Assessment of Post-COVID-19 Changes in Brain—Clinical and Imaging Evaluation Using MRI Vessel Wall Imaging and Complementary MRI Methods
Abstract
:1. Introduction
2. Materials and Methods
- Group I: post-COVID-19 group—23 patients who were hospitalized for COVID-19 after a positive SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) or antigen test. They were assessed 6 months after the infection.
- Group II: control group—20 patients from the general population who did not suffer from COVID-19, with a negative SARS-CoV-2 RT-PCR or antigen test.
2.1. VWI MRI Acquisition
2.2. Image Analysis
2.3. Statistical Methods
2.4. Ethical Issues
3. Results
3.1. Baseline Characteristics
3.2. Descriptive Statistics of Group I
3.3. Descriptive Statistics of Group II
3.4. The Pearson Correlation of Radiological Features with Various Clinical Data in Group I
3.5. Point-Biserial Correlation of Radiological Features with Various Clinical Data in Group I
4. Discussion
5. Conclusions
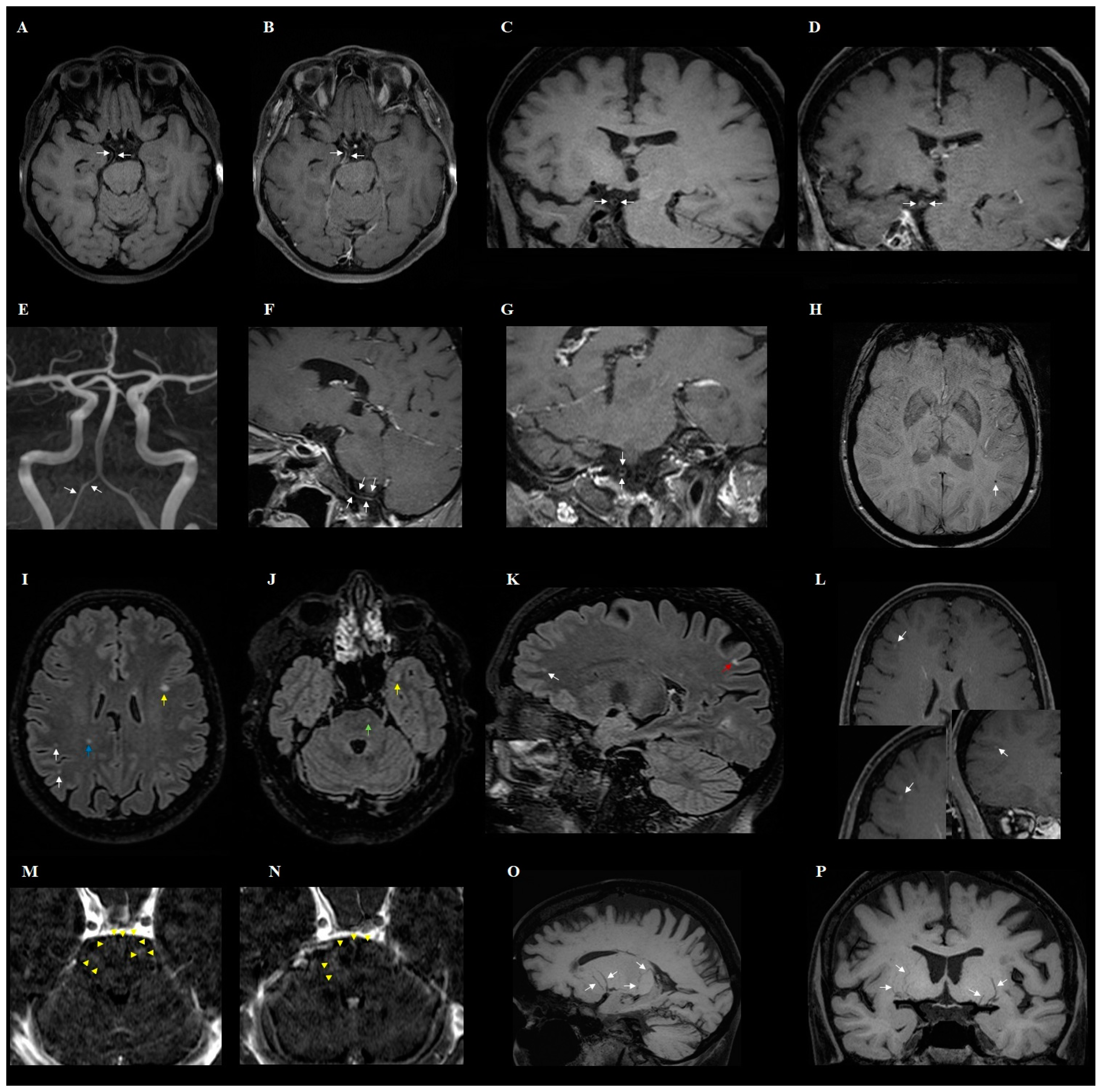
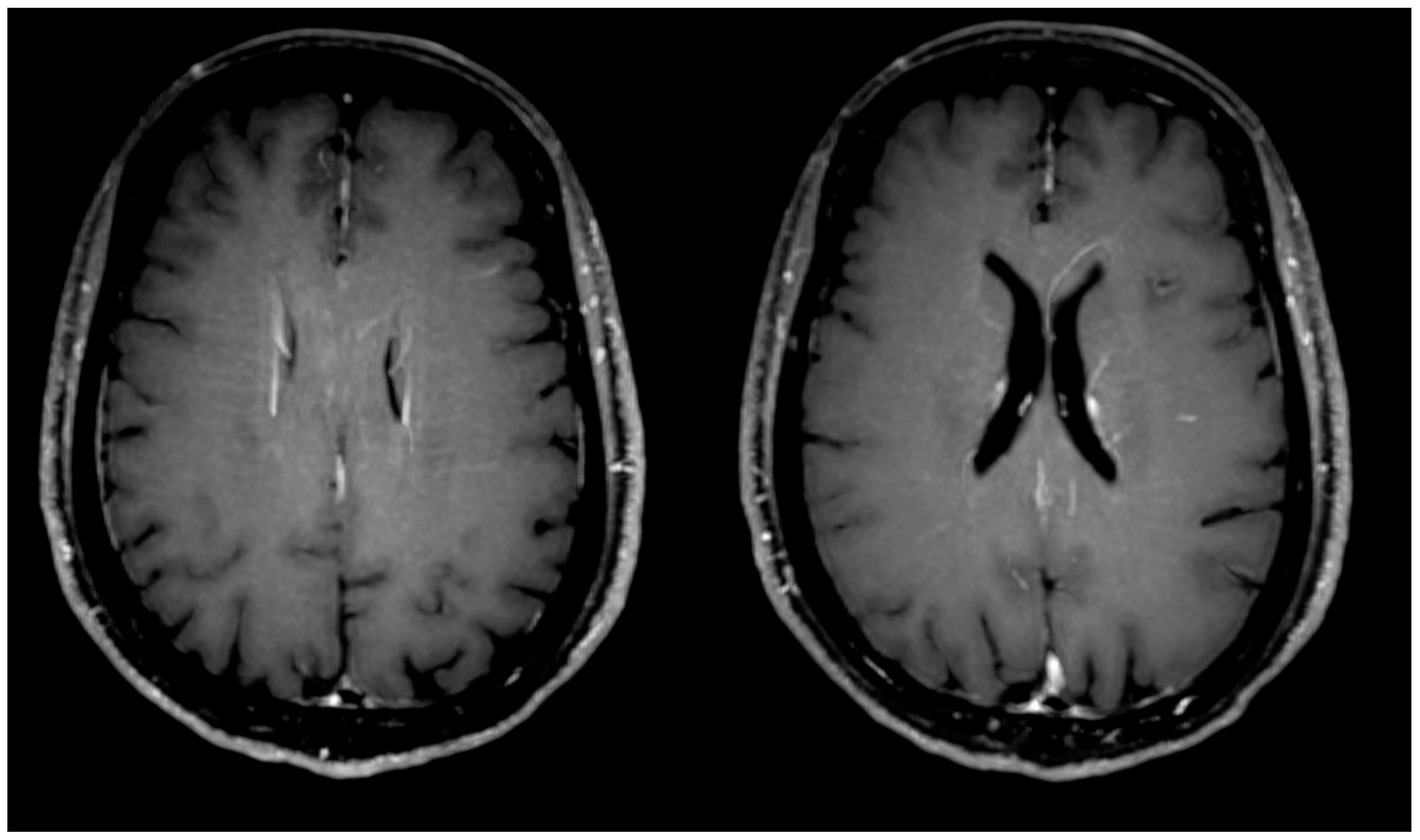
- The changes observed in post-COVID-19 patients were: hyperintense foci (in the white matter of the brain hemispheres, in the lower parts of the temporal lobes, and in the structures of the posterior cranial fossa), presence of engorgement of deep medullary veins or perivascular enhancement, presence of inflammatory (concentric) vessel thickening in VWI images, changes in hippocampus size according to the MTA scale, presence of cortical atrophy, and thickening of the mucous membrane of the paranasal sinuses.
