Retinopathy of Prematurity: Incidence, Risk Factors, and Treatment Outcomes in a Tertiary Care Center
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Assessment
2.3. Statistical Analysis
3. Results
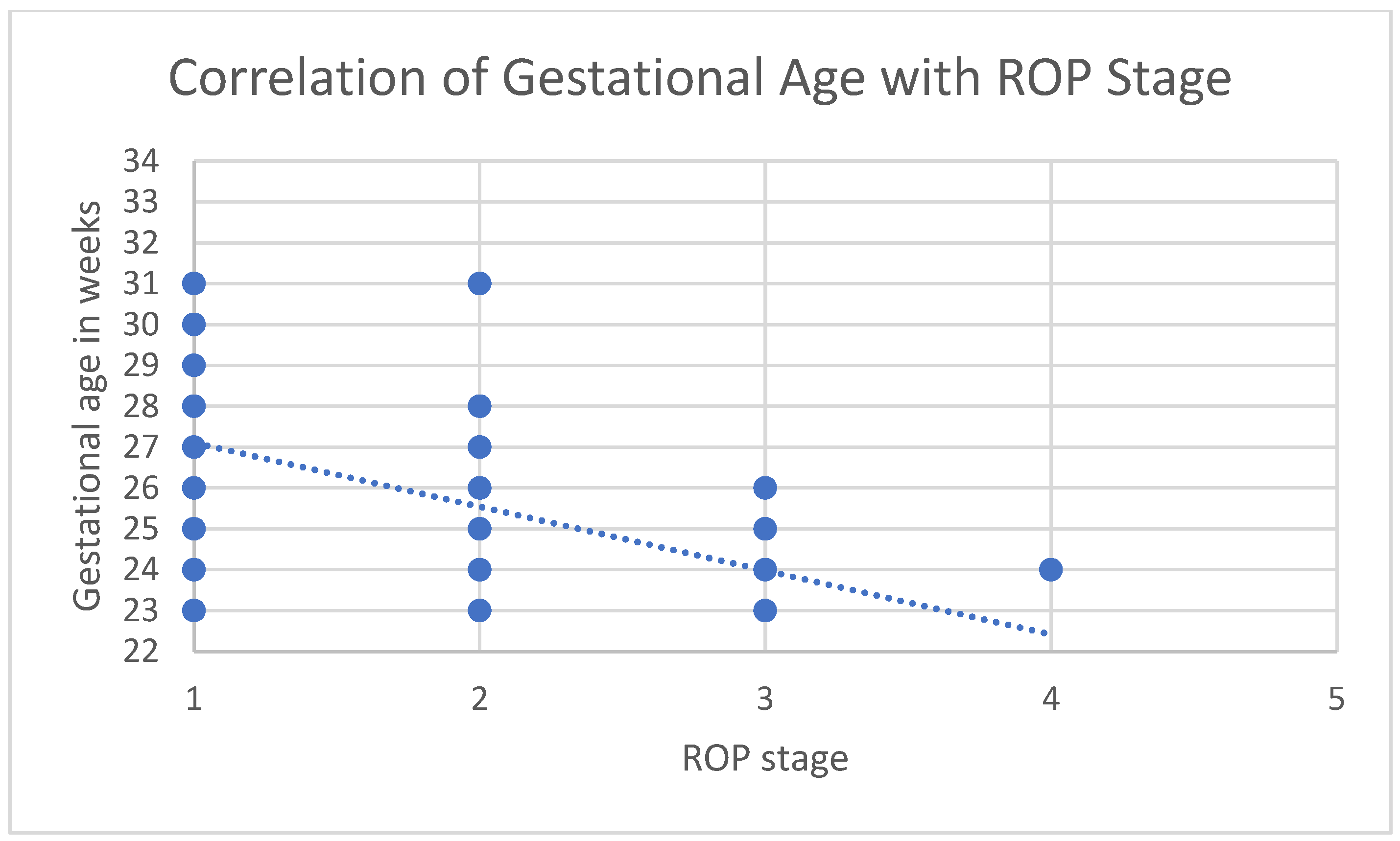
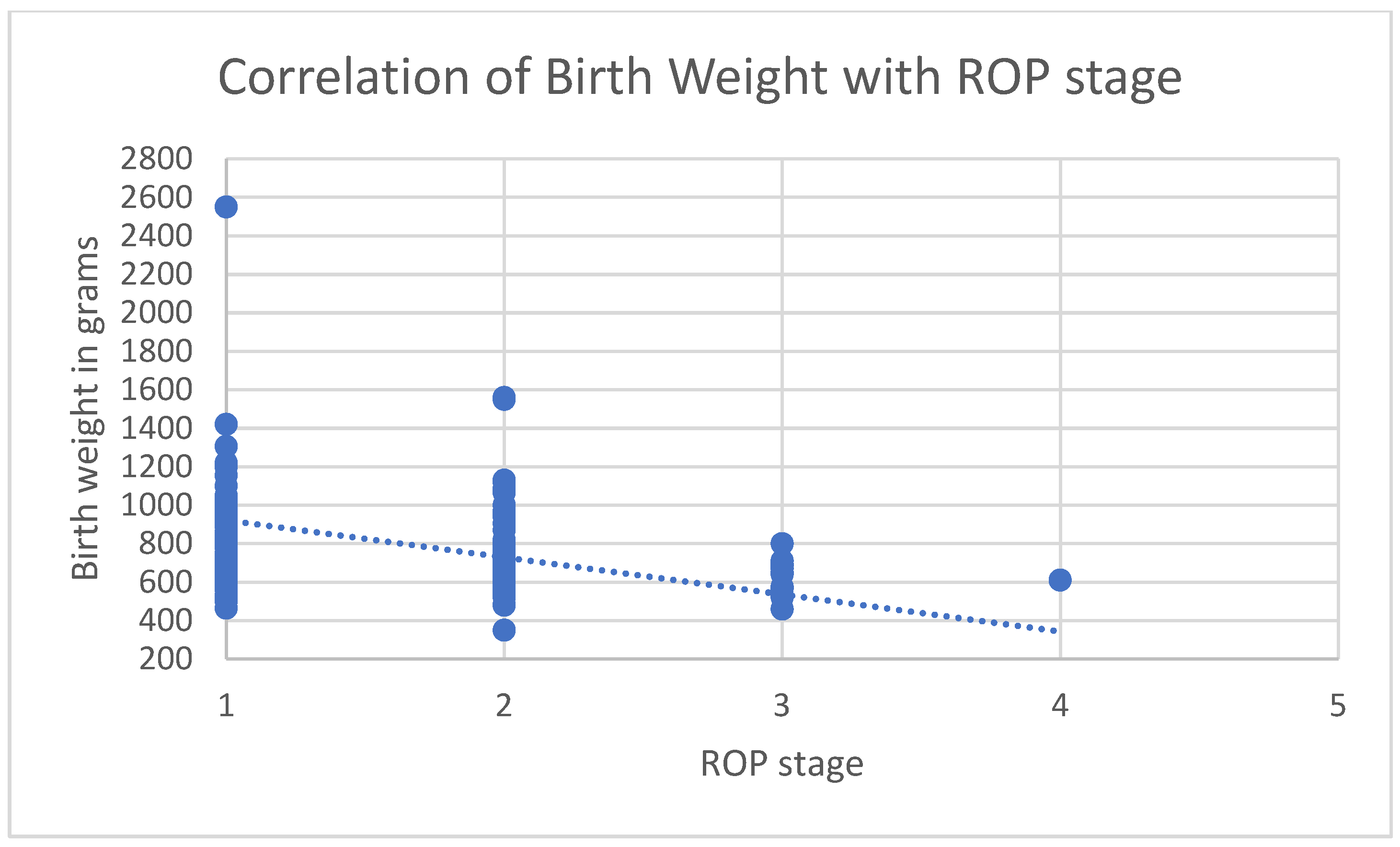
3.1. Correlations
3.2. Treatment of ROP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esser, J.; Gareis, O.; Lang, G.E.; Recker, D.; Spraul, C.W.; Wagner, P. Augenheilkunde, 6th ed.; Lang, G.K., Ed.; Georg Thieme Verlag: Stuttgart, Germany; New York, NY, USA, 2019; p. 442S. [Google Scholar]
- Stahl, A.; Lagrèze, W.A.; Agostini, H.T. Pathogenese der Frühgeborenenretinopathie. Der Ophthalmol. 2012, 109, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.H. Pathogenesis of retinopathy of prematurity. In Seminars in Neonatology; WB Saunders: Philadelphia, PA, USA, 2003; Volume 8, pp. 469–473. [Google Scholar]
- Kong, L.; Fry, M.; Al-Samarraie, M.; Gilbert, C.; Steinkuller, P.G. An update on progress and the changing epidemiology of causes of childhood blindness worldwide. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus JAAPOS 2012, 16, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.H.; Chang, E.Y.; Beck, K.; Hadfield, B.R.; Quinn, A.R.; Harper, C.A. 80 Years of vision: Preventing blindness from retinopathy of prematurity. J. Perinatol. 2021, 41, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Sabri, K.; Ells, A.L.; Lee, E.Y.; Dutta, S.; Vinekar, A. Retinopathy of Prematurity: A Global Perspective and Recent Developments. Pediatrics 2022, 150, e2021053924. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.E.; Lang, G.K.; Esser, J. (Eds.) Schlaglicht Augenheilkunde: Kinderophthalmologie, 1st ed.; Schlaglicht Augenheilkunde; Thieme: Stuttgart, Germany, 2015; p. 451 S. [Google Scholar]
- Hübler, A.; Jorch, G.; Arenz, S.; Avenarius, S.; Bachmaier, N.; Berger, A.; Bittrich, H.-J.; Brockmann, P.E.; Brune, T.; Bührer, C.; et al. Neonatologie [Internet], 2nd ed.; Aktualisierte und Erweiterte Auflage; Thieme Verlag: Stuttgart, Germany, 2019; Available online: https://www.thieme-connect.de/products/ebooks/book/10.1055/b-006-149522 (accessed on 11 May 2022).
