A Systematic Review of Dietary Lifestyle Interventions for Neuropathic Pain
Abstract
:1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Outcome Measures
2.4. Data Sources
2.5. Search Strategy
2.6. Study Selection
2.7. Data Extraction
2.8. Statistical Analysis
2.9. Risk of Bias and Certainty of Evidence
3. Results
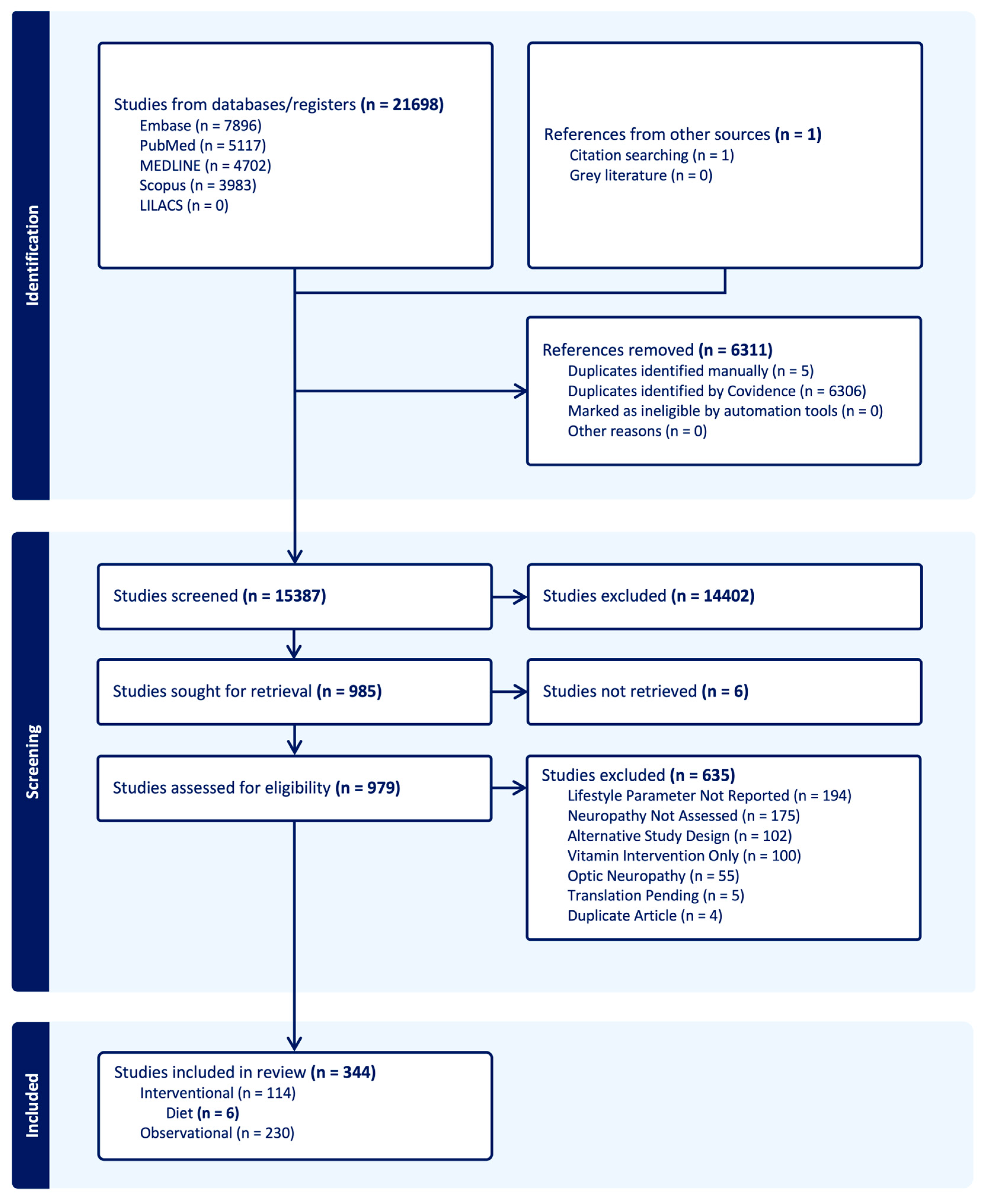
3.1. Literature Search
3.2. Included Studies
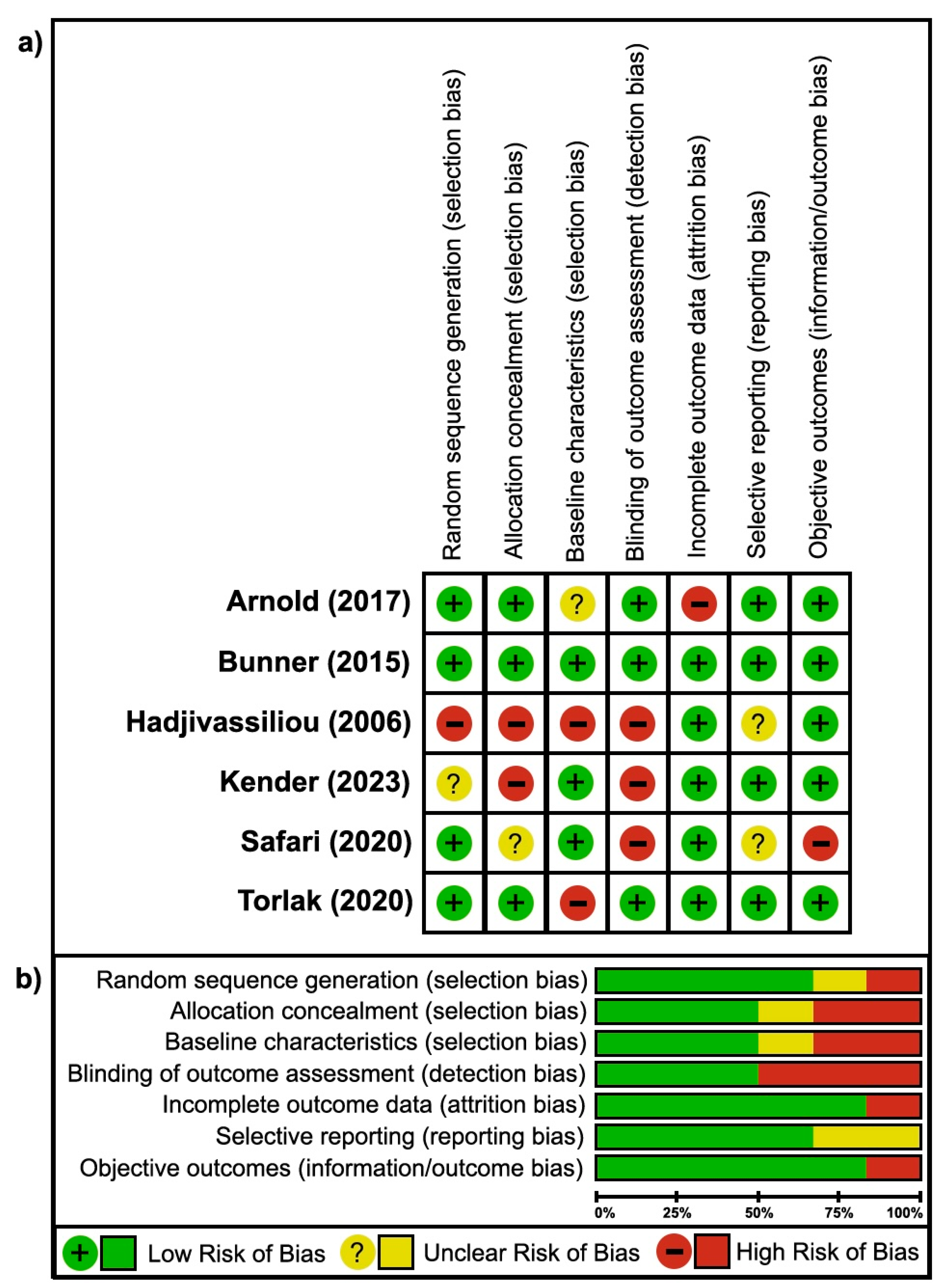
3.3. Risk of Bias
4. Discussion
4.1. Summary of Findings
4.1.1. Summary of Findings-Efficacy
4.1.2. Summary of Findings: Safety and Tolerability
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Glossary
| BPI | Brief Pain Inventory |
| EORTC QLQ C-30 | European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30 |
| FACT-NTX | Functional Assessment of Cancer TherapyNeurotoxicity |
| GPS | Gracely Pain Scale |
| GRADE | Grading of Recommendations, Assessment, Development and Evaluation |
| LANSS | Leeds Assessment of Neuropathic Symptoms and Signs |
| MPQ | McGill Pain Questionnaire |
| MDNS | Michigan Diabetic Neuropathy Score |
| MNSI | Michigan Neuropathy Screening Instrument |
| NCV | nerve conduction velocity |
| NP | neuropathic pain |
| NPS | Neuropathic Pain Scale |
| NQOL | Neuropathy Quality of Life |
| NSS | Neuropathy Symptom Score |
| NTSS | Neuropathy Total Symptom Score |
| NPRS | Numeric Pain Rating Scale |
| PN | peripheral neuropathy |
| PPI | Present Pain Intensity |
| PSS | Pain Severity Scale |
| PNQ | Patient Neurotoxicity Questionnaire |
| QST | quantitative sensory testing |
| RCT | randomized controlled trial |
| ROB | risk of bias |
| SF36 | Short Form-36 Health Survey |
| SPNS | Subjective Peripheral Neuropathy Screening |
| T2DM | Type 2 Diabetes Mellitus |
| mTCNS | modified Toronto Clinical Neuropathy Score |
| TNS | Total Neuropathy Score |
| VAS | Visual Analog Scale |
References
- Smith, B.H.; Hébert, H.L.; Veluchamy, A. Neuropathic pain in the community: Prevalence, impact, and risk factors. Pain 2020, 161, S127–S137. [Google Scholar] [CrossRef] [PubMed]
- de Greef, B.T.A.; Hoeijmakers, J.G.J.; Gorissen-Brouwers, C.M.L.; Geerts, M.; Faber, C.G.; Merkies, I.S.J. Associated conditions in small fiber neuropathy—a large cohort study and review of the literature. Eur. J. Neurol. 2018, 25, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Gierthmühlen, J.; Baron, R. Neuropathic Pain. Semin. Neurol. 2016, 36, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Kerstman, E.; Ahn, S.; Battu, S.; Tariq, S.; Grabois, M. Neuropathic pain. Handb. Clin. Neurol. 2013, 110, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Zilliox, L.A. Neuropathic pain. CONTINUUM Lifelong Learn. Neurol. 2017, 23, 512–532. [Google Scholar] [CrossRef]