- In addition to the characteristic changes in COVID-19, it was observed that the presence of atherosclerotic (eccentric) vessel thickening in VWI and the width of the third ventricle depend on the age of the patient.
- Despite thickening of vessels and changes that might suggest inflammation in VWI in post-COVID-19 patients, lesions in typical CNS vasculitis are much more extensive than in post-COVID-19 syndrome. Large-scale studies are warranted to demonstrate the role of VWI in imaging of post-COVID-19 changes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Li, P.; Yang, X.; Zhong, S.; Manyande, A.; Feng, M. The impact of novel coronavirus SARS-CoV-2 among healthcare workers in hospitals: An aerial overview. Am. J. Infect. Control 2020, 48, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Kakamad, F.H.; Mahmood, S.O.; Rahim, H.M.; Abdulla, B.A.; Abdullah, H.O.; Othman, S.; Mohammed, S.H.; Kakamad, S.H.; Mustafa, S.M.; Salih, A.M. Post covid-19 invasive pulmonary Aspergillosis: A case report. Int. J. Surg. Case Rep. 2021, 82, 105865. [Google Scholar] [CrossRef]
- Okrzeja, J.; Garkowski, A.; Kubas, B.; Moniuszko-Malinowska, A. Imaging and neuropathological findings in patients with Post COVID-19 Neurological Syndrome—A review. Front. Neurol. 2023, 14, 1136348. [Google Scholar] [CrossRef]
- Rogers, J.P.; Watson, C.J.; Badenoch, J.; Cross, B.; Butler, M.; Song, J.; Hafeez, D.; Morrin, H.; Rengasamy, E.R.; Thomas, L.; et al. Neurology and neuropsychiatry of COVID-19: A systematic review and meta-analysis of the early literature reveals frequent CNS manifestations and key emerging narratives. J. Neurol. Neurosurg. Psychiatry 2021, 92, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Josephson, S.A.; Kamel, H. Neurology and COVID-19. JAMA 2020, 324, 1139–1140. [Google Scholar] [CrossRef]
- Chou, S.H.; Beghi, E.; Helbok, R.; Moro, E.; Sampson, J.; Altamirano, V.; Mainali, S.; Bassetti, C.; Suarez, J.I.; McNett, M.; et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19-a report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw. Open 2021, 4, e2112131. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.; Valencia, H.S.; Barbella-Aponte, R.A.; Collado-Jiménez, R.; Ayo-Martín, Ó.; Barrena, C.; Molina-Nuevo, J.D.; García-García, J.; Lozano-Setién, E.; Alcahut-Rodriguez, C.; et al. Cerebrovascular disease in patients with COVID-19: Neuroimaging, histological and clinical description. Brain 2020, 143, 3089–3103. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Choudhary, N.; Vyas, S.; Modi, M.; Raj, S.; Kumar, A.; Sankhyan, N.; Suthar, R.; Saini, A.G.; Goyal, M.K.; Ahuja, C.K.; et al. MR vessel wall imaging in tubercular meningitis. Neuroradiology 2021, 63, 1627–1634. [Google Scholar] [CrossRef]
- Cheng-Ching, E.; Jones, S.; Hui, F.K.; Man, S.; Gilden, D.; Bhimraj, A.; Uchino, K. High-resolution MRI vessel wall imaging in varicella zoster virus vasculopathy. J. Neurol. Sci. 2015, 351, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Vyas, S.; Ahuja, C.K.; Modi, M.; Sankhyan, N.; Suthar, R.; Sahu, J.K.; Goyal, M.K.; Prabhakar, A.; Singh, P. MR vessel wall imaging in cerebral bacterial and fungal infections. Neuroradiology 2021, 64, 453–464. [Google Scholar] [CrossRef]
