Abstract
Background/Objectives: Hepatocellular carcinoma (HCC) is a leading cause of cancer-related mortality worldwide, primarily developing in the context of chronic liver disease. Traditional prevention has focused on liver-specific interventions like antiviral therapies and surveillance. However, extrahepatic factors also significantly contribute to HCC risk. This review explores comprehensive strategies for HCC prevention, including both hepatic and extrahepatic factors. Methods: An extensive literature search of peer-reviewed articles up to October 2024 was conducted, focusing on studies addressing HCC prevention strategies. Studies that focused on both hepatic and extrahepatic factors were included. Data were extracted and synthesized to provide an overview of current prevention strategies and their effectiveness in reducing HCC incidence. Results: Hepatitis B vaccination and antiviral treatments for hepatitis B and C significantly reduce HCC incidence. Lifestyle modifications—such as reducing alcohol consumption, maintaining a healthy weight through diet and exercise, and smoking cessation—are crucial in lowering HCC risk. Environmental measures to limit exposure to aflatoxins and other hazards also contribute to prevention. Regular surveillance of high-risk groups enables early detection and improves survival rates. Emerging strategies like immunotherapy and gene therapy show potential for further reducing HCC risk. Conclusions: A comprehensive approach combining medical interventions, lifestyle changes, and environmental controls is essential for effectively decreasing HCC incidence globally. Implementing these combined measures could significantly reduce the global burden of HCC.
1. Introduction
Hepatocellular carcinoma (HCC), the most common primary liver cancer, represents a significant global health challenge due to its high morbidity and mortality rates. As the third leading cause of cancer-related deaths worldwide, HCC accounted for an estimated 529,202 new cases and 483,875 deaths in 2021, according to the Global Burden of Disease Study, 2021 [1]. The global burden of HCC continues to rise, driven by the rising prevalence of chronic liver diseases and metabolic disorders.
The incidence and mortality rates of HCC vary geographically, with the highest occurrences reported in East Asia and sub-Saharan Africa [2]. This variation is largely attributed to the prevalence of chronic hepatitis B virus (HBV) infection in these regions [3]. Chronic HBV infection remains a predominant etiological factor for HCC, responsible for approximately 50% of cases worldwide [4]. In contrast, the incidence of HCC in Western countries has been rising due to factors such as chronic hepatitis C virus (HCV) infection, alcohol-associated liver disease (ALD), and metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as nonalcoholic fatty liver disease (NAFLD) [5,6]. HBV contributes to hepatocarcinogenesis through direct viral effects, such as integration into the host genome, and indirect effects like chronic inflammation leading to cirrhosis. Similarly, chronic HCV infection increases HCC risk by inducing chronic liver inflammation and fibrosis, eventually progressing to cirrhosis [7].
A recent epidemiological study in the Asia–Pacific region showed an increase in HCC cases caused by steatotic liver disease (SLD), including both ALD and MASLD. This finding aligns with global trends, where the increased prevalence of metabolic syndrome, alcohol consumption, and SLD is often attributed to the widespread adoption of Westernized diets in many countries [8]. Similar to Western countries, these proportions are expected to shift over the next 2–3 decades, with a decline in HCV-related HCC cases and an increase in those linked to metabolic syndrome [9].
ALD remains a significant contributor to HCC development, particularly in regions with high alcohol consumption. Prolonged excessive alcohol intake leads to liver cirrhosis, creating a conducive environment for the malignant transformation of hepatocytes [10]. The emergence of MASLD and its progressive form, metabolic dysfunction-associated steatohepatitis (MASH), has become a growing concern. These conditions are associated with metabolic syndrome components such as obesity, type 2 diabetes, dyslipidemia, and hypertension [11]. MASLD is now recognized as one of the leading causes of chronic liver disease worldwide and is increasingly associated with HCC development, even in the absence of cirrhosis [12]. In addition, obesity and diabetes not only contribute to MASLD but also independently elevate the risk of HCC through mechanisms involving insulin resistance and chronic inflammation [13]. Moreover, autoimmune liver disease, though a less common cause of HCC, has been associated with a lower risk of HCC compared to chronic viral hepatitis, potentially due to differences in the regulatory T cell background [14].
Exposure to aflatoxins, toxic metabolites produced by Aspergillus species contaminating food supplies, is another significant risk factor for HCC, especially in developing countries with inadequate food storage facilities. Aflatoxin B₁ has been implicated in inducing mutations in the p53 tumor suppressor gene, thereby promoting hepatocarcinogenesis [15]. Genetic and metabolic disorders, including hereditary hemochromatosis, alpha-1 antitrypsin deficiency, and Wilson’s disease, also predispose individuals to chronic liver injury and subsequent HCC development [16]. Environmental and lifestyle factors also contribute to HCC risk; for example, tobacco smoking has been associated with an elevated risk of liver cancer due to the presence of carcinogens that can induce DNA damage [17].
Understanding the multifactorial etiology of HCC is essential for developing effective prevention strategies. This comprehensive review aims to explore current approaches to preventing HCC by addressing these diverse risk factors. By focusing on primary prevention through vaccination, antiviral therapies, lifestyle modifications, and environmental interventions, as well as secondary prevention via surveillance and early detection in high-risk populations, we aim to contribute to the reduction in HCC incidence and mortality worldwide.
2. Primary Prevention Strategies
2.1. Vaccination Programs
Hepatitis B Virus Vaccination
Vaccination against HBV is one of the most effective primary prevention measures for HCC. Universal HBV vaccination programs have significantly reduced the prevalence of HBV infection and the incidence of HBV-related HCC, particularly in endemic regions [18]. A landmark study in Taiwan demonstrated that the incidence of HCC in children decreased substantially following the implementation of a nationwide HBV vaccination program [19]. The World Health Organization (WHO) recommends that all infants receive the HBV vaccine within 24 h of birth, followed by at least two additional doses, to decrease mother-to-child transmission (MTCT) globally [20]. Therefore, HBV vaccination remains an essential component of global health strategies aimed at reducing the burden of HCC. Additionally, infants born to hepatitis B surface antigen-positive mothers should receive hepatitis B immunoglobulin (HBIG) within 12–24 h of birth [21].
2.2. Hepatitis C Virus Vaccine Development
Currently, there is no approved vaccine for hepatitis C virus (HCV) due to the virus’s high genetic variability and the complex immune responses required for protection [22]. However, ongoing research aims to develop an effective HCV vaccine by inducing a broad spectrum of neutralizing antibodies and a strong cellular immune response to overcome these challenges. Such a vaccine could significantly reduce new infections and, consequently, HCV-related hepatocellular carcinoma (HCC) cases by preventing chronic infection and subsequent liver disease progression [23]. Recently, a phase 1–2 randomized, double-blind, placebo-controlled trial evaluated an HCV vaccine regimen using a recombinant chimpanzee adenovirus 3 vector, followed by a booster with modified vaccinia Ankara, in adults at risk for HCV infection. While the vaccine was safe, induced HCV-specific T-cell responses, and reduced peak HCV RNA levels, it did not significantly prevent chronic HCV infection compared to placebo [24].
4. Lifestyle Modifications
4.1. Alcohol Consumption Reduction
Chronic alcohol intake is a well-established risk factor for HCC, as it leads to liver cirrhosis and promotes carcinogenesis through mechanisms such as oxidative stress, inflammation, and impaired immune surveillance [44]. Studies have demonstrated that decreasing alcohol consumption lowers the incidence of HCC, particularly in populations with high rates of ALD [45]. Currently, there is no longer a so-called ‘safe amount’ of alcohol intake [46]. A large database study included 28 million individuals globally indicated that the level of alcohol consumption that minimizes harm from alcohol is zero [47]. Similarly, a 4.9-year follow-up study of 58,927 Korean MASLD patients reported that light and moderate drinkers were also associated with worsening fibrosis scores [48]. Another study also supported alcohol abstinence, particularly in patients who had metabolic syndrome [49]. Public health interventions aimed at reducing alcohol use—including taxation, regulation of availability, and educational campaigns—have been effective in diminishing alcohol-related harm and, consequently, the risk of HCC.
4.2. Weight Management and Physical Activity
Obesity and metabolic syndrome are associated with MASLD, which can progress to metabolic dysfunction-associated steatohepatitis (MASH) and subsequently increase the risk of HCC [50]. Engaging in regular physical activity and maintaining a healthy body weight have been shown to reduce hepatic fat accumulation, improve insulin sensitivity, and decrease liver inflammation, thereby lowering the risk of HCC development [51,52]. In patients with MASLD, a weight loss of at least 7–10% is recommended to achieve significant improvements in steatosis and fibrosis [53]. Furthermore, a meta-analysis found that physical activity reduces liver cancer risk and mortality in a dose-dependent manner [54]. Engaging in at least two hours of physical activity per week is necessary to reduce liver cancer mortality.