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Bennett, M.I.; Rayment, C.; Hjermstad, M.; Aass, N.; Caraceni, A.; Kaasa, S. Prevalence and aetiology of neuropathic pain in cancer patients: A systematic review. Pain 2012, 153, 359–365. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Derry, S.; Bell, R.F.; Rice, A.S.; Tölle, T.R.; Phillips, T.; Moore, R.A. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef]
- Lunn, M.P.; Hughes, R.A.; Wiffen, P.J. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst. Rev. 2014, 2014, CD007115. [Google Scholar] [CrossRef]
- Moore, R.A.; Derry, S.; Aldington, D.; Cole, P.; Wiffen, P.J. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2012, 12, CD008242. [Google Scholar] [CrossRef]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419838383. [Google Scholar] [CrossRef]
- Moore, R.A.; McQuay, H.J. Prevalence of opioid adverse events in chronic non-malignant pain: Systematic review of randomised trials of oral opioids. Arthritis Res. Ther. 2005, 7, R1046. [Google Scholar] [CrossRef] [PubMed]
- Binder, A.; Baron, R. The pharmacological therapy of chronic neuropathic pain. Dtsch. Arztebl. Int. 2016, 113, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Huang, Y. Advances in the treatment of neuropathic pain. Adv. Exp. Med. Biol. 2016, 904, 117–129. [Google Scholar] [CrossRef]
- Hurley, R.W.; Adams, M.C.B.; Benzon, H.T. Neuropathic pain: Treatment guidelines and updates. Curr. Opin. Anaesthesiol. 2013, 26, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Binder, A.; Wasner, G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef]
- Ghavami, H.; Radfar, M.; Soheily, S.; Shamsi, S.A.; Khalkhali, H.R. Effect of lifestyle interventions on diabetic peripheral neuropathy in patients with type 2 diabetes, result of a randomized clinical trial. Agri 2018, 30, 165–170. [Google Scholar] [CrossRef]
- Bunner, A.E.; Wells, C.L.; Gonzales, J.; Agarwal, U.; Bayat, E.; Barnard, N.D. A dietary intervention for chronic diabetic neuropathy pain: A randomized controlled pilot study. Nutr. Diabetes. 2015, 5, e158. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015, 3, 866–875. [Google Scholar] [CrossRef]
- Barnard, N.D.; Katcher, H.I.; Jenkins, D.J.A.; Cohen, J.; Turner-McGrievy, G. Vegetarian and vegan diets in type 2 diabetes management. Nutr. Rev. 2009, 67, 255–263. [Google Scholar] [CrossRef]