- Uginet, M.; Breville, G.; Hofmeister, J.; Machi, P.; Lalive, P.H.; Rosi, A.; Fitsiori, A.; Vargas, M.I.; Assal, F.; Allali, G.; et al. Cerebrovascular Complications and Vessel Wall Imaging in COVID-19 Encephalopathy—A Pilot Study. Clin. Neuroradiol. 2021, 32, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Cheron, J.; Wyndham-Thomas, C.; Sadeghi, N.; Naeije, G. Response of Human Immunodeficiency Virus-Associated Cerebral Angiitis to the Combined Antiretroviral Therapy. Front. Neurol. 2017, 8, 95. [Google Scholar] [CrossRef]
- Fan, T.H.; Khoury, J.; Cho, S.-M.; Bhimraj, A.; Shoskes, A.; Uchino, K. Cerebrovascular complications and vasculopathy in patients with herpes simplex virus central nervous system infection. J. Neurol. Sci. 2020, 419, 117200. [Google Scholar] [CrossRef] [PubMed]
- Caldas, A.C.; Geraldes, R.; Neto, L.; Canhão, P.; Melo, T.P. Central nervous system vasculitis associated with hepatitis C virus infection: A brain MRI-supported diagnosis. J. Neurol. Sci. 2014, 336, 152–154. [Google Scholar] [CrossRef]
- Afsahi, A.M.; Norbash, A.M.; Syed, S.F.; Sedaghat, M.; Afsahi, G.; Shahidi, R.; Tajabadi, Z.; Bagherzadeh-Fard, M.; Karami, S.; Yarahmadi, P.; et al. Brain MRI findings in neurologically symptomatic COVID-19 patients: A systematic review and meta-analysis. J. Neurol. 2023, 270, 5131–5154. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 14 January 2024).
- Wickham, H.; Averick, M.; Bryan, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef]
- Manca, R.; De Marco, M.; Ince, P.G.; Venneri, A. Heterogeneity in Regional Damage Detected by Neuroimaging and Neuropathological Studies in Older Adults With COVID-19: A Cognitive-Neuroscience Systematic Review to Inform the Long-Term Impact of the Virus on Neurocognitive Trajectories. Front. Aging Neurosci. 2021, 13, 646908. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Fabbris, C.; Polesel, J.; Emanuelli, E.; Tirelli, G.; Spinato, G.; Hopkins, C. Two-Year Prevalence and Recovery Rate of Altered Sense of Smell or Taste in Patients with Mildly Symptomatic COVID-19. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.J.; Han, R.; Zhao, J.J.; Tan, N.K.W.; Quah, E.S.; Tan, C.J.; Chan, Y.H.; Teo, N.W.; Charn, T.C.; See, A.; et al. Prognosis and persistence of smell and taste dysfunction in patients with COVID-19: Meta-analysis with parametric cure modelling of recovery curves. BMJ 2022, 378, e069503. [Google Scholar] [CrossRef]
- Okrzeja, J.; Sołomacha, S.; Alimowski, M.; Sowa, P.; Dubatówka, M.; Łapińska, M.; Kiszkiel, Ł.; Szczerbiński, Ł.; Laskowski, P.P.; Czupryna, P.; et al. Assessment of smell disturbances 6 months after COVID-19 in Polish population. Sci Rep. 2024, 14, 11251. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Choudhary, N.; Modi, M.; Sankhyan, N.; Suthar, R.; Saini, A.G.; Bansal, A.; Sharma, N.; Singh, P. High-resolution intracranial vessel wall imaging in cerebral viral infections evaluations. Neuroradiology 2021, 64, 915–924. [Google Scholar] [CrossRef]
- Mandell, D.; Mossa-Basha, M.; Qiao, Y.; Hess, C.; Hui, F.; Matouk, C.; Johnson, M.; Daemen, M.; Vossough, A.; Edjlali, M.; et al. Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology. Am. J. Neuroradiol. 2016, 38, 218–229. [Google Scholar] [CrossRef]