4.3. Dietary Interventions
Dietary interventions play a significant role in the primary prevention of HCC by modulating risk factors such as obesity, metabolic syndrome, and MASLD, as well as directly influencing liver carcinogenesis through bioactive compounds [55]. Hypercaloric diets high in trans and saturated fats, cholesterol, and fructose-sweetened beverages increase visceral adiposity and promote hepatic lipid accumulation, leading to MASLD [56]. Consuming a diet rich in fruits, vegetables, and whole grains—as emphasized in the Mediterranean diet—reduces the risk of HCC due to the antioxidant and anti-inflammatory properties of these foods, which reducing oxidative stress and inflammation, key processes in hepatic carcinogenesis [57]. Limiting the intake of red and processed meats is recommended, as they contain heme iron and carcinogenic compounds formed during processing and high-temperature cooking—such as heterocyclic amines and polycyclic aromatic hydrocarbons—that have been linked to increased HCC risk [58].
Regular consumption of coffee and green tea has been inversely associated with HCC incidence as demonstrated in Table 2, possibly due to their antioxidant compounds like chlorogenic acids, caffeine, and catechins such as epigallocatechin gallate (EGCG), which exhibit anti-inflammatory and anti-carcinogenic properties [59,60]. Omega-3 fatty acids, found in fatty fish and certain plant oils, have demonstrated protective effects against HCC development by modulating liver fat metabolism, reducing hepatic steatosis, and exerting anti-inflammatory and anti-fibrotic effects [61].

Table 2.
Incidence of hepatocellular carcinoma in regular coffee and green tea consumers compared to non-drinkers.
4.4. Smoking Cessation
Tobacco smoking is an established independent risk factor for HCC, with meta-analyses demonstrating that smokers have a significantly higher risk of developing HCC compared to non-smokers [17]. This risk is further amplified in patients with MASLD [68]. Carcinogenic compounds in tobacco smoke contribute to hepatocarcinogenesis through mechanisms such as oxidative stress induction, DNA damage, and promotion of hepatic inflammation and fibrosis [69]. Notably, individuals who quit smoking more than 30 years ago exhibit an HCC risk nearly equivalent to that of never-smokers [70].
5. Environmental Control
5.1. Reducing Aflatoxin Exposure
Aflatoxin B₁, produced by Aspergillus species and primarily transmitted through contaminated food, is a significant risk factor for HCC in developing countries [15]. Strategies to reduce aflatoxin exposure—such as improving agricultural practices, ensuring proper food storage, and utilizing processing methods to prevent fungal contamination—have been effective in decreasing HCC incidence. Implementing regulations to monitor and control aflatoxin levels in food supplies is also critical for reducing exposure in affected populations [71].
5.2. Occupational and Environmental Hazard Management
Exposure to industrial chemicals such as vinyl chloride, arsenic, and polychlorinated biphenyls (PCBs) has been associated with an increased risk of HCC development [72,73]. Occupational settings involving the handling of these carcinogenic substances require stringent safety protocols—including the use of personal protective equipment and proper ventilation systems—to minimize exposure. Environmental measures, such as regulating industrial emissions and preventing contamination of water and soil, are also essential in reducing the prevalence of HCC linked to environmental toxins.
5.3. Management of Genetic and Metabolic Disorders
Genetic disorders such as hereditary hemochromatosis, alpha-1 antitrypsin deficiency and Wilson’s disease can lead to chronic liver injury and increase HCC risk [74]. Early diagnosis through genetic screening and appropriate management, including phlebotomy for hemochromatosis, can prevent disease progression [75,76].
6. Pharmacological Interventions
6.1. Aspirin
Aspirin demonstrates its chemopreventive effects primarily through the inhibition of cyclooxygenase-2 (COX-2), which diminishes the synthesis of pro-inflammatory prostaglandins and reduces chronic liver inflammation, a key driver of hepatocarcinogenesis [77]. Additionally, its antiplatelet properties disrupt platelet–tumor cell interactions that facilitate tumor growth and metastasis [78]. Several studies and recent meta-analyses have demonstrated that regular aspirin use is associated with a significant reduction in HCC risk as demonstrated in Table 3, particularly among individuals with chronic liver disease [79,80,81,82,83,84,85,86,87,88,89,90,91]. However, long-term aspirin therapy may increase the risk of gastrointestinal bleeding and hemorrhagic stroke, necessitating a careful risk–benefit assessment [92].

Table 3.
Incidence of hepatocellular carcinoma in aspirin users compared to non-users.
6.2. Statin
As hydroxymethylglutaryl-CoA (HMG-CoA) reductase inhibitors, statins lower cholesterol levels and exhibit anti-inflammatory and immunomodulatory effects that may help inhibit carcinogenesis. They also induce apoptosis and inhibit the proliferation of hepatic cancer cells by modulating the mevalonate pathway and suppressing oncogenic signaling pathways, such as Ras/Raf/MEK/ERK [93]. Numerous studies have consistently demonstrated that statin users with chronic liver disease have a lower risk of developing HCC compared to non-users as demonstrated in Table 4. However, despite these benefits, statin use can lead to myopathy or, in severe cases, rhabdomyolysis, particularly in patients with decompensated cirrhosis, who may receive low-dose statins and require intensive monitoring. A recent study suggests that the protective effects of statins against HCC may be primarily associated with lipophilic statins, such as simvastatin and atorvastatin, while hydrophilic statins, including pravastatin and rosuvastatin, showed no significant benefit [94]. Similarly, a recent meta-analysis found that lipophilic statins had a greater chemopreventive effect on HCC risk compared to hydrophilic statins, with an adjusted odds ratio of 0.51 (95% CI: 0.46–0.57) versus 0.77 (95% CI: 0.58–1.02) [95].

Table 4.
Incidence of hepatocellular carcinoma in statin users compared to non-users.
6.3. Metformin
Metformin, a widely used antidiabetic medication, has shown potential in reducing HCC incidence through multiple mechanisms. It activates AMP-activated protein kinase (AMPK), which inhibits the mammalian target of rapamycin (mTOR) pathway, thereby suppressing tumor cell growth and proliferation [104]. Metformin also lowers insulin and insulin-like growth factor (IGF) levels, which are implicated in hepatocarcinogenesis [105]. Clinical evidence from studies indicates that diabetic patients treated with metformin have a significantly reduced risk of developing HCC as demonstrated in Table 5. However, metformin use may be associated with lactic acidosis, particularly in patients with renal impairment, necessitating careful patient selection [106].

Table 5.
Incidence of hepatocellular carcinoma in metformin users compared to non-users.
6.4. Glucagon-like Peptide-1 (GLP-1) Agonists
GLP-1 agonists have emerged as potential chemopreventive agents for HCC, particularly in patients with type 2 diabetes. These agents enhance insulin sensitivity, reduce hyperinsulinemia, and exhibit anti-inflammatory properties, collectively contributing to a lower risk of hepatocarcinogenesis [113]. Additionally, GLP-1 agonists induce apoptosis in hepatic cancer cells through the modulation of the PI3K/Akt pathway [113]. Several studies have reported a significant reduction in HCC incidence among GLP-1 agonist users [114]. A recent large cohort study by Wang et al. demonstrated that GLP-1 agonists are associated with a lower risk of incident HCC in patients with type 2 diabetes, with hazard ratios of 0.20 (95% CI: 0.14–0.31), 0.39 (95% CI: 0.21–0.69), and 0.63 (95% CI: 0.26–1.50) when compared with insulin, sulfonylureas, and metformin, respectively [114]. However, potential adverse effects, including gastrointestinal disturbances and an increased risk of pancreatitis, warrant careful consideration and monitoring. Figure 1 summarizes the mechanism of action of GLP-1 agonists in HCC prevention.
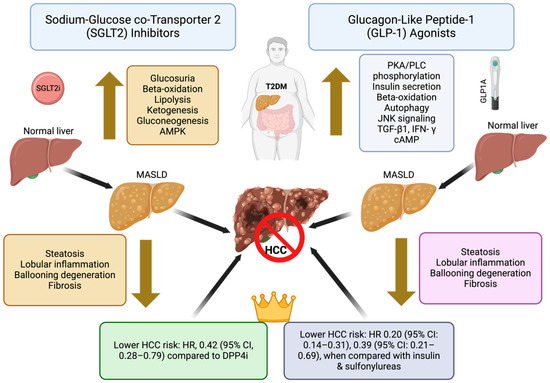
Figure 1.