- Gortner, L.; Meyer, S. (Eds.) Duale Reihe Pädiatrie [Internet], 5th ed.; Georg Thieme Verlag: Stuttgart, Germany, 2018; Available online: https://eref.thieme.de/10.1055/b-005-145246 (accessed on 13 May 2022).
- Ancel, P.Y.; Goffinet, F.; EPIPAGE-2 Writing Group; Kuhn, P.; Langer, B.; Matis, J.; Hernandorena, X.; Chabanier, P.; Joly-Pedespan, L.; Lecomte, B.; et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: Results of the EPIPAGE-2 cohort study. JAMA Pediatr. 2015, 169, 230–238. [Google Scholar] [CrossRef]
- Busik, V.; Lorenz, B.; Mais, C.; Jäger, M.; Friedburg, C.; Andrassi-Darida, M.; Ehrhardt, H.; Hubert, M. 10 Jahre Screening auf Frühgeborenenretinopathie (2009–2019). Die Ophthalmol. 2023, 120, 920–931. [Google Scholar] [CrossRef]
- Valentine, P.H.; Jackson, J.C.; Kalina, R.E.; Woodrum, D.E. Increased survival of low birth weight infants: Impact on the incidence of retinopathy of prematurity. Pediatrics 1989, 84, 442–445. [Google Scholar] [CrossRef]
- Gilbert, C.; Malik, A.N.J.; Nahar, N.; Das, S.K.; Visser, L.; Sitati, S.; Ademola-Popoola, D.S. Epidemiology of ROP update—Africa is the new frontier. In Seminars in Perinatology; WB Saunders: Philadelphia, PA, USA, 2019; Volume 43, pp. 317–322. [Google Scholar]
- Bhatnagar, A.; Skrehot, H.C.; Bhatt, A.; Herce, H.; Weng, C.Y. Epidemiology of Retinopathy of Prematurity in the US From 2003 to 2019. JAMA Ophthalmol. 2023, 141, 479–485. [Google Scholar] [CrossRef]
- Ahmed, I.; Hoyek, S.; Patel, N.A. Global Disparities in Retinopathy of Prematurity: A Literature Review. In Seminars in Ophthalmology; Taylor & Francis: Abingdon, UK, 2023; Volume 38, pp. 151–157. [Google Scholar]
- Gilbert, C.; Rahi, J.; Eckstein, M.; O’Sullivan, J.; Foster, A. Retinopathy of prematurity in middle-income countries. Lancet 1997, 350, 12–14. [Google Scholar] [CrossRef]
- Stahl, A.; Göpel, W. Screening and Treatment in Retinopathy of Prematurity. Dtsch. Arztebl. Int. 2015, 112, 730–735. [Google Scholar] [CrossRef]