- Cai, Z.-Y.; Zhuang, F.-J.; Chen, Y.; He, W.-B. Prevalence of white matter hyperintensities increases with age. Neural Regen. Res. 2018, 13, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Guenter, W.; Betscher, E.; Bonek, R. Predictive Value of the Third Ventricle Width for Neurological Status in Multiple Sclerosis. J. Clin. Med. 2022, 11, 2841. [Google Scholar] [CrossRef]
- Cogswell, P.M.; Lants, S.K.; Davis, L.T.; Donahue, M.J. Vessel wall and lumen characteristics with age in healthy participants using 3T intracranial vessel wall magnetic resonance imaging. J. Magn. Reson. Imaging 2019, 50, 1452–1460. [Google Scholar] [CrossRef]
- Md Noh, M.S.F.; Abdul Rashid, A.M.; Mohd Zain, N.R. The Spectrum of Vessel Wall Imaging (VWI) Findings in COVID-19-Associated Neurological Syndromes: A Review. Cureus 2023, 15, e37296. [Google Scholar] [CrossRef]
- Mazzacane, F.; Zito, A.; Magno, S.; Persico, A.; Mazzoleni, V.; Asteggiano, C.; Rognone, E.; Pichiecchio, A.; Padovani, A.; Cavallini, A.; et al. Vessel wall magnetic resonance imaging in COVID-19-associated cryptogenic ischemic stroke. Eur. J. Neurol. 2021, 29, 615–619. [Google Scholar] [CrossRef]
| Radiological Results | Mean | SD | SE | Range |
|---|---|---|---|---|
| Hippocampus size according to the MTA scale | 0.83 | 0.58 | 0.12 | (0–4) |
| GCA scale | 0.83 | 0.39 | 0.08 | (0–3) |
| Width of third ventricle (in mm) | 4.93 | 1.88 | 0.39 | (2–9) |
| Radiological Results | n | % | ||
| Hyperintense foci in the white matter of the brain hemispheres | 20 | 87% | ||
| Hyperintense foci—paracortical or in the cortex of the cerebral hemispheres | 6 | 26% | ||
| Hyperintense foci in the corpus callosum | 3 | 13% | ||
| Hyperintense foci in the lower parts of the temporal lobes | 7 | 30% | ||
| Hyperintense foci in the basal ganglia or in the thalamus | 3 | 13% | ||
| Hyperintense foci in the structures of the posterior cranial fossa (brain stem and cerebellum) | 7 | 30% | ||
| Presence of cerebral microbleeds in SWI | 3 | 13% | ||
| Presence of engorgement of deep medullary veins or perivascular enhancement (Figure 2) | 3 | 13% | ||
| Enhancement of the pia mater in T1-weighted images (space black blood) | 3 | 13% | ||
| Presence of inflammatory (concentric) vessel thickening in VWI images | 7 | 30% | ||
| Presence of atherosclerotic (eccentric) vessel thickening in VWI images | 11 | 48% | ||
| Presence of cortical atrophy (accentuation of pericerebral fluid spaces) | 19 | 83% | ||
| Thickening of the mucous membrane of the paranasal sinuses | 15 | 65% | ||
| Laboratory/Clinical Results | Mean | SD | SE | Range |
|---|---|---|---|---|
| SpO2—beginning of hospitalization | 90.62 | 5.98 | 1.49 | (78–96) |
| Remdesivir—duration of treatment | 0.71 | 1.57 | 0.38 | (0–4) |
| Tocilizumab—duration of treatment | 0.06 | 0.24 | 0.06 | (0–1) |
| Dexaven—duration of treatment | 4.59 | 6.1 | 1.48 | (0–20) |
| Heparin (prophylactically)—duration of treatment | 7.18 | 4.25 | 1.03 | (0–16) |
| WBC—beginning of hospitalization (in count per microliter) | 5.83 | 1.97 | 0.48 | (2.74–8.84) |
| Neutrophils—beginning of hospitalization (in count per microliter) | 3.88 | 2.02 | 0.49 | (1.31–8.17) |
| Lymphocytes—beginning of hospitalization (in count per microliter) | 1.4 | 0.77 | 0.19 | (0.36–3.26) |
| PLT—beginning of hospitalization (in count per microliter) | 211.41 | 62.95 | 15.27 | (125–379) |
| AST—beginning of hospitalization (in IU/L) | 40.24 | 36.95 | 8.96 | (15–154) |