Summary of the mechanisms of action of SGLT2 inhibitors and GLP-1 analogs for HCC prevention. AMPK—5′-adenosine monophosphate-activated protein kinase; cAMP—cyclic adenosine monophosphate; GLP-1—glucagon-like peptide-1; HCC—hepatocellular carcinoma; IFN-γ—interferon gamma; JNK—c-Jun N-terminal kinase; MASLD—metabolic dysfunction-associated steatotic liver disease; PKA—protein kinase A; PLC—phospholipase C; SGLT2i—sodium-glucose cotransporter 2 inhibitor; TGF-β1—transforming growth factor beta 1.
6.5. Sodium-Glucose Co-Transporter 2 (SGLT2) Inhibitors
SGLT2 inhibitors, primarily used for managing type 2 diabetes (T2DM), improve glycemic control and reduce insulin resistance, both of which are implicated in the development of HCC, as shown in the mechanisms summarized in Figure 1. These agents also exert anti-inflammatory and anti-fibrotic effects, helping to reduce liver damage, particularly in patients with MASLD [115]. Emerging studies suggest that SGLT2 inhibitors may lower the risk of HCC by improving non-invasive markers of steatosis and even fibrosis in patients with T2DM [116]. Recently, a large retrospective cohort study from Hong Kong compared the risk of new-onset HCC in patients with T2DM treated with SGLT2 inhibitors versus dipeptidyl peptidase-4 inhibitors (DPP4i). After propensity score matching and adjustments, SGLT2-inhibitor use was associated with a significantly lower risk of HCC (HR, 0.42; 95% CI, 0.28–0.79) compared to DPP4i, with this protective effect also observed in patients with cirrhosis, advanced fibrosis, HBV, and HCV infections. The findings were consistent across different risk models and sensitivity analyses [117].
6.6. Thiazolidinediones
Thiazolidinediones, such as pioglitazone, are insulin sensitizers used in T2DM management and have been studied for their role in reducing HCC risk. These drugs activate peroxisome proliferator-activated receptor gamma (PPARγ), which enhances insulin sensitivity, reduces liver steatosis, and has anti-inflammatory effects, which may contribute to a reduced risk of HCC [118]. Some observational studies suggest that pioglitazone use is associated with a reduced incidence of HCC, particularly in diabetic patients [119]. A recent meta-analysis by Arvind et al. demonstrated that thiazolidinedione use is associated with a reduced risk of HCC in patients with type 2 diabetes (aOR = 0.92, 95% CI: 0.86–0.97; I2 = 43%) [119]. However, concerns about the potential risk of bladder cancer and cardiovascular complications with long-term pioglitazone use warrant careful consideration [120].
6.7. Angiotensin-Converting Enzyme (ACE) Inhibitors and Angiotensin Receptor Blockers (ARBs)
ACE inhibitors and ARBs have anti-fibrotic and anti-inflammatory properties, which can reduce liver fibrosis, a major precursor to HCC [121]. One meta-analysis suggested that patients using ACE inhibitors or ARBs have a lower risk of developing HCC [122].
6.8. Vitamin D Supplements
Vitamin D has anti-inflammatory, immunomodulatory, and anti-proliferative effects, which may reduce the risk of HCC [123]. A meta-analysis conducted by Yi et al. demonstrated that vitamin D deficiency is significantly associated with an increased risk of liver cancer, with a pooled relative risk (RR) of 2.16 (95% CI: 1.20–3.88; p = 0.01), and supplementation could theoretically lower this risk by modulating pathways involved in cell proliferation and inflammation [124,125]. However, results from vitamin D supplementation trials on liver outcomes are limited.
6.9. Nutraceuticals and Herbal Supplements
Nutraceuticals and herbal supplements have garnered attention for their potential role in preventing HCC due to their antioxidant, anti-inflammatory, and anti-proliferative properties. Compounds such as curcumin, found in turmeric, and resveratrol, found in grapes, have shown promise in preclinical studies by inhibiting tumor growth, reducing oxidative stress, and modulating inflammatory pathways linked to liver [126,127]. Silymarin, derived from milk thistle, has also demonstrated hepatoprotective effects by reducing liver fibrosis and inflammation, thereby potentially lowering the risk of HCC [128]. Despite its general reputation for hepatoprotective properties, curcumin has been linked to documented cases of drug-induced liver injury (DILI) [129]. Additionally, herbal supplements can interact with conventional medications and vary in quality and potency, underscoring the importance of regulation and standardization.
7. Secondary Prevention Strategies
Target Populations for Surveillance [130]
Surveillance for HCC is recommended for individuals at high risk of developing the disease [131]. According to international guidelines, the following groups should undergo regular surveillance: patients with cirrhosis of any etiology except for those with Child–Pugh class C cirrhosis who are not on the waiting list for liver transplantation; individuals with chronic HBV infection, even without cirrhosis, in certain subgroups, including males aged at least 40 years, females aged at least 50 years, and patients with a family history of HCC at any age; and patients with chronic HCV infection with advanced fibrosis (Metavir stage F3) in the absence of cirrhosis. Information about the incidence of HCC in patients with non-viral chronic liver diseases without cirrhosis—such as alcohol-associated steatohepatitis and MASH, autoimmune liver disease, genetic haemochromatosis, alpha-1 antitrypsin deficiency, and Wilson’s disease—is limited [29]. The recommended surveillance strategy is liver ultrasound every six months, with or without the measurement of serum alpha-fetoprotein (AFP), to facilitate early detection and improve survival outcomes [29]. Studies have demonstrated that surveillance leads to earlier detection of HCC, increased eligibility for curative treatments, and reduction in mortality compared to those who did not undergo surveillance [132,133].
The strategy of HCC surveillance was based on a randomized controlled trial from China involving 18,816 CHB patients [133]. In the study, the patients were divided into two groups, a surveillance group (9373 patients) and a control (9443 patients) group; patients in the surveillance group received ultrasonography of the liver and AFP at six-month intervals. HCC-related mortality was significantly lower in the screened group (83.2 per 100,000) than in the control group (131.5 per 100,000), with a mortality rate ratio of 0.63 (95% CI 0.41–0.98), indicating that surveillance every six months reduced HCC mortality by 37%. Currently, according to international practice guidelines, surveillance for HCC should be undertaken in the high-risk group of patients, particularly in cirrhosis, as up to 90% of HCC occurs in cirrhotic patients [74,134,135,136]. Recently, a meta-analysis of over 93,000 HCC patients showed that approximately one-third of MASLD-related HCC occurred in patients without cirrhosis [137]. Of note, the proportion of MASLD-related HCC undergoing HCC surveillance was lower than that of HCC from other causes, and the tumors were usually detected at a larger size. These findings highlight the necessity of HCC surveillance among MASLD patients, especially in the absence of cirrhosis [137].
8. Screening Modalities
8.1. Imaging Techniques
Ultrasound (US): Ultrasound is the most widely used screening tool due to its non-invasive nature, availability, and cost-effectiveness. It is recommended every six months for high-risk patients [138]. Increasingly, a higher proportion of HCC cases are being diagnosed through structured surveillance programs, particularly those adhering to a semi-annual screening schedule. Regular use of ultrasound in these programs enables the early detection of HCC, allowing for timely intervention and improved patient outcomes [139].
Contrast-enhanced ultrasound (CEUS): CEUS enhances lesion characterization by providing superior visualization of tumor vascularity [140]. International guidelines recommend CEUS when conventional ultrasound results are inconclusive due to its higher sensitivity and specificity [74]. However, its application is limited by availability and the requirement for specialized training.
Computed tomography (CT): CT is a widely used imaging modality for the diagnosis and staging of HCC. Multi-phase contrast-enhanced CT, particularly with arterial and portal venous phases, enables the visualization of the characteristic vascular patterns of HCC [141]. HCC lesions typically exhibit hyperenhancement during the arterial phase and washout during the portal venous or delayed phases, which are critical imaging features for diagnosis [141]. Despite its advantages, the use of CT for routine HCC screening is limited due to concerns about radiation exposure and its relatively lower sensitivity in detecting small lesions (<1 cm) compared to MRI [134].