- Li, L.; Gao, Y.; Chen, W.; Han, M. Screening for retinopathy of prematurity in North China. BMC Ophthalmol. 2022, 22, 251. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.H.; Shin, Y.U.; Cho, H. Retinopathy of prematurity: A review of epidemiology and current treatment strategies. Clin. Exp. Pediatr. 2021, 65, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.P.; Bründer, M.C.; Petrak, M.; Jehle, V.; Lagrèze, W.A.; Holz, F.G.; Stahl, A.; Krohne, T.U. Frühgeborenenretinopathie-Screening: Trends über die vergangenen 5 Jahre an zwei deutschen Universitätskliniken. Ophthalmologe 2018, 115, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Holmström, G.; Tornqvist, K.; Al-Hawasi, A.; Nilsson, Å.; Wallin, A.; Hellström, A. Increased frequency of retinopathy of prematurity over the last decade and significant regional differences. Acta Ophthalmol. 2018, 96, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Gerull, R.; Brauer, V.; Bassler, D.; Laubscher, B.; Pfister, R.E.; Nelle, M.; Müller, B.; Gerth-Kahlert, C.; Adams, M. Incidence of retinopathy of prematurity (ROP) and ROP treatment in Switzerland 2006-2015: A population-based analysis. Arch. Dis. Child.-Fetal Neonatal Ed. 2018, 103, F337–F342. [Google Scholar] [CrossRef]
- Akman, S.H.; Pfeil, J.M.; Stahl, A.; Ehlers, S.; Böhne, C.; Bohnhorst, B.; Framme, C.; Brockmann, D.; Bajor, A.; Jacobsen, C.; et al. Epidemiologie und Therapie der behandlungsbedürftigen Frühgeborenenretinopathie. Die Hannoveraner Daten im Retina.net ROP-Register von 2001 bis 2017. Der Ophthalmol. 2022, 119, 497–505. [Google Scholar] [CrossRef]
- Walz, J.M.; Rop-Register-Studiengruppe, R.; Bemme, S.; Reichl, S.; Akman, S.; Breuß, H.; Süsskind, D.; Glitz, B.; Müller, V.C.; Wagenfeld, L.; et al. Behandelte Frühgeborenenretinopathie in Deutschland. Ophthalmologe 2018, 115, 476–488. [Google Scholar] [CrossRef]
- Bowe, T.; Nyamai, L.; Ademola-Popoola, D.; Amphornphruet, A.; Anzures, R.; Cernichiaro-Espinosa, L.A.; Duke, R.; Duran, F.; Martinez-Castellanos, M.A.; Multani, D.P.K.; et al. The current state of retinopathy of prematurity in India, Kenya, Mexico, Nigeria, Philippines, Romania, Thailand, and Venezuela. Digit. J. Ophthalmol. DJO 2019, 25, 49–58. [Google Scholar] [CrossRef]