| ALT—beginning of hospitalization (in IU/L) | 45.06 | 43.17 | 10.47 | (10–168) |
| Creatinine—beginning of hospitalization (in mg/dL) | 0.75 | 0.22 | 0.05 | (0.48–1.35) |
| D-dimers—beginning of hospitalization (in µg/L) | 755.4 | 521.67 | 134.69 | (160–1811) |
| Ferritin—beginning of hospitalization (in µg/L) | 695.17 | 853.43 | 220.35 | (10.9–2838) |
| Fibrinogen—beginning of hospitalization (in mg/dL) | 483.47 | 153.8 | 37.3 | (242–824) |
| WBC—end of hospitalization (in count per microliter) | 6.73 | 1.98 | 0.51 | (3.77–10.73) |
| Neutrophils—end of hospitalization (in count per microliter) | 3.75 | 1.57 | 0.4 | (1.65–6.57) |
| Lymphocytes—end of hospitalization (in count per microliter) | 2.11 | 0.68 | 0.18 | (1.31–3.37) |
| PLT—end of hospitalization (in count per microliter) | 255.53 | 99.33 | 25.65 | (134–429) |
| AST—end of hospitalization (in IU/L) | 32.31 | 15.64 | 4.34 | (17–65) |
| ALT—end of hospitalization (in IU/L) | 46.17 | 21.57 | 6.23 | (15–87) |
| Creatinine—end of hospitalization (in mg/dL) | 0.74 | 0.14 | 0.04 | (0.53–0.98) |
| D-dimers—end of hospitalization (in µg/L) | 632 | 477.46 | 123.28 | (157–2005) |
| Ferritin—end of hospitalization (in µg/L) | 782.03 | 762.25 | 254.08 | (9.6–2556) |
| Fibrinogen—end of hospitalization (in mg/dL) | 349.75 | 89.78 | 25.92 | (214–501) |
| Length of hospitalization (days) | 8.53 | 4.6 | 1.12 | (1–20) |
| How many doses of the vaccine? | 1 | 1.12 | 0.25 | (0–3) |
| Laboratory/clinical Results | n | % | ||
| Post-COVID-19 headaches | 6 | 26% | ||
| Post-COVID-19 vertigo | 7 | 30% | ||
| Post-COVID-19 memory disorders | 16 | 70% | ||
| Post-COVID-19 concentration disorders | 6 | 26% | ||
| Post-COVID-19 dyspnea | 10 | 43% | ||
| Post-COVID-19 cough | 2 | 9% | ||
| Post-COVID-19 smell disorders | 2 | 9% | ||
| Post-COVID-19 taste disorders | 2 | 9% | ||
| Post-COVID-19 thromboembolic episode | 9 | 39% | ||
| Vaccinated against COVID-19 | 10 | 43% | ||
| Radiological Results | Mean | SD | SE | Range |
|---|---|---|---|---|
| Hippocampus size according to the MTA scale | 0.4 | 0.5 | 0.11 | (0–1) |
| GCA scale | 0.75 | 0.44 | 0.1 | (0–1) |
| Width of third ventricle (in mm) | 4.28 | 1.85 | 0.41 | (2–8) |
| Radiological Results | n | % | ||
| Hyperintense foci in the white matter of the brain hemispheres | 15 | 75% | ||
| Hyperintense foci—paracortical or in the cortex of the cerebral hemispheres | 2 | 10% | ||
| Hyperintense foci in the lower parts of the temporal lobes | 4 | 20% | ||
| Hyperintense foci in the structures of the posterior cranial fossa (brain stem and cerebellum) | 3 | 15% | ||
| Presence of engorgement of deep medullary veins or perivascular enhancement | 1 | 5% | ||
| Presence of inflammatory (concentric) vessel thickening in VWI images | 1 | 5% | ||
| Presence of atherosclerotic (eccentric) vessel thickening in VWI images | 11 | 55% | ||
| Presence of cortical atrophy (accentuation of pericerebral fluid spaces) | 15 | 75% | ||
| Thickening of the mucous membrane of the paranasal sinuses | 10 | 50% | ||
| Radiological Features | Clinical Data | Pearson Correlation Coefficient | p Value |
| Width of third ventricle (in mm) | Age | 0.62 | 0.01 |
| Width of third ventricle (in mm) | Dexaven—duration of treatment | 0.56 | 0.02 |
| Width of third ventricle (in mm) | Dexaven | 0.56 | 0.02 |