Magnetic resonance imaging (MRI): MRI, particularly with hepatobiliary contrast agents like gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA), is considered superior to CT for HCC screening, particularly in detecting early-stage and small lesions [142]. This modality is particularly advantageous in cases lacking the typical vascular pattern, as it enables the identification of small HCC lesions through the characteristic double hypo-intensity in the portal-venous and hepatobiliary phases. This distinctive feature provides a significant diagnostic advantage for early-stage detection, thereby improving patient outcomes [143]. MRI provides better soft tissue contrast and can distinguish between benign and malignant lesions more accurately than CT. In addition to conventional sequences, diffusion-weighted imaging (DWI) enhances the ability to detect and characterize small tumors, and dynamic contrast-enhanced imaging identifies the vascular characteristics of HCC, similar to CT [144]. Although MRI is generally more sensitive and specific for early HCC, its higher cost and limited availability in some regions remain challenges in routine screening programs.
8.2. Biomarkers
Alpha-fetoprotein (AFP): AFP is the most studied serum biomarker for HCC surveillance. However, its sensitivity and specificity are suboptimal when used alone, as elevated levels can be seen in other liver diseases [145]. Combining AFP measurement with ultrasound improves detection rates [146].
Des-gamma-carboxy prothrombin (DCP) and AFP-L3: These biomarkers have shown promise in detecting HCC but are not widely adopted due to limited availability and higher costs [147].
Emerging biomarkers: Research is ongoing to identify novel biomarkers, such as circulating tumor DNA, microRNAs, and proteins, to improve early detection [148].
9. Risk Stratification Models
Risk stratification models identify individuals at the highest risk of developing HCC, allowing for more personalized surveillance strategies. These models integrate demographic, clinical, and laboratory variables to accurately predict HCC risk. By utilizing risk scores, healthcare providers can tailor the intensity of surveillance, with higher risk patients receiving more frequent or advanced imaging modalities to enhance early detection and improve outcomes.
Examples of Risk Scores
- REACH-B score: Developed for HBV-infected patients without cirrhosis, incorporating age, gender, ALT levels, HBeAg status, and HBV DNA levels [149].
- PAGE-B score: Used for HBV patients on antiviral therapy, including age, gender, and platelet count [149].
- aMAP score: Applicable to both HBV and HCV patients, considering age, male gender, albumin-bilirubin grade, and platelet count [150,151].
- GALAD score: Combines gender, age, AFP-L3, AFP, and DCP to enhance predictive accuracy for HCC in diverse populations [152].
- GAAD score: Incorporates gender, age, AFP, and DCP levels to improve HCC risk prediction, particularly in at-risk populations such as those with chronic liver disease [153].
10. Conclusions
HCC continues to represent a significant global health burden due to its high incidence and mortality rates. This comprehensive review highlights that effective prevention strategies are diverse, including primary measures such as vaccination, antiviral therapies, lifestyle modifications, and environmental control, alongside secondary prevention strategies like regular surveillance in high-risk populations, as summarized in Figure 2. Pharmacological interventions, including aspirin, statins, and metformin, offer promising chemopreventive benefits. Moving forward, it is imperative to enhance global efforts to expand access to preventive measures, invest in public health education, and foster research into novel preventive therapies. A coordinated, worldwide approach is essential to further reduce the incidence and improve outcomes in HCC.
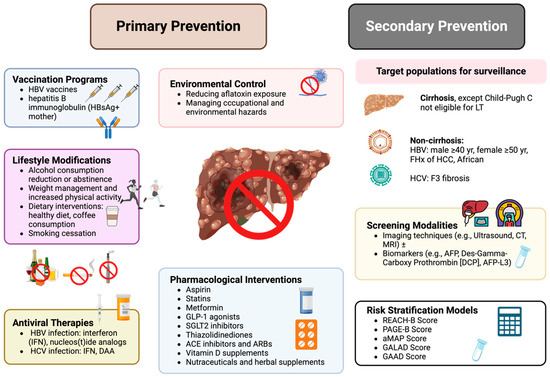
Figure 2.
Summary of overall strategies for HCC prevention. HBV—hepatitis B virus; HCV—hepatitis C virus; HCC—hepatocellular carcinoma; IFN—interferon; DAA—direct-acting antiviral; GLP-1—glucagon-like peptide-1; SGLT2—sodium-glucose cotransporter 2; ACE—angiotensin-converting enzyme; ARB—angiotensin II receptor blocker; AFP—alpha-fetoprotein; DCP—des-gamma-carboxy prothrombin; REACH-B—risk estimation for hepatocellular carcinoma in chronic hepatitis B; PAGE-B—platelet, age, gender-HBV score; aMAP—age, male gender, albumin-bilirubin score, and platelet count; GALAD—gender, age, AFP, AFP-L3, and DCP; GAAD—Gender, Age, AFP, and AFP-L3.
Author Contributions
Conceptualization, N.P. and A.K.; writing—original draft preparation, N.P., S.S., P.D., S.-Y.C., L.S. and J.B.; writing—review and editing, C.R., P.S. and A.K.; visualization, C.R. and P.S.; supervision, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was exempt from the Ethics committee in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and approved by the Human Research Ethics Unit (HREU) of the Faculty of Medicine, Prince of Songkla University (REC number: 67-504-14-1).
Informed Consent Statement
Patient consent was waived because of the study’s retrospective design.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
Apichat Kaewdech received research grants or support from Roche, Roche Diagnostics, and Abbott Laboratories, and honoraria from Roche, Roche Diagnostics, Abbott Laboratories, and Esai. The other authors have no relevant conflicts of interest to declare.
References
- Tan, E.Y.; Danpanichkul, P.; Yong, J.N.; Yu, Z.; Tan, D.J.H.; Lim, W.H.; Koh, B.; Lim, R.Y.Z.; Tham, E.K.J.; Mitra, K.; et al. Liver Cancer in 2021: Global Burden of Disease Study. J. Hepatol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F.; Chen, D.S.; Dusheiko, G.M.; Janssen, H.L.A.; Lau, D.T.Y.; Locarnini, S.A.; Peters, M.G.; Lai, C.L. Hepatitis B virus infection. Nat. Rev. Dis. Primers 2018, 4, 18035. [Google Scholar] [CrossRef] [PubMed]
- Maucort-Boulch, D.; de Martel, C.; Franceschi, S.; Plummer, M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int. J. Cancer 2018, 142, 2471–2477. [Google Scholar] [CrossRef]
- Singal, A.G.; Kanwal, F.; Llovet, J.M. Global trends in hepatocellular carcinoma epidemiology: Implications for screening, prevention and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 864–884. [Google Scholar] [CrossRef]
- Kaewdech, A.; Sripongpun, P. Navigating the Nomenclature of Liver Steatosis: Transitioning from NAFLD to MAFLD and MASLD—Understanding Affinities and Differences. Siriraj Med. J. 2024, 76, 234–243. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Picchio, C.A.; Colombo, M. Hepatocellular Carcinoma Prevention in the Era of Hepatitis C Elimination. Int. J. Mol. Sci. 2023, 24, 14404. [Google Scholar] [CrossRef]
- Danpanichkul, P.; Suparan, K.; Sukphutanan, B.; Kaeosri, C.; Tothanarungroj, P.; Sirimangklanurak, S.; Kalligeros, M.; Polpichai, N.; Pang, Y.; Wijarnpreecha, K.; et al. Changes in the epidemiological trends of primary liver cancer in the Asia-Pacific region. Sci. Rep. 2024, 14, 19544. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Kanwal, F. Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology 2014, 60, 1767–1775. [Google Scholar] [CrossRef]
- Huang, D.Q.; Mathurin, P.; Cortez-Pinto, H.; Loomba, R. Global epidemiology of alcohol-associated cirrhosis and HCC: Trends, projections and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 37–49. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1991. [Google Scholar] [CrossRef]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef]
- Shin, H.S.; Jun, B.G.; Yi, S.W. Impact of diabetes, obesity, and dyslipidemia on the risk of hepatocellular carcinoma in patients with chronic liver diseases. Clin. Mol. Hepatol. 2022, 28, 773–789. [Google Scholar] [CrossRef]
- Granito, A.; Muratori, L.; Lalanne, C.; Quarneti, C.; Ferri, S.; Guidi, M.; Lenzi, M.; Muratori, P. Hepatocellular carcinoma in viral and autoimmune liver diseases: Role of CD4+ CD25+ Foxp3+ regulatory T cells in the immune microenvironment. World J. Gastroenterol. 2021, 27, 2994–3009. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, F. Global burden of aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Chanprasert, S.; Scaglia, F. Adult liver disorders caused by inborn errors of metabolism: Review and update. Mol. Genet. Metab. 2015, 114, 1–10. [Google Scholar] [CrossRef]
- Marti-Aguado, D.; Clemente-Sanchez, A.; Bataller, R. Cigarette smoking and liver diseases. J. Hepatol. 2022, 77, 191–205. [Google Scholar] [CrossRef]
- Chang, M.H. Hepatitis B virus infection. Semin. Fetal Neonatal Med. 2007, 12, 160–167. [Google Scholar] [CrossRef]
- Chang, M.H.; You, S.L.; Chen, C.J.; Liu, C.J.; Lee, C.M.; Lin, S.M.; Chu, H.C.; Wu, T.C.; Yang, S.S.; Kuo, H.S.; et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: A 20-year follow-up study. J. Natl. Cancer Inst. 2009, 101, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Hepatitis B vaccines: WHO position paper–July 2017. Wkly. Epidemiol. Rec. 2017, 92, 369–392.