- Crane, M.G.; Sample, C. Regression of diabetic neuropathy with total vegetarian (vegan) diet. J. Nutr. Med. 1994, 4, 431–439. [Google Scholar] [CrossRef]
- Bjørklund, G.; Aaseth, J.; Doşa, M.D.; Pivina, L.; Dadar, M.; Pen, J.J.; Chirumbolo, S. Has human diet a role in reducing nociception related to inflammation and chronic pain? Nutrition 2019, 66, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Mostacci, B.; Liguori, R.; Cicero, A.F. Nutraceutical Approach to Peripheral Neuropathies: Evidence from Clinical Trials. Curr. Drug Metab. 2018, 19, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Mirian, A.; Aljohani, Z.; Grushka, D.; Florendo-Cumbermack, A. Diagnosis and management of patients with polyneuropathy. CMAJ Can. Med. Assoc. J. 2023, 195, E227–E233. [Google Scholar] [CrossRef] [PubMed]
- Aas, A.M.; Axelsen, M.; Churuangsuk, C.; Hermansen, K.; Kendall, C.W.C.; Kahleova, H.; Khan, T.; Lean, M.E.J.; Mann, J.I.; Pedersen, E. Evidence-based European recommendations for the dietary management of diabetes. Diabetologia 2023, 66, 965–985. [Google Scholar] [CrossRef]
- Rowin, J. Integrative neuromuscular medicine: Neuropathy and neuropathic pain: Consider the alternatives. Muscle Nerve 2019, 60, 124–136. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Government of Canada. Canada’s Dietary Guidelines for Health Professionals and Policy Makers. Published online 2019. Available online: https://food-guide.canada.ca/en/guidelines/ (accessed on 30 April 2024).
- BDA: The Association of UK Dietitians. Vegetarian, Vegan, and Plant-Based Diet: Food Fact Sheet. Available online: https://www.bda.uk.com/resource/vegetarian-vegan-plant-based-diet.html (accessed on 17 January 2021).
- Klowak, M.; Boggild, A.K. The efficacy of a whole foods, plant-based dietary lifestyle intervention for the treatment of peripheral neuropathic pain in leprosy: A randomized control trial protocol. Front. Nutr. 2023, 10, 1196470. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Poquet, N.; Lin, C. The Brief Pain Inventory (BPI). J. Physiother. 2016, 62, 52. [Google Scholar] [CrossRef]
- McTaggart-Cowan, H.; King, M.T.; Norman, R.; Costa, D.S.J.; Pickard, A.S.; Regier, D.A.; Viney, R.; Peacock, S.J. The EORTC QLU-C10D: The Canadian Valuation Study and Algorithm to Derive Cancer-Specific Utilities from the EORTC QLQ-C30. MDM Policy Pract. 2019, 4, 2381468319842532. [Google Scholar] [CrossRef]
- Functional Assessment of Cancer Therapy / Gynecologic Oncology Group—Neurotoxicity. FACT-GOG-NTX. Published online 2024. Available online: https://www.facit.org/measures/fact-gog-ntx (accessed on 30 April 2024).