| Width of third ventricle (in mm) | Nasal oxygen cannula | 0.52 | 0.03 |
| Radiological Features | Clinical Data | Correlation Coefficient | p Value |
|---|---|---|---|
| Hyperintense foci in the structures of the posterior cranial fossa (brain stem, cerebellum) | Dexaven—duration of treatment | 0.76 | 0.01 |
| Hyperintense foci in the structures of the posterior cranial fossa (brain stem, cerebellum) | Heparin (prophylactically)—duration of treatment | 0.72 | 0.01 |
| Presence of atherosclerotic (eccentric) vessel thickening in VWI images | Age | 0.59 | 0.01 |
| Hyperintense foci in the structures of the posterior cranial fossa (brain stem, cerebellum) | Dexaven | 0.59 | 0.01 |
| Hyperintense foci in the structures of the posterior cranial fossa (brain stem, cerebellum) | Oxygen nasal cannula | 0.59 | 0.01 |
| Hyperintense foci in the corpus callosum | Heparin (prophylactically)—duration of treatment | 0.56 | 0.02 |
| Hyperintense foci in the structures of the posterior cranial fossa (brain stem, cerebellum) | SpO2—beginning of hospitalization | −0.56 | 0.02 |
| Presence of inflammatory (concentric) vessel thickening in VWI images | Tocilizumab | 0.54 | 0.03 |
| Presence of inflammatory (concentric) vessel thickening in VWI images | Tocilizumab—duration of treatment | 0.54 | 0.03 |
| Hyperintense foci in the lower parts of the temporal lobes | Dexaven | 0.54 | 0.03 |
| Hyperintense foci in the lower parts of the temporal lobes | Dexaven—duration of treatment | 0.53 | 0.03 |
| Hyperintense foci in the corpus callosum | Dexaven—duration of treatment | 0.52 | 0.03 |
| Hyperintense foci in the lower parts of the temporal lobes | SpO2—beginning of hospitalization | −0.51 | 0.04 |
| Presence of inflammatory (concentric) vessel thickening in VWI images | Oxygen nasal cannula | 0.49 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okrzeja, J.; Alimowski, M.; Garkowski, A.; Hładuński, M.; Kubas, B.; Adamczuk, J.; Czupryna, P.; Narejko, K.; Moniuszko-Malinowska, A. Assessment of Post-COVID-19 Changes in Brain—Clinical and Imaging Evaluation Using MRI Vessel Wall Imaging and Complementary MRI Methods. J. Clin. Med. 2024, 13, 6884. https://doi.org/10.3390/jcm13226884
Okrzeja J, Alimowski M, Garkowski A, Hładuński M, Kubas B, Adamczuk J, Czupryna P, Narejko K, Moniuszko-Malinowska A. Assessment of Post-COVID-19 Changes in Brain—Clinical and Imaging Evaluation Using MRI Vessel Wall Imaging and Complementary MRI Methods. Journal of Clinical Medicine. 2024; 13(22):6884. https://doi.org/10.3390/jcm13226884
Chicago/Turabian StyleOkrzeja, Jakub, Maciej Alimowski, Adam Garkowski, Marcin Hładuński, Bożena Kubas, Justyna Adamczuk, Piotr Czupryna, Karolina Narejko, and Anna Moniuszko-Malinowska. 2024. "Assessment of Post-COVID-19 Changes in Brain—Clinical and Imaging Evaluation Using MRI Vessel Wall Imaging and Complementary MRI Methods" Journal of Clinical Medicine 13, no. 22: 6884. https://doi.org/10.3390/jcm13226884
APA StyleOkrzeja, J., Alimowski, M., Garkowski, A., Hładuński, M., Kubas, B., Adamczuk, J., Czupryna, P., Narejko, K., & Moniuszko-Malinowska, A. (2024). Assessment of Post-COVID-19 Changes in Brain—Clinical and Imaging Evaluation Using MRI Vessel Wall Imaging and Complementary MRI Methods. Journal of Clinical Medicine, 13(22), 6884. https://doi.org/10.3390/jcm13226884