- Wong, G.L.; Ong, J.P.; Kaewdech, A.; Su, T.H. Halfway through HBV elimination—Are we not waiting? Int. J. Infect. Dis. 2023, 134, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.R.; Barnes, E.; Cox, A.L. Approaches, Progress, and Challenges to Hepatitis C Vaccine Development. Gastroenterology 2019, 156, 418–430. [Google Scholar] [CrossRef]
- Rehermann, B. Hepatitis C virus versus innate and adaptive immune responses: A tale of coevolution and coexistence. J. Clin. Investig. 2009, 119, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Page, K.; Melia, M.T.; Veenhuis, R.T.; Winter, M.; Rousseau, K.E.; Massaccesi, G.; Osburn, W.O.; Forman, M.; Thomas, E.; Thornton, K.; et al. Randomized Trial of a Vaccine Regimen to Prevent Chronic HCV Infection. N. Engl. J. Med. 2021, 384, 541–549. [Google Scholar] [CrossRef]
- Charatcharoenwitthaya, P.; Kaewdech, A.; Piratvisuth, T. Controversies in Treating Chronic HBV: The Role of PEG-interferon-alfa. Clin. Liver Dis. 2021, 25, 741–762. [Google Scholar] [CrossRef]
- Kaewdech, A.; Sripongpun, P. Challenges in the discontinuation of chronic hepatitis B antiviral agents. World J. Hepatol. 2021, 13, 1042–1057. [Google Scholar] [CrossRef]
- Kaewdech, A.; Assawasuwannakit, S.; Sripongpun, P.; Chamroonkul, N.; Tangkijvanich, P.; Piratvisuth, T. Clinical Utility of SCALE-B to Predict Hepatitis B Virus Relapse, Hepatitis B Surface Antigen Loss After Antiviral Cessation in Asian Patients After 2-Year Follow-up. Front. Med. 2022, 9, 859430. [Google Scholar] [CrossRef] [PubMed]
- Kaewdech, A.; Tangkijvanich, P.; Sripongpun, P.; Witeerungrot, T.; Jandee, S.; Tanaka, Y.; Piratvisuth, T. Hepatitis B surface antigen, core-related antigen and HBV RNA: Predicting clinical relapse after NA therapy discontinuation. Liver Int. 2020, 40, 2961–2971. [Google Scholar] [CrossRef]
- Lampertico, P.; Agarwal, K.; Berg, T.; Buti, M.; Janssen, H.L.; Papatheodoridis, G.; Zoulim, F.; Tacke, F. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Papatheodoridis, G.V.; Chan, H.L.; Hansen, B.E.; Janssen, H.L.; Lampertico, P. Risk of hepatocellular carcinoma in chronic hepatitis B: Assessment and modification with current antiviral therapy. J. Hepatol. 2015, 62, 956–967. [Google Scholar] [CrossRef]
- Ren, H.; Huang, Y. Effects of pegylated interferon-α based therapies on functional cure and the risk of hepatocellular carcinoma development in patients with chronic hepatitis B. J. Viral Hepat. 2019, 26 (Suppl. S1), 5–31. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Yang, J.; Hu, K.; Huang, Y. The effectiveness of TDF versus ETV on incidence of HCC in CHB patients: A meta analysis. BMC Cancer 2019, 19, 511. [Google Scholar] [CrossRef]
- Choi, W.M.; Choi, J.; Lim, Y.S. Effects of Tenofovir vs Entecavir on Risk of Hepatocellular Carcinoma in Patients With Chronic HBV Infection: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 246–258.e249. [Google Scholar] [CrossRef]
- Cheung, K.S.; Mak, L.Y.; Liu, S.H.; Cheng, H.M.; Seto, W.K.; Yuen, M.F.; Lai, C.L. Entecavir vs Tenofovir in Hepatocellular Carcinoma Prevention in Chronic Hepatitis B Infection: A Systematic Review and Meta-Analysis. Clin. Transl. Gastroenterol. 2020, 11, e00236. [Google Scholar] [CrossRef]
- Dave, S.; Park, S.; Murad, M.H.; Barnard, A.; Prokop, L.; Adams, L.A.; Singh, S.; Loomba, R. Comparative Effectiveness of Entecavir Versus Tenofovir for Preventing Hepatocellular Carcinoma in Patients with Chronic Hepatitis B: A Systematic Review and Meta-Analysis. Hepatology 2021, 73, 68–78. [Google Scholar] [CrossRef]
- Tan, D.J.H.; Ng, C.H.; Tay, P.W.L.; Syn, N.; Muthiah, M.D.; Lim, W.H.; Tang, A.S.P.; Lim, K.E.; Lim, G.E.H.; Tamaki, N.; et al. Risk of Hepatocellular Carcinoma With Tenofovir vs Entecavir Treatment for Chronic Hepatitis B Virus: A Reconstructed Individual Patient Data Meta-analysis. JAMA Netw. Open 2022, 5, e2219407. [Google Scholar] [CrossRef]
- Pawlotsky, J.M. New hepatitis C therapies: The toolbox, strategies, and challenges. Gastroenterology 2014, 146, 1176–1192. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005.e1001. [Google Scholar] [CrossRef]
- Morgan, R.L.; Baack, B.; Smith, B.D.; Yartel, A.; Pitasi, M.; Falck-Ytter, Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: A meta-analysis of observational studies. Ann. Intern. Med. 2013, 158, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Del Prete, V.; Turco, A.; Buccino, R.V.; Nacchiero, M.C.; Muscatiello, N. Long-term liver stiffness assessment in hepatitis C virus patients undergoing antiviral therapy: Results from a 5-year cohort study. J. Gastroenterol. Hepatol. 2018, 33, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Mariño, Z.; Perelló, C.; Iñarrairaegui, M.; Ribeiro, A.; Lens, S.; Díaz, A.; Vilana, R.; Darnell, A.; Varela, M.; et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef]
- Conti, F.; Buonfiglioli, F.; Scuteri, A.; Crespi, C.; Bolondi, L.; Caraceni, P.; Foschi, F.G.; Lenzi, M.; Mazzella, G.; Verucchi, G.; et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J. Hepatol. 2016, 65, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Waziry, R.; Hajarizadeh, B.; Grebely, J.; Amin, J.; Law, M.; Danta, M.; George, J.; Dore, G.J. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J. Hepatol. 2017, 67, 1204–1212. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol. Chem. 2006, 387, 349–360. [Google Scholar] [CrossRef]
- Rehm, J.; Samokhvalov, A.V.; Shield, K.D. Global burden of alcoholic liver diseases. J. Hepatol. 2013, 59, 160–168. [Google Scholar] [CrossRef]
- Eslam, M.; Sarin, S.K.; Wong, V.W.; Fan, J.G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef]
- Griswold, M.G.; Fullman, N.; Hawley, C.; Arian, N.; Zimsen, S.R.; Tymeson, H.D.; Venkateswaran, V.; Tapp, A.D.; Forouzanfar, M.H.; Salama, J.S.; et al. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef]
- Chang, Y.; Cho, Y.K.; Kim, Y.; Sung, E.; Ahn, J.; Jung, H.S.; Yun, K.E.; Shin, H.; Ryu, S. Nonheavy Drinking and Worsening of Noninvasive Fibrosis Markers in Nonalcoholic Fatty Liver Disease: A Cohort Study. Hepatology 2019, 69, 64–75. [Google Scholar] [CrossRef]
- Åberg, F.; Helenius-Hietala, J.; Puukka, P.; Färkkilä, M.; Jula, A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology 2018, 67, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Dyson, J.; Jaques, B.; Chattopadyhay, D.; Lochan, R.; Graham, J.; Das, D.; Aslam, T.; Patanwala, I.; Gaggar, S.; Cole, M.; et al. Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 2014, 60, 110–117. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Overweight, obesity and risk of liver cancer: A meta-analysis of cohort studies. Br. J. Cancer 2007, 97, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Kaewdech, A.; Assawasuwannakit, S.; Churuangsuk, C.; Chamroonkul, N.; Sripongpun, P. Effect of smartphone-assisted lifestyle intervention in MASLD patients: A randomized controlled trial. Sci. Rep. 2024, 14, 13961. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Lee, J. Associations between Physical Activity and Liver Cancer Risks and Mortality: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public. Health 2020, 17, 8943. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef]