- Gracely, R.H.; Dubner, R. Reliability and vapidity of verbal descriptor scales of painfulness. Pain 1987, 29, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M. The LANSS Pain Scale: The Leeds assessment of neuropathic symptoms and signs. Pain 2001, 92, 147–157. Available online: www.elsevier.nl/locate/pain (accessed on 30 April 2024). [CrossRef]
- Melzack, R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain 1975, 1, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Stevens, M.J.; Thomas, P.K.; Brown, M.B.; Canal, N.; Greene, D.A. A Practical Two-Step Quantitative Clinical and Electrophysiological Assessment for the Diagnosis and Staging of Dianetic Neuropathy. Diabetes Care 1994, 17, 1281–1289. Available online: http://diabetesjournals.org/care/article-pdf/17/11/1281/515039/17-11-1281.pdf (accessed on 30 April 2024). [CrossRef]
- Herman, W.H.; Pop-Busui, R.; Braffett, B.H.; Martin, C.L.; Cleary, P.A.; Albers, J.W.; Feldman, E.L.; The DCCT/EDIC Research Group. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type1 diabetes: Results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet. Med. 2012, 29, 937–944. [Google Scholar] [CrossRef]
- Galer, B.S.; Jensen, M.P. Development and preliminary validation of a pain measure specific to neuropathic pain: The Neuropathic Pain Scale. Neurology 1997, 48, 332–338. [Google Scholar] [CrossRef]
- Vileikyte, L.; Peyrot, M.; Bundy, C.; Rubin, R.R.; Leventhal, H.; Mora, P.; Shaw, J.E.; Baker, P.; Boulton, A.J. The Development and Validation of a Neuropathy-and Foot Ulcer-Specific Quality of Life Instrument. Diabetes Care 2003, 26, 2549–2555. Available online: http://diabetesjournals.org/care/article-pdf/26/9/2549/665146/dc0903002549.pdf (accessed on 30 April 2024). [CrossRef]
- Meijer, J.W.; Smit, A.J.; Sonderen, E.V.; Groothoff, J.W.; Eisma, W.H.; Links, T.P. Symptom scoring systems to diagnose distal polyneuropathy in diabetes: The Diabetic Neuropathy Symptom score. Diabet. Med. 2002, 11, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Bastyr, E.J. Development and Validity Testing of the Neuropathy Total Symptom Score-6: Questionnaire for the Study of Sensory Symptoms of Diabetic Peripheral Neuropathy. Clin. Ther. 2005, 27, 1278–1294. [Google Scholar] [CrossRef]
- Farrar, J.T.; Young, J.P.B.; Lamoreaux, L.; Werth, J.L.; Poole, R.M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001, 94, 149–158. Available online: https://www.elsevier.com/locate/pain (accessed on 30 April 2024).
- Albabtain, M.; Alharbi, H.; Alobiad, N.; Alhasan, J.A.; Alruhaimi, M.; Alnefisah, M.; Alateeq, S.; Alghosoon, H.; Alarfaj, S.J.; Arafat, A.; et al. Pain perception assessment using the short-form McGill pain questionnaire after cardiac surgery. Saudi J. Anaesth. 2020, 14, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Hitt, J.; Lee, R.; Elkin, P. Pain severity scale: A methodology for classifying postoperative pain severity by surgical procedure. Surg. Open Sci. 2023, 12, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Tsoleridis, T.; Chloropoulou, P.; Tsaroucha, A.; Vadalouca, A.; Siafaka, I.; Vogiatzaki, T. Validation of the Patient Neurotoxicity Questionnaire for Patients Suffering from Chemotherapy-Induced Peripheral Neuropathy in Greek. Cureus 2021, 13, e14324. [Google Scholar] [CrossRef]
- Rolfson, O.; Chenok, K.E.; Bohm, E.; Lübbeke, A.; Denissen, G.; Dunn, J.; Lyman, S.; Franklin, P.; Dunbar, M.; Overgaard, S.; et al. Patient-reported outcome measures in arthroplasty registries: Report of the Patient-Reported Outcome Measures Working Group of the International Society of Arthroplasty Registries: Part I. Overview and rationale for patient-reported outcome measures. Acta Orthop. 2016, 87, 3–8. [Google Scholar] [CrossRef]
- Mcarthur, J.H. The Reliability and Validity of the Subjective Peripheral Neuropathy Screen. J. Assoc. Nurses AIDS Care 1998, 9, 84–94. [Google Scholar] [CrossRef]
- Bril, V.; Tomioka, S.; Buchanan, R.A.; Perkins, B.A. Reliability and validity of the modified Toronto Clinical Neuropathy Score in diabetic sensorimotor polyneuropathy. Diabet. Med. 2009, 26, 240–246. [Google Scholar] [CrossRef]
- Smith, E.M.L.; Beck, S.L.; Cohen, J. The total neuropathy score: A tool for measuring chemotherapy-induced peripheral neuropathy. Oncol. Nurs. Forum 2008, 35, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Langley, G.B.; Sheppeard, H. The visual analogue scale: Its use in pain measurement. Rheumatol. Int. 1985, 5, 145–148. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490–1494. [Google Scholar] [CrossRef]
- Joanna Briggs Institute. Checklist for Systematic Reviews and Research Syntheses. Available online: https://jbi.global/critical-appraisal-tools (accessed on 30 April 2024).
- Arnold, R.; Pianta, T.J.; Pussell, B.A.; Kirby, A.; O’brien, K.; Sullivan, K.; Holyday, M.; Cormack, C.; Kiernan, M.C.; Krishnan, A.V. Randomized, controlled trial of the effect of dietary potassium restriction on nerve function in CKD. Clin. J. Am. Soc. Nephrol. 2017, 12, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Kandler, R.H.; Chattopadhyay, A.K.; Davies-Jones, A.G.B.; Jarratt, J.A.; Sanders, D.S.; Sharrack, B.; Grünewald, R.A. Dietary treatment of gluten neuropathy. Muscle Nerve 2006, 34, 762–766. [Google Scholar] [CrossRef]
- Kender, Z.; von Rauchhaupt, E.; Schwarz, D.; Tsilingiris, D.; Schimpfle, L.; Bartl, H.; Longo, V.D.; Bendszus, M.; Kopf, S.; Herzig, S.; et al. Six-month periodic fasting does not affect somatosensory nerve function in type 2 diabetes patients. Front. Endocrinol. 2023, 14, 1143799. [Google Scholar] [CrossRef] [PubMed]
- Torlak, M.S.; Bagcaci, S.; Akpinar, E.; Okutan, O.; Nazli, M.S.; Kuccukturk, S. The effect of intermittent diet and/or physical therapy in patients with chronic low back pain: A single-blinded randomized controlled trial. Explore 2022, 18, 76–81. [Google Scholar] [CrossRef]
- Safari, M.B.; Nozad, A.; Ghaffari, F.; Ghavamzadeh, S.; Alijaniha, F.; Naseri, M. Efficacy of a Short-Term Low-Calorie Diet in Overweight and Obese Patients with Chronic Sciatica: A Randomized Controlled Trial. J. Altern. Complement. Med. 2020, 26, 508–514. [Google Scholar] [CrossRef] [PubMed]
- The World Bank. World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 30 October 2024).