- Tiryaki Demir, S. Evaluation of Treatment Models in the Treatment of Retinopathy of Prematurity. SiSli Etfal Hastan Tip Bul. Med. Bull. Sisli Hosp. 2019, 53, 290–295. Available online: http://www.sislietfaltip.org/jvi.aspx?un=SETB-60465 (accessed on 17 May 2022). [CrossRef]
- Maier, R.F.; Hummler, H.; Kellner, U.; Krohne, T.U.; Lawrenz, B.; Lorenz, B.; Mitschdörfer, B.; Roll, C.; Stahl, A. Augenärztliche Screening-Untersuchung bei Frühgeborenen. Ophthalmol. Z. Dtsch. Ophthalmol. Ges. 2021, 118 (Suppl. 2), 117–131. [Google Scholar]
- Stahl, A.; Krohne, T.U.; Eter, N.; Oberacher-Velten, I.; Guthoff, R.; Meltendorf, S.; Ehrt, O.; Aisenbrey, S.; Roider, J.; Gerding, H.; et al. Comparing Alternative Ranibizumab Dosages for Safety and Efficacy in Retinopathy of Prematurity. JAMA Pediatr. 2018, 172, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Shih, C.P.; Lien, R.; Wang, N.K.; Chen, Y.P.; Chao, A.N.; Chen, K.-J.; Chen, T.-L.; Hwang, Y.-S.; Lai, C.-C. Serum vascular endothelial growth factor after Bevacizumab or Ranibizumab treatment for retinopathy of prematurity. Retina 2017, 37, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Lepore, D.; Fielder, A.; Fleck, B.; Reynolds, J.D.; Chiang, M.F.; Li, J.; Liew, M.; Maier, R.; Zhu, Q.; et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): An open-label randomised controlled trial. Lancet 2019, 394, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Mintz-Hittner, H.A.; Kennedy, K.A.; Chuang, A.Z.; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl. J. Med. 2011, 364, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.K.; Hubbard, G.B.; Hutchinson, A.K.; Lambert, S.R. Outcomes after Intravitreal Bevacizumab versus Laser Photocoagulation for Retinopathy of Prematurity: A 5-Year Retrospective Analysis. Ophthalmology 2015, 122, 1008–1015. [Google Scholar] [CrossRef]
- Hu, J.; Blair, M.P.; Shapiro, M.J.; Lichtenstein, S.J.; Galasso, J.M.; Kapur, R. Reactivation of Retinopathy of Prematurity After Bevacizumab Injection. Arch. Ophthalmol. 2012, 130, 1000–1006. [Google Scholar] [CrossRef]
- Deutsche Ophthalmologische Gesellschaft e. V. (DOG); Retinologische Gesellschaft e. V. (RG); Berufsverband der Augenärzte Deutschlands e. V. (BVA). Stellungnahme der Deutschen Ophthalmologischen Gesellschaft, der Retinologischen Gesellschaft und des Berufsverbands der Augenärzte Deutschlands zur Anti-VEGF-Therapie der Frühgeborenenretinopathie. Klin. Monatsblätter Augenheilkd. 2020, 117, 873–885. [Google Scholar]