- Lim, J.S.; Mietus-Snyder, M.; Valente, A.; Schwarz, J.M.; Lustig, R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 251–264. [Google Scholar] [CrossRef]
- Moussa, I.; Day, R.S.; Li, R.; Du, X.L.; Kaseb, A.O.; Jalal, P.K.; Daniel-MacDougall, C.; Hatia, R.I.; Abdelhakeem, A.; Rashid, A.; et al. Dietary Patterns and Hepatocellular Carcinoma Risk among US Adults. Nutrients 2021, 13, 2011. [Google Scholar] [CrossRef]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Mante Angua, K.; Rosner, B.A.; Barnett, J.B. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Bosetti, C.; Tavani, A.; Gallus, S.; La Vecchia, C. Coffee reduces risk for hepatocellular carcinoma: An updated meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1413–1421.e1411. [Google Scholar] [CrossRef]
- Ui, A.; Kuriyama, S.; Kakizaki, M.; Sone, T.; Nakaya, N.; Ohmori-Matsuda, K.; Hozawa, A.; Nishino, Y.; Tsuji, I. Green tea consumption and the risk of liver cancer in Japan: The Ohsaki Cohort study. Cancer Causes Control 2009, 20, 1939–1945. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liang, D.; Li, J.; Liu, Z.; Zhou, F.; Wang, T.; Ma, S.; Wang, G.; Chen, B.; Chen, W. Coffee, Green Tea Intake, and the Risk of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis of Observational Studies. Nutr. Cancer 2023, 75, 1295–1308. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, O.J.; Roderick, P.; Buchanan, R.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee, including caffeinated and decaffeinated coffee, and the risk of hepatocellular carcinoma: A systematic review and dose-response meta-analysis. BMJ Open 2017, 7, e013739. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Wada, K.; Konishi, K.; Goto, Y.; Mizuta, F.; Koda, S.; Hori, A.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N.; et al. Coffee, Green Tea, and Caffeine Intake and Liver Cancer Risk: A Prospective Cohort Study. Nutr. Cancer 2018, 70, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, V.W.; Wilkens, L.R.; Lu, S.C.; Hernandez, B.Y.; Le Marchand, L.; Henderson, B.E. Association of coffee intake with reduced incidence of liver cancer and death from chronic liver disease in the US multiethnic cohort. Gastroenterology 2015, 148, 118–125; quiz e115. [Google Scholar] [CrossRef]
- Lai, G.Y.; Weinstein, S.J.; Albanes, D.; Taylor, P.R.; McGlynn, K.A.; Virtamo, J.; Sinha, R.; Freedman, N.D. The association of coffee intake with liver cancer incidence and chronic liver disease mortality in male smokers. Br. J. Cancer 2013, 109, 1344–1351. [Google Scholar] [CrossRef]
- Gelatti, U.; Covolo, L.; Franceschini, M.; Pirali, F.; Tagger, A.; Ribero, M.L.; Trevisi, P.; Martelli, C.; Nardi, G.; Donato, F. Coffee consumption reduces the risk of hepatocellular carcinoma independently of its aetiology: A case-control study. J. Hepatol. 2005, 42, 528–534. [Google Scholar] [CrossRef]
- Yoo, J.J.; Park, M.Y.; Cho, E.J.; Yu, S.J.; Kim, S.G.; Kim, Y.J.; Kim, Y.S.; Yoon, J.H. Smoking Increases the Risk of Hepatocellular Carcinoma and Cardiovascular Disease in Patients with Metabolic-Associated Fatty Liver Disease. J. Clin. Med. 2023, 12, 3336. [Google Scholar] [CrossRef]
- El-Zayadi, A.R. Heavy smoking and liver. World J. Gastroenterol. 2006, 12, 6098–6101. [Google Scholar] [CrossRef]
- Petrick, J.L.; Campbell, P.T.; Koshiol, J.; Thistle, J.E.; Andreotti, G.; Beane-Freeman, L.E.; Buring, J.E.; Chan, A.T.; Chong, D.Q.; Doody, M.M.; et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Br. J. Cancer 2018, 118, 1005–1012. [Google Scholar] [CrossRef]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 2013, 14, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cheng, S.; Zhang, D. Association of inorganic arsenic exposure with liver cancer mortality: A meta-analysis. Environ. Res. 2014, 135, 120–125. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Niederau, C.; Fischer, R.; Pürschel, A.; Stremmel, W.; Häussinger, D.; Strohmeyer, G. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology 1996, 110, 1107–1119. [Google Scholar] [CrossRef]
- Roberts, E.A.; Schilsky, M.L. Diagnosis and treatment of Wilson disease: An update. Hepatology 2008, 47, 2089–2111. [Google Scholar] [CrossRef]
- Cervello, M.; Montalto, G. Cyclooxygenases in hepatocellular carcinoma. World J. Gastroenterol. 2006, 12, 5113–5121. [Google Scholar] [CrossRef]
- Hayashi, T.; Shibata, M.; Oe, S.; Miyagawa, K.; Honma, Y.; Harada, M. Antiplatelet Therapy Improves the Prognosis of Patients with Hepatocellular Carcinoma. Cancers 2020, 12, 3215. [Google Scholar] [CrossRef]
- Iqbal, U.; Dennis, B.B.; Li, A.A.; Cholankeril, G.; Kim, D.; Khan, M.A.; Ahmed, A. Use of anti-platelet agents in the prevention of hepatic fibrosis in patients at risk for chronic liver disease: A systematic review and meta-analysis. Hepatol. Int. 2019, 13, 84–90. [Google Scholar] [CrossRef]
- Lee, T.Y.; Hsu, Y.C.; Tseng, H.C.; Yu, S.H.; Lin, J.T.; Wu, M.S.; Wu, C.Y. Association of Daily Aspirin Therapy With Risk of Hepatocellular Carcinoma in Patients With Chronic Hepatitis B. JAMA Intern. Med. 2019, 179, 633–640. [Google Scholar] [CrossRef]
- Lee, M.; Chung, G.E.; Lee, J.H.; Oh, S.; Nam, J.Y.; Chang, Y.; Cho, H.; Ahn, H.; Cho, Y.Y.; Yoo, J.J.; et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology 2017, 66, 1556–1569. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.C.; Chang, J.; Kim, K.; Park, S.M. Aspirin Use and Risk of Hepatocellular Carcinoma in a National Cohort Study of Korean Adults. Sci. Rep. 2018, 8, 4968. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Duberg, A.S.; Aleman, S.; Chung, R.T.; Chan, A.T.; Ludvigsson, J.F. Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality. N. Engl. J. Med. 2020, 382, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zuo, L.; Lin, Z.; Yang, Z.; Chen, R.; Xu, Y. The relationship between aspirin consumption and hepatocellular carcinoma: A systematic review and meta-analysis. Eur. J. Med. Res. 2023, 28, 226. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.W.; Yong, J.N.; Tan, D.J.H.; Fu, C.E.; Lim, W.H.; Xiao, J.; Chan, K.E.; Tan, C.; Goh, X.L.; Chee, D.; et al. Meta-analysis: Chemoprevention of hepatocellular carcinoma with statins, aspirin and metformin. Aliment. Pharmacol. Ther. 2023, 57, 600–609. [Google Scholar] [CrossRef]
- Tan, J.L.; Sidhu-Brar, S.; Woodman, R.; Chinnaratha, M.A. Regular Aspirin Use Is Associated with a Reduced Risk of Hepatocellular Carcinoma (HCC) in Chronic Liver Disease: A Systematic Review and Meta-analysis. J. Gastrointest. Cancer 2023, 54, 325–331. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Liu, C.; Wang, W.; Shi, J.; Dang, S. Aspirin Use and the Risk of Hepatocellular Carcinoma: A Meta-analysis. J. Clin. Gastroenterol. 2022, 56, e293–e302. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, T.; Sun, Y.; Li, C.; Ding, X.; Zhu, Y.; Li, L.; Fan, Z. Systematic Review and Meta-analysis: Association of Aspirin With Incidence of Hepatocellular Carcinoma. Front. Pharmacol. 2022, 13, 764854. [Google Scholar] [CrossRef]
- Liao, R.; Zhou, B.Y.; Wu, Z.J. Association between Aspirin and Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 2480–2481. [Google Scholar] [CrossRef]