- De Los Santos-Arteaga, M.; Sierra-Domínguez, S.A.; Fontanella, G.H.; Delgado-García, J.M.; Ngel, A.; Carrión, M. Analgesia induced by dietary restriction is mediated by the κ-opioid system. J. Neurosci. 2003, 23, 11120–11126. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Pluta, R.; Januszewski, S. Ketogenic diet and epilepsy. Nutrients 2019, 11, 2510. [Google Scholar] [CrossRef]
- Jang, S.-P.; Park, S.-H.; Jung, J.-S.; Lee, H.-J.; Hong, J.-W.; Lee, J.-Y.; Suh, H.-W. Characterization of changes of pain behavior and signal transduction system in food-deprived mice. Anim. Cells Syst. 2018, 22, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Storoni, M.; Plant, G.T. The Therapeutic Potential of the Ketogenic Diet in Treating Progressive Multiple Sclerosis. Mult Scler Int. 2015, 2015, 681289. [Google Scholar] [CrossRef]
- Nozad, A.; Safari, M.B.; Saboory, E.; Derafshpoor, L.; Moghaddam, P.M.; Ghaffari, F.; Naseri, M. Caloric restriction and formalin-induced inflammation: An experimental study in rat model. Iran. Red Crescent Med. J. 2015, 17, e22590. [Google Scholar] [CrossRef]
- De Carvalho, T. Calorie restriction or dietary restriction: How far they can protect the brain against neurodegenerative diseases? Neural Regen Res. 2022, 17, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Phoon, R.K.S.; Pussell, B.A.; Charlesworth, J.A.; Bostock, H.; Kiernan, M.C. Altered motor nerve excitability in end-stage kidney disease. Brain 2005, 128, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.; Pussell, B.A.; Howells, J.; Grinius, V.; Kiernan, M.C.; Lin, C.S.-Y.; Krishnan, A.V. Evidence for a causal relationship between hyperkalaemia and axonal dysfunction in end-stage kidney disease. Clin. Neurophysiol. 2014, 125, 179–185. [Google Scholar] [CrossRef]
- Faris, M.A.-I.E.; Kacimi, S.; Al-Kurd, R.A.; Fararjeh, M.A.; Bustanji, Y.K.; Mohammad, M.K.; Salem, M.L. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr. Res. 2012, 32, 947–955. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Fann, D.Y.W.; Ng, G.Y.Q.; Poh, L.; Arumugam, T.V. Positive effects of intermittent fasting in ischemic stroke. Exp. Gerontol. 2017, 89, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Pellecchia, M.; Scala, R.; Perretti, A.; De Michele, G.; Santoro, L.; Filla, A.; Ciacci, C.; Barone, P. Cerebellar ataxia associated with subclinical celiac disease responding to gluten-free diet. Neurology 1999, 53, 1606. [Google Scholar] [CrossRef]
- Klowak, M.; Boggild, A.K. A review of nutrition in neuropathic pain of leprosy. Ther. Adv. Infect. Dis. 2022, 9, 20499361221102663. [Google Scholar] [CrossRef]
| Author (Year) | Setting | N | Mean Age (SD) | Range | Sex N (F:M) | Population/Etiology | Lifestyle | Outcomes (Mean ± SD) |
|---|---|---|---|---|---|---|---|---|
| Bunner (2015) [18] | US | 34 | Int: 57 (6); Con: 58 (6) | Int: 8:9; Con: 11:6 | T2DM + PN | Low-fat plant-based diet + 1000 mcg vitamin B12/day Ψ for 5 months | Efficacy: Improvement of pain on MPQ (22.6 ± 11 vs. 13.5 ± 10 **), MNSI (7.5 ± 2.5 vs. 5.3 ± 2.5 **), and NTSS (10.7 ± 4.9 vs. 6.8 ± 4.5 **) within int. group, and in the change in MPQ (−9.1 ± 11.4 vs. −0.9 ± 11.3 *), MNSI (−2.2 ± 2.4 vs. −0.6 ± 1.5 *), and feet conductance (0.7 ± 10.5 vs. −11.7 ± 13.2 *) between groups | |
| Safety: No AE observed. | ||||||||
| Tolerability: ~76% adherence. | ||||||||
| Kender (2023) [58] | Germany | 31 | Int: 66.6 (5.8); Con: 67.1 (5.9) | 50–75 | Int: 5:12; Con: 5:9 | T2DM | Plant-based fasting-mimicking diet for 1 week/month for 6 months | Efficacy: Improvement in tibial motor nerve conduction velocity (37.23 ± 2.38 vs. 32.89 ± 3.05 *), and HPT (−0.76 ± 0.37 vs. −1.10 ± 0.30 *) within con. group, and tibial nerve compound muscle action potential (7.79 ± 1.24 vs. 9.21 ± 1.45 *) within int. group |
| Safety: Mentioned “low” but AEs not specified | ||||||||
| Tolerability: High adherence and no L2FU | ||||||||
| Safari (2020) [60] | Iran | 96 | Int: 39.67 (10.66); Con: 40.21 (10.46) | Int: 26–59; Con: 24–60 | Int: 20:28; Con: 21:27 | Chronic Sciatica + NP | Low calorie diet for 30 days | Efficacy: Improvement in MPQ sensory (6.73 ± 1.41 vs. 4.46 ± 1.71 ***), affective (0.98 ± 0.64 vs. 0.50 ± 0.62 **), total (7.71 ± 1.69 vs. 4.96 ± 2.02 ***) scores, and PPI (2.23 ± 0.47 vs. 2 ± 0.68 ***) within int. group, PPI (2 ± 0.68 vs. 1.79 ± 1.3 *) within con. group, and MPQ sensory (4.46 ± 1.71 vs. 5.74 ± 2.11 *), affective (0.50 ± 0.62 vs. 0.87 ± 0.85 **), total (4.96 ± 2.02 vs. 6.62 ± 2.53 ***) scores, and PPI (1.02 ± 0.98 vs. 1.79 ± 1.3 **) between groups adjusted for baseline |
| Safety: Not mentioned | ||||||||