- Stahl, A. Studienüberblick zur Frühgeborenenretinopathie. Der Ophthalmologe 2018, 115, 456–463. [Google Scholar] [CrossRef]
- Geloneck, M.M.; Chuang, A.Z.; Clark, W.L.; Hunt, M.G.; Norman, A.A.; Packwood, E.A.; Tawansy, K.A.; Mintz-Hittner, H.A.; for the BEAT-ROP Cooperative Group. Refractive Outcomes Following Bevacizumab Monotherapy Compared With Conventional Laser Treatment: A Randomized Clinical Trial. JAMA Ophthalmol. 2014, 132, 1327–1333. [Google Scholar] [CrossRef]
- Marlow, N.; Stahl, A.; Lepore, D.; Fielder, A.; Reynolds, J.D.; Zhu, Q.; Weisberger, A.; Stiehl, D.P.; Fleck, B. 2-year outcomes of ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW extension study): Prospective follow-up of an open label, randomised controlled trial. Lancet Child Adolesc. Health 2021, 5, 698–707. [Google Scholar] [CrossRef]
- Fortes Filho, J.B.; Eckert, G.U.; Valiatti, F.B.; dos Santos, P.G.B.; da Costa, M.C.; Procianoy, R.S. The influence of gestational age on the dynamic behavior of other risk factors associated with retinopathy of prematurity (ROP). Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 893–900. [Google Scholar] [CrossRef]
- Seiberth, V.; Linderkamp, O. Risk Factors in Retinopathy of Prematurity: A Multivariate Statistical Analysis. Ophthalmologica 2000, 214, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, P.; Kistner, A.; Andersson, E.M.; Hansen Pupp, I.; Holmström, G.; Ley, D.; Niklasson, A.; Smith, L.E.H.; Wu, C.; Hellström, A.; et al. Low Birth Weight Is a Risk Factor for Severe Retinopathy of Prematurity Depending on Gestational Age. PLoS ONE 2014, 9, e109460. [Google Scholar] [CrossRef] [PubMed]
- Engström, E.; Niklasson, A.; Wikland, K.A.; Ewald, U.; Hellström, A. The Role of Maternal Factors, Postnatal Nutrition, Weight Gain, and Gender in Regulation of Serum IGF-I among Preterm Infants. Pediatr. Res. 2005, 57, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Enninga, E.A.L.; Nevala, W.K.; Creedon, D.J.; Markovic, S.N.; Holtan, S.G. Fetal Sex-Based Differences in Maternal Hormones, Angiogenic Factors, and Immune Mediators During Pregnancy and the Postpartum Period. Am. J. Reprod. Immunol. 