- Ma, S.; Qu, G.; Sun, C.; Liu, H.; Jiang, Y.; Li, N.; Wu, B.; Gao, J.; Feng, L.; Xie, P.; et al. Does aspirin reduce the incidence, recurrence, and mortality of hepatocellular carcinoma? A GRADE-assessed systematic review and dose-response meta-analysis. Eur. J. Clin. Pharmacol. 2023, 79, 39–61. [Google Scholar] [CrossRef]
- Bian, W.; Bian, W.; Li, Q.; Li, Y. Aspirin in Patients with Viral Hepatitis: Systematic Review and Meta-Analysis of Observational Studies. J. Gastrointest. Cancer 2024, 55, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Serebruany, V.L.; Malinin, A.I.; Eisert, R.M.; Sane, D.C. Risk of bleeding complications with antiplatelet agents: Meta-analysis of 338,191 patients enrolled in 50 randomized controlled trials. Am. J. Hematol. 2004, 75, 40–47. [Google Scholar] [CrossRef]
- Mullen, P.J.; Yu, R.; Longo, J.; Archer, M.C.; Penn, L.Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nat. Rev. Cancer 2016, 16, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Duberg, A.S.; Aleman, S.; Hagstrom, H.; Nguyen, L.H.; Khalili, H.; Chung, R.T.; Ludvigsson, J.F. Lipophilic Statins and Risk for Hepatocellular Carcinoma and Death in Patients With Chronic Viral Hepatitis: Results From a Nationwide Swedish Population. Ann. Intern. Med. 2019, 171, 318–327. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wang, M.; Shi, J.; Jia, X.; Dang, S. A Meta-Analysis of Statin Use and Risk of Hepatocellular Carcinoma. Can. J. Gastroenterol. Hepatol. 2022, 2022, 5389044. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Rafsanjani, M.R.; Rahimi, R.; Heidari-Soureshjani, S.; Darvishi, M.; Adeli, O.A.; Abbaszadeh, S. Statin Use and Hepatocellular Carcinoma Risk: A Comprehensive Meta- Analysis and Systematic Review. Recent. Pat. Anticancer. Drug Discov. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, S.; Liu, D.; Wang, Y.; Tan, Y. Statin can reduce the risk of hepatocellular carcinoma among patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2023, 35, 353–358. [Google Scholar] [CrossRef]
- Tsai, P.C.; Huang, C.F.; Yeh, M.L.; Hsieh, M.H.; Kuo, H.T.; Hung, C.H.; Tseng, K.C.; Lai, H.C.; Peng, C.Y.; Wang, J.H.; et al. Metformin and statins reduce hepatocellular carcinoma risk in chronic hepatitis C patients with failed antiviral therapy. Clin. Mol. Hepatol. 2024, 30, 468–486. [Google Scholar] [CrossRef]
- Vell, M.S.; Loomba, R.; Krishnan, A.; Wangensteen, K.J.; Trebicka, J.; Creasy, K.T.; Trautwein, C.; Scorletti, E.; Seeling, K.S.; Hehl, L.; et al. Association of Statin Use With Risk of Liver Disease, Hepatocellular Carcinoma, and Liver-Related Mortality. JAMA Netw. Open 2023, 6, e2320222. [Google Scholar] [CrossRef]
- Sinn, D.H.; Kang, D.; Park, Y.; Kim, H.; Hong, Y.S.; Cho, J.; Gwak, G.Y. Statin use and the risk of hepatocellular carcinoma among patients with chronic hepatitis B: An emulated target trial using longitudinal nationwide population cohort data. BMC Gastroenterol. 2023, 23, 366. [Google Scholar] [CrossRef]
- Zou, B.; Odden, M.C.; Nguyen, M.H. Statin Use and Reduced Hepatocellular Carcinoma Risk in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 435–444.e436. [Google Scholar] [CrossRef] [PubMed]
- Kraglund, F.; Christensen, D.H.; Eiset, A.H.; Villadsen, G.E.; West, J.; Jepsen, P. Effects of statins and aspirin on HCC risk in alcohol-related cirrhosis: Nationwide emulated trials. Hepatol. Commun. 2023, 7, e0013. [Google Scholar] [CrossRef]
- Pinyopornpanish, K.; Al-Yaman, W.; Butler, R.S.; Carey, W.; McCullough, A.; Romero-Marrero, C. Chemopreventive Effect of Statin on Hepatocellular Carcinoma in Patients With Nonalcoholic Steatohepatitis Cirrhosis. Am. J. Gastroenterol. 2021, 116, 2258–2269. [Google Scholar] [CrossRef]
- Memmott, R.M.; Mercado, J.R.; Maier, C.R.; Kawabata, S.; Fox, S.D.; Dennis, P.A. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev. Res. 2010, 3, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Zi, F.; Zi, H.; Li, Y.; He, J.; Shi, Q.; Cai, Z. Metformin and cancer: An existing drug for cancer prevention and therapy. Oncol. Lett. 2018, 15, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Lipska, K.J.; Mayo, H.; Bailey, C.J.; McGuire, D.K. Metformin in patients with type 2 diabetes and kidney disease: A systematic review. Jama 2014, 312, 2668–2675. [Google Scholar] [CrossRef]
- Li, Q.; Xu, H.; Sui, C.; Zhang, H. Impact of metformin use on risk and mortality of hepatocellular carcinoma in diabetes mellitus. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101781. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, C.; Fang, L.; Zhao, H.C.; Yao, S.K. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: A meta-analysis. Scand. J. Gastroenterol. 2013, 48, 78–87. [Google Scholar] [CrossRef]
- Tsai, P.C.; Kuo, H.T.; Hung, C.H.; Tseng, K.C.; Lai, H.C.; Peng, C.Y.; Wang, J.H.; Chen, J.J.; Lee, P.L.; Chien, R.N.; et al. Metformin reduces hepatocellular carcinoma incidence after successful antiviral therapy in patients with diabetes and chronic hepatitis C in Taiwan. J. Hepatol. 2023, 78, 281–292. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int. 2018, 38, 2018–2027. [Google Scholar] [CrossRef]
- Chen, C.I.; Kuan, C.F.; Fang, Y.A.; Liu, S.H.; Liu, J.C.; Wu, L.L.; Chang, C.J.; Yang, H.C.; Hwang, J.; Miser, J.S.; et al. Cancer risk in HBV patients with statin and metformin use: A population-based cohort study. Medicine 2015, 94, e462. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Hsu, C.C.; Wahlqvist, M.L.; Tsai, H.N.; Chang, Y.H.; Huang, Y.C. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: A representative population prospective cohort study of 800,000 individuals. BMC Cancer 2011, 11, 20. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Zhang, Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 721135. [Google Scholar] [CrossRef]
- Wang, L.; Berger, N.A.; Kaelber, D.C.; Xu, R. Association of GLP-1 Receptor Agonists and Hepatocellular Carcinoma Incidence and Hepatic Decompensation in Patients With Type 2 Diabetes. Gastroenterology 2024, 167, 689–703. [Google Scholar] [CrossRef]
- Bica, I.C.; Stoica, R.A.; Salmen, T.; Janež, A.; Volčanšek, Š.; Popovic, D.; Muzurovic, E.; Rizzo, M.; Stoian, A.P. The Effects of Sodium-Glucose Cotransporter 2-Inhibitors on Steatosis and Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease or Steatohepatitis and Type 2 Diabetes: A Systematic Review of Randomized Controlled Trials. Medicina 2023, 59, 1136. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Xu, X.; Guo, L.; Li, J.; Li, L. Effect of SGLT2 Inhibitors on Type 2 Diabetes Mellitus With Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2021, 12, 635556. [Google Scholar] [CrossRef]
- Chou, O.H.I.; Ning, J.; Chan, R.N.C.; Chung, C.T.; Huang, H.; Ng, K.; Dee, E.C.; Lee, S.; Kaewdech, A.; Chow, A.K.M.; et al. Lower Risks of New-Onset Hepatocellular Carcinoma in Patients With Type 2 Diabetes Mellitus Treated With SGLT2 Inhibitors Versus DPP4 Inhibitors. J. Natl. Compr. Canc Netw. 2024, 22, e237118. [Google Scholar] [CrossRef] [PubMed]
- Yki-Järvinen, H. Thiazolidinediones. N. Engl. J. Med. 2004, 351, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Arvind, A.; Memel, Z.N.; Philpotts, L.L.; Zheng, H.; Corey, K.E.; Simon, T.G. Thiazolidinediones, alpha-glucosidase inhibitors, meglitinides, sulfonylureas, and hepatocellular carcinoma risk: A meta-analysis. Metabolism 2021, 120, 154780. [Google Scholar] [CrossRef]