| Tolerability: 100% adherence and no L2FU | ||||||||
| Arnold (2017) [56] | Australia | 47 | Int: 67; Con: 66 | 52–69 | Int: 10:13; Con: 7:17 | Stage 3/4 Chronic Kidney Disease | Potassium- reduced diet (1 mmol/kg/day) § for 2 years | Efficacy: Improvement in the change in TNS (0.4 ± 2.2 vs. 2.8 ± 3.3 **) and nerve excitability score (5.1 ± 2.8 vs. −2.3 ± 2.2 *) between groups |
| Safety: No AE observed. | ||||||||
| Tolerability: 8.7% L2FU in int. group & 12.5% L2FU in con. group. | ||||||||
| Hadjivassiliou (2006) [57] | UK | 35 | Int: 67.2 (2); Con: 70.9 (1.9) | Gluten Sensitivity † + PN | Gluten-free diet including counselling from expert dietician for 1 year | Efficacy: Improvement in the change in sural sensory nerve action potential amplitude within the int. group (1.39 ± 0.22 vs. 2.15 ± 0.43 ***), con. group (1.39 ± 0.47 vs. 0.96 ± 0.29 **), and between groups (0.76 ± 0.31 vs. −0.42 ± 0.25 *) | ||
| Safety: Not mentioned. | ||||||||
| Tolerability: High adherence. | ||||||||
| Torlak (2020) [59] | Turkey | 60 | Diet Group: 50.3 (1.64); Diet + PT Group: 54.30 (1.38); PT Group: 54.85 (3.81) | Diet Group: 10:10; Diet + PT Group: 10:10; PT Group: 10:10 | Chronic Lower Back Pain + NP | Intermittent high protein diet (2 days/week) and Mediterranean diet (5 days/week) for 5 weeks | Efficacy: Improvement in VAS (8.3 ± 0.36 vs. 4.7 ± 0.41 ***; 7.45 ± 0.44 vs. 4.7 ± 0.42 ***; 6.65 ± 0.31 vs. 3.1 ± 0.59 ***) and LANSS (4.8 ± 0.88 vs. 2.3 ± 0.59 ***; 10.6 ± 0.88 vs. 7.1 ± 0.76 ***; 5.1 ± 0.42 vs. 2.6 ± 0.36 ***) within diet group, diet + PT group, and PT group, respectively | |
| Safety: Mentioned “low” but AEs not specified | ||||||||
| Tolerability: 100% adherence and no L2FU |
| Patient or Population: Patients with T2DM and Peripheral Neuropathy Setting: High-Income Countries (United States) (Bunner et al., 2015) [18] Intervention: Low-Fat Plant-Based diet, Plus Vitamin B12 (1000 mcg/Day) Supplementation Comparison: Standard Care | |||||
|---|---|---|---|---|---|
| Outcomes | Anticipated Absolute Effects * | № of Participants (Studies) | Certainty of Evidence (GRADE) | Comments | |
| Risk with Standard Care | Risk with Diet (95% CI) | ||||
| MPQ-SF | The mean change in MPQ-SF was 0 | MD 8.2 lower (−15.83, −0.57) | 34 (1 study) | ⨁⨁⨁⨁ High | Dietary lifestyle intervention reduced pain severity. |
| VAS | The mean change in VAS was 0 | MD 0.8 higher (−1.15, 2.75) | 34 (1 study) | ⨁⨁⨁⨁ High | No difference in VAS. |
| MNSI-Q | The mean change in MNSI-Q was 0 | MD 1.6 lower (−2.95, −0.25) | 34 (1 study) | ⨁⨁⨁⨁ High | Dietary lifestyle intervention reduced neuropathy severity. |
| MNSI-PA | The mean change in MNSI-PA was 0 | MD 0.3 higher (−0.91, 1.51) | 34 (1 study) | ⨁⨁⨁⨁ High | No difference in MNSI-PA. |
| NTSS | The mean change in NTSS was 0 | MD 0.7 lower (−3.33, 1.93) | 34 (1 study) | ⨁⨁⨁⨁ High | No difference in NTSS. |
| Feet Conductance (uS) | The mean change in feet conductance (uS) was 0 | MD 12.4 higher (1.95, 22.85) | 10 (1 study) | ⨁⨁⨁⨁ High | Dietary lifestyle intervention improved foot conductance. |
| Hands Conductance (uS) | The mean change in hands conductance (uS) was 0 | MD 8.9 higher (−2.36, 20.16) | 10 (1 study) | ⨁⨁⨁⨁ High | No difference in hand conductance. |
| Patient or population: patients with T2DM and neuropathic pain Setting: high-income countries (United States) (Kender et al., 2023) [58] Intervention: plant-based fasting-mimicking diet Comparison: standard care | |||||
| Outcomes | Values Reported in Original Manuscript † | № of Participants (studies) | Certainty of Evidence (GRADE) | Comments | |
| Intervention Group | Control Group | ||||
| NSS | Pre: 5.4 ± 0.8 Post: 4.1 ± 1.0 | Pre: 5.5 ± 0.9 Post: 5.6 ± 0.9 | 31 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Cold Detection Threshold | Pre: −1.13 ± 0.28 Post: −1.59 ± 0.25 | Pre: −1.73 ± 0.35 Post: −1.96 ± 0.37 | 31 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Warm Detection Threshold | Pre: −0.77 ± 0.23 Post: −0.95 ± 0.24 | Pre: −1.37 ± 0.21 Post: −1.46 ± 0.30 | 31 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Thermal Sensory Limen | Pre: −1.06 ± 0.20 Post: −1.00 ± 0.19 | Pre: −1.57 ± 0.26 Post: −1.21 ± 0.30 | 31 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Cold Pain Threshold | Pre: −0.45 ± 0.21 Post: −0.28 ± 0.20 | Pre: −0.42 ± 0.23 Post: −0.46 ± 0.22 | 31 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Heat Pain Threshold | Pre: −0.02 ± 0.36 Post: −0.47 ± 0.41 | Pre: −0.76 ± 0.37 Post: −1.10 ± 0.30 | 31 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) reported a statistically significant difference within control group (p < 0.05). |
