faces of Immune Thrombocytopenia: Mechanisms, Therapies, and Clinical Challenges in Oncological Patients" Journal of Clinical Medicine 13, no. 22: 6738. https://doi.org/10.3390/jcm13226738
APA StyleKos, M., Tomaka, P., Mertowska, P., Mertowski, S., Wojnicka, J., Błażewicz, A., Grywalska, E., & Bojarski, K. (2024). The Many Faces of Immune Thrombocytopenia: Mechanisms, Therapies, and Clinical Challenges in Oncological Patients. Journal of Clinical Medicine, 13(22), 6738. https://doi.org/10.3390/jcm13226738