- Lewis, J.D.; Habel, L.A.; Quesenberry, C.P.; Strom, B.L.; Peng, T.; Hedderson, M.M.; Ehrlich, S.F.; Mamtani, R.; Bilker, W.; Vaughn, D.J.; et al. Pioglitazone Use and Risk of Bladder Cancer and Other Common Cancers in Persons With Diabetes. Jama 2015, 314, 265–277. [Google Scholar] [CrossRef]
- Barone, M.; Viggiani, M.T.; Losurdo, G.; Principi, M.; Leo, A.D. Systematic review: Renin-angiotensin system inhibitors in chemoprevention of hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 2524–2538. [Google Scholar] [CrossRef] [PubMed]
- Asgharzadeh, F.; Jafarzadeh-Esfehani, R.; Hassanian, S.M.; Ferns, G.A.; Avan, A.; Khazaei, M. Renin-angiotensin System Inhibitors and Development of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Curr. Pharm. Des. 2020, 26, 5079–5085. [Google Scholar] [CrossRef]
- Ding, N.; Yu, R.T.; Subramaniam, N.; Sherman, M.H.; Wilson, C.; Rao, R.; Leblanc, M.; Coulter, S.; He, M.; Scott, C.; et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell 2013, 153, 601–613. [Google Scholar] [CrossRef]
- Fedirko, V.; Duarte-Salles, T.; Bamia, C.; Trichopoulou, A.; Aleksandrova, K.; Trichopoulos, D.; Trepo, E.; Tjønneland, A.; Olsen, A.; Overvad, K.; et al. Prediagnostic circulating vitamin D levels and risk of hepatocellular carcinoma in European populations: A nested case-control study. Hepatology 2014, 60, 1222–1230. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, L.; Tu, X. Effect of Vitamin D Deficiency on Liver Cancer Risk: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2021, 22, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Hegde, M.; Parama, D.; Girisa, S.; Kumar, A.; Daimary, U.D.; Garodia, P.; Yenisetti, S.C.; Oommen, O.V.; Aggarwal, B.B. Role of Turmeric and Curcumin in Prevention and Treatment of Chronic Diseases: Lessons Learned from Clinical Trials. ACS Pharmacol. Transl. Sci. 2023, 6, 447–518. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef]
- Polyak, S.J.; Morishima, C.; Shuhart, M.C.; Wang, C.C.; Liu, Y.; Lee, D.Y. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology 2007, 132, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Halegoua-DeMarzio, D.; Navarro, V.; Ahmad, J.; Avula, B.; Barnhart, H.; Barritt, A.S.; Bonkovsky, H.L.; Fontana, R.J.; Ghabril, M.S.; Hoofnagle, J.H.; et al. Liver Injury Associated with Turmeric-A Growing Problem: Ten Cases from the Drug-Induced Liver Injury Network [DILIN]. Am. J. Med. 2023, 136, 200–206. [Google Scholar] [CrossRef]
- Hennedige, T.; Venkatesh, S.K. Imaging of hepatocellular carcinoma: Diagnosis, staging and treatment monitoring. Cancer Imaging 2013, 12, 530–547. [Google Scholar] [CrossRef]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Pillai, A.; Tiro, J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: A meta-analysis. PLoS Med. 2014, 11, e1001624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.H.; Yang, B.H.; Tang, Z.Y. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Tan, D.J.H.; Ng, C.H.; Lin, S.Y.; Pan, X.H.; Tay, P.; Lim, W.H.; Teng, M.; Syn, N.; Lim, G.; Yong, J.N.; et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: A systematic review and meta-analysis. Lancet Oncol. 2022, 23, 521–530. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Organisation for Research and Treatment of Cancer (EORTC). EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef]
- Bucci, L.; Garuti, F.; Lenzi, B.; Pecorelli, A.; Farinati, F.; Giannini, E.G.; Granito, A.; Ciccarese, F.; Rapaccini, G.L.; Di Marco, M.; et al. The evolutionary scenario of hepatocellular carcinoma in Italy: An update. Liver Int. 2017, 37, 259–270. [Google Scholar] [CrossRef]
- Guo, L.H.; Xu, H.X. Contrast-Enhanced Ultrasound in the Diagnosis of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma: Controversy over the ASSLD Guideline. Biomed. Res. Int. 2015, 2015, 349172. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Li, X.Q.; Wang, X.; Zhao, D.W.; Sun, J.; Liu, J.J.; Lin, D.D.; Yang, G.; Liu, H.; Xia, Z.Y.; Jia, C.Y.; et al. Application of Gd-EOB-DTPA-enhanced magnetic resonance imaging (MRI) in hepatocellular carcinoma. World J. Surg. Oncol. 2020, 18, 219. [Google Scholar] [CrossRef] [PubMed]
- Granito, A.; Galassi, M.; Piscaglia, F.; Romanini, L.; Lucidi, V.; Renzulli, M.; Borghi, A.; Grazioli, L.; Golfieri, R.; Bolondi, L. Impact of gadoxetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance on the non-invasive diagnosis of small hepatocellular carcinoma: A prospective study. Aliment. Pharmacol. Ther. 2013, 37, 355–363. [Google Scholar] [CrossRef]
- Gluskin, J.S.; Chegai, F.; Monti, S.; Squillaci, E.; Mannelli, L. Hepatocellular Carcinoma and Diffusion-Weighted MRI: Detection and Evaluation of Treatment Response. J. Cancer 2016, 7, 1565–1570. [Google Scholar] [CrossRef]
- Hanif, H.; Ali, M.J.; Susheela, A.T.; Khan, I.W.; Luna-Cuadros, M.A.; Khan, M.M.; Lau, D.T. Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J. Gastroenterol. 2022, 28, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1701. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, M.A.; Abbas, Z.; Amjad, S.; Shahid, B.; Altaf, A.; Siyal, M. Des-gamma-carboxy prothrombin and alpha-fetoprotein levels as biomarkers for hepatocellular carcinoma and their correlation with radiological characteristics. World J. Gastrointest. Pathophysiol. 2024, 15, 90893. [Google Scholar] [CrossRef]
- Fares, S.; Wehrle, C.J.; Hong, H.; Sun, K.; Jiao, C.; Zhang, M.; Gross, A.; Allkushi, E.; Uysal, M.; Kamath, S.; et al. Emerging and Clinically Accepted Biomarkers for Hepatocellular Carcinoma. Cancers 2024, 16, 1453. [Google Scholar] [CrossRef]
- Costa, A.P.M.; da Silva, M.; Castro, R.S.; Sampaio, A.L.O.; Alencar Júnior, A.M.; da Silva, M.C.; Ferreira, A.S.P. PAGE-B and REACH-B Predicts the Risk of Developing Hepatocellular Carcinoma in Chronic Hepatitis B Patients from Northeast, Brazil. Viruses 2022, 14, 732. [Google Scholar] [CrossRef]
- Fan, R.; Papatheodoridis, G.; Sun, J.; Innes, H.; Toyoda, H.; Xie, Q.; Mo, S.; Sypsa, V.; Guha, I.N.; Kumada, T.; et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J. Hepatol. 2020, 73, 1368–1378. [Google Scholar] [CrossRef]
- Chaiwiriyawong, S.; Assawasuwannakit, S.; Feuangwattana, P.; Sripongpun, P.; Chamroonkul, N.; Piratvisuth, T.; Kaewdech, A. Clinical Utility of the aMAP Score for Predicting Hepatocellular Carcinoma Development in Patients with Chronic Hepatitis B. Diagnostics 2024, 14, 1325. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Addissie, B.D.; Mara, K.C.; Harmsen, W.S.; Dai, J.; Zhang, N.; Wongjarupong, N.; Ali, H.M.; Ali, H.A.; Hassan, F.A.; et al. GALAD Score for Hepatocellular Carcinoma Detection in Comparison with Liver Ultrasound and Proposal of GALADUS Score. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Piratvisuth, T.; Hou, J.; Tanwandee, T.; Berg, T.; Vogel, A.; Trojan, J.; De Toni, E.N.; Kudo, M.; Eiblmaier, A.; Klein, H.G.; et al. Development and clinical validation of a novel algorithmic score (GAAD) for detecting HCC in prospective cohort studies. Hepatol. Commun. 2023, 7, e0317. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).