| Pain Pressure Threshold | Pre: 0.10 ± 0.29 Post: −0.09 ± 0.22 | Pre: −0.38 ± 0.36 Post: −0.47 ± 0.41 | 31 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Mechanical Pain Threshold | Pre: 1.81 ± 0.48 Post: 2.01 ± 0.55 | Pre: 0.71 ± 0.66 Post: 0.36 ± 0.60 | 31 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Mechanical Pain Sensitivity | Pre: 0.65 ± 0.30 Post: 0.77 ± 0.35 | Pre: 0.18 ± 0.49 Post: 0.46 ± 0.52 | 31 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Wind-up Ratio | Pre: −0.17 ± 0.23 Post: 0.04 ± 0.23 | Pre: −0.16 ± 0.24 Post: 0.59 ± 0.53 | 31 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Mechanical Detection Threshold | Pre: −0.30 ± 0.68 Post: −0.78 ± 0.41 | Pre: −1.30 ± 0.64 Post: −1.52 ± 0.52 | 31 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Vibration Detection Threshold | Pre: −1.70 ± 0.65 Post: −1.79 ± 0.62 | Pre: −3.80 ± 0.79 Post: −1.91 ± 0.89 | 31 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Peroneal Compound Muscle Action Potential (uV) | Pre: 5.50 ± 0.97 Post: 4.77 ± 1.09 | Pre: 3.41 ± 0.79 Post: 3.61 ± 0.72 | 30 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Peroneal Motor Nerve Conduction Velocity (m/s) | Pre: 38.65 ± 1.86 Post: 37.71 ± 1.94 | Pre: 37.39 ± 2.28 Post: 37.08 ± 1.94 | 30 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Sural Sensory Nerve Action Potential Amplitude (uV) | Pre: 3.96 ± 1.06 Post: 2.88 ± 0.69 | Pre: 2.46 ± 0.64 Post: 2.10 ± 0.44 | 30 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Sural Sensory Nerve Conduction Velocity (m/s) | Pre: 38.18 ± 1.81 Post: 37.12 ± 2.04 | Pre: 38.54 ± 3.37 Post: 38.54 ± 2.46 | 30 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Tibial Compound Muscle Action Potential (uV) | Pre: 7.79 ± 1.24 Post: 9.21 ± 1.45 | Pre: 6.35 ± 1.47 Post: 6.38 ± 1.49 | 30 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) reported a statistically significant difference within intervention group (p < 0.05). |
| Tibial Motor Nerve Conduction Velocity (m/s) | Pre: 39.29 ± 1.55 Post: 36.29 ± 2.38 | Pre: 37.23 ± 2.38 Post: 32.89 ± 3.05 | 30 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) reported a statistically significant difference within control group (p < 0.05). |
| Sciatic Nerve Fractional Anisotropy | Pre: 0.37 ± 0.02 Post: 0.40 ± 0.02 | Pre: 0.37 ± 0.05 Post: 0.37 ± 0.04 | 13 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Sciatic Nerve T2-Time | Pre: 72.85 ± 3.51 Post: 67.88 ± 3.35 | Pre: 76.94 ± 7.17 Post: 75.21 ± 4.81 | 13 (1 study) | ⨁⨁◯◯ Low | Kender et al. (2023) did not report statistically significant findings. |
| Patient or population: patients with chronic sciatica and neuropathic pain Setting: low-income countries (Iran) (Safari et al., 2020) [60] Intervention: low-calorie diet Comparison: standard care | |||||
| Outcomes | Values Reported in Original Manuscript † | № of Participants (Studies) | Certainty of Evidence (GRADE) | Comments | |
| Intervention Group | Control Group | ||||
| MPQ-SF Sensory | Pre: 6.73 ± 1.41 Post: 4.46 ± 1.71 | Pre: 5.77 ± 1.45 Post: 5.74 ± 2.11 | 96 (1 study) | ⨁⨁◯◯ Low | Safari et al. (2020) reported a statistically significant difference within intervention group (p < 0.001) and between groups (p = 0.015). |
| MPQ-SF Affective | Pre: 0.98 ± 0.64 Post: 0.50 ± 0.62 | Pre: 0.90 ± 0.63 Post: 0.87 ± 0.85 | 96 (1 study) | ⨁⨁◯◯ Low | Safari et al. (2020) reported a statistically significant difference within intervention group (p = 0.002) and between groups (p = 0.002). |
| MPQ-SF Total | Pre: 7.71 ± 1.69 Post: 4.96 ± 2.02 | Pre: 6.63 ± 1.44 Post: 6.62 ± 2.53 | 96 (1 study) | ⨁⨁◯◯ Low | Safari et al. (2020) reported a statistically significant difference within intervention group (p < 0.001) and between groups (p = 0.001). |
| MPQ-SF PPI | Pre: 2.23 ± 0.47 Post: 2 ± 0.68 | Pre: 2 ± 0.68 Post: 1.79 ± 1.3 | 96 (1 study) | ⨁⨁◯◯ Low | Safari et al. (2020) reported a statistically significant difference within intervention group (p = 0.001), control group (p = 0.013), and between groups (p = 0.006). |
| Patient or population: patients with stage 3/4 chronic kidney disease and peripheral neuropathy Setting: high-income countries (Australia) (Arnold et al., 2017) [56] Intervention: potassium reduced diet (1 mmol/kg/day) Comparison: standard care | |||||
| Outcomes | Anticipated absolute effects * | № of Participants (studies) | Certainty of Evidence (GRADE) | Comments | |
| Risk with Standard Care | Risk with Diet (95% CI) | ||||
| TNS | The mean change in TNS was 0 | MD 2.4 lower (−4, −30.8) | 47 (1 study) | ⨁⨁⨁⨁ High | Dietary lifestyle intervention improved neuropathy severity. Certainty upgraded due to large effect size. |
| Median Nerve Composite Excitability Score | The mean change in median nerve composite excitability score was 0 | MD 7.4 higher (5.96, 8.84) | 47 (1 study) | ⨁⨁⨁⨁ High | Dietary lifestyle intervention improved composite nerve excitability score. Certainty upgraded due to very large effect size. |
| Outcomes | Values Reported in Original Manuscript † | № of Participants (Studies) | Certainty of Evidence (GRADE) | Comments | |
| Intervention Group | Control Group | ||||
| SF36-Physical Function (median with IQR) | Pre: 75 (53–90) Post: 70 (40–80) | Pre: 60 (30–95) Post: 60 (26–94) | 47 (1 study) | ⨁⨁⨁◯ Moderate | Arnold et al. (2017) did not report statistically signfiicant findings. |
| Sural Sensory Nerve Action Potential Amplitude (uV) | Pre: 7.1 ± 10.5 Post: 6.2 ± 7.8 | Pre: 8.9 ± 9.4 Post: 7.6 ± 9.3 | 47 (1 study) | ⨁⨁⨁◯ Moderate | Arnold et al. (2017) did not report statistically signfiicant findings. |
| Patient or population: patients with gluten sensitivity and peripheral neuropathy Setting: high-income countries (United Kingdom) (Hadjivassiliou et al., 2006) [57] Intervention: gluten free diet Comparison: standard care | |||||
| Outcomes | Anticipated absolute effects * (95% CI) | № of Participants (Studies) | Certainty of Evidence (GRADE) | Comments | |
| Risk with Standard Care | Risk with Diet (95% CI) | ||||
| Sural Sensory Nerve Action Potential Amplitude (uV) | The mean change in sural sensory nerve action potential amplitude (uV) was 0 | MD 1.18 higher (0.98, 1.38) | 35 (1 study) | ⨁⨁◯◯ Low | Dietary lifestyle intervention improved sural sensory nerve action potential amplitude. |
| Sural Sensory Nerve Conduction Velocity (m/s) | The mean change in sural sensory nerve conduction velocity (m/s) was 0 | MD 2.26 higher (−1.00, 3.52) | 35 (1 study) | ⨁⨁⨁◯ Moderate | No difference in sural sensory nerve conduction velocity. Certainty upgraded due to large effect size. |
| Outcomes | Values Reported in Original Manuscript † | № of Participants (Studies) | Certainty of Evidence (GRADE) | Comments | |
| Intervention Group | Control Group | ||||
| Subjective Neuropathy Perception | 16/25 (64%) reported improvement | 8/10 (80%) reported worsening | 35 (1 study) | ⨁⨁◯◯ Low | Hadjivassiliou et al. (2006) report patients in the control group were statistically significantly less likely to feel their neuropathy had improved (p < 0.0006). |
| Patient or population: patients with chronic lower back pain and neuropathic pain Setting: high-income countries (Turkey) (Torlak et al., 2020) [59] Intervention: intermittent high protein diet and mediterannean diet Comparison: standard care | |||||
| Outcomes | Values Reported in Original Manuscript † | № of Participants (Studies) | Certainty of Evidence (GRADE) | Comments | |
| Diet + PT Group | PT Alone Group | ||||
| LANSS | Pre: 10.6 ± 0.88 Post: 7.1 ± 0.76 | Pre: 5.1 ± 0.42 Post: 2.6 ± 0.36 | 40 (1 study) | ⨁⨁⨁◯ Moderate | Torlak et al. (2020) reported a statistically significant difference within diet + PT group (p < 0.001) and within PT alone group (p < 0.001). |
| VAS | Pre: 7.45 ± 0.44 Post: 4.7 ± 0.42 | Pre: 6.65 ± 0.31 Post: 3.1 ± 0.59 | 40 (1 study) | ⨁⨁⨁◯ Moderate | Torlak et al. (2020) reported a statistically significant difference within diet + PT group (p < 0.001) and within PT alone group (p < 0.001). |
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect; Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klowak, M.; Lau, R.; Mohammed, M.N.; Birago, A.; Samson, B.; Ahmed, L.; Renee, C.; Meconnen, M.; Sam, M.; Boggild, A.K. A Systematic Review of Dietary Lifestyle Interventions for Neuropathic Pain. J. Clin. Med. 2024, 13, 6766. https://doi.org/10.3390/jcm13226766
Klowak M, Lau R, Mohammed MN, Birago A, Samson B, Ahmed L, Renee C, Meconnen M, Sam M, Boggild AK. A Systematic Review of Dietary Lifestyle Interventions for Neuropathic Pain. Journal of Clinical Medicine. 2024; 13(22):6766. https://doi.org/10.3390/jcm13226766
Chicago/Turabian StyleKlowak, Michael, Rachel Lau, Mariyam N. Mohammed, Afia Birago, Bethel Samson, Layla Ahmed, Camille Renee, Milca Meconnen, Mahmud Sam, and Andrea K. Boggild. 2024. "A Systematic Review of Dietary Lifestyle Interventions for Neuropathic Pain" Journal of Clinical Medicine 13, no. 22: 6766. https://doi.org/10.3390/jcm13226766
APA StyleKlowak, M., Lau, R., Mohammed, M. N., Birago, A., Samson, B., Ahmed, L., Renee, C., Meconnen, M., Sam, M., & Boggild, A. K. (2024). A Systematic Review of Dietary Lifestyle Interventions for Neuropathic Pain. Journal of Clinical Medicine, 13(22), 6766. https://doi.org/10.3390/jcm13226766