2015, 73, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, A.H.A.A.; Mohamed, G.B.; Othman, M.F. Retinopathy of Prematurity: A Study of Prevalence and Risk Factors. Middle East Afr. J. Ophthalmol. 2012, 19, 289–294. [Google Scholar] [CrossRef]
- Feghhi, M.; Altayeb, S.M.H.; Haghi, F.; Kasiri, A.; Farahi, F.; Dehdashtyan, M.; Movasaghi, M.; Rahim, F. Incidence of Retinopathy of Prematurity and Risk Factors in the South-Western Region of Iran. Middle East Afr. J. Ophthalmol. 2012, 19, 101–106. [Google Scholar] [CrossRef]
- Darlow, B.A.; Hutchinson, J.L.; Henderson-Smart, D.J.; Donoghue, D.A.; Simpson, J.M.; Evans, N.J.; Australian and New Zealand Neonatal Network. Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics 2005, 115, 990–996. [Google Scholar] [CrossRef]
- Blondel, B.; Kogan, M.D.; Alexander, G.R.; Dattani, N.; Kramer, M.S.; Macfarlane, A.; Wen, S.W. The Impact of the Increasing Number of Multiple Births on the Rates of Preterm Birth and Low Birthweight: An International Study. Am. J. Public Health 2002, 92, 1323–1330. [Google Scholar] [CrossRef]
- Blumenfeld, L.C.; Siatkowski, R.M.; Johnson, R.A.; Feuer, W.J.; Flynn, J.T. Retinopathy of prematurity in multiple-gestation pregnancies. Am. J. Ophthalmol. 1998, 125, 197–203. [Google Scholar] [CrossRef]
- Friling, R.; Rosen, S.D.; Monos, T.; Karplus, M.; Yassur, Y. Retinopathy of prematurity in multiple-gestation, very low birth weight infants. J. Pediatr. Ophthalmol. Strabismus 1997, 34, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; He, L.; Liu, X.H.; Wang, Y.M.; Liu, J.Q. Analysis of risk factors for retinopathy of prematurity. Int. J. Ophthalmol. 2011, 4, 631–633. [Google Scholar] [PubMed]
- Motta, M.; Filho, J.; Coblentz, J.; Fiorot, C. Multiple pregnancies and its relationship with the development of retinopathy of prematurity (ROP). Clin. Ophthalmol. 2011, 5, 1783–1787. [Google Scholar]
- Gschließer, A.; Stifter, E.; Neumayer, T.; Moser, E.; Papp, A.; Dorner, G.; Schmidt-Erfurth, U. Twin-twin transfusion syndrome as a possible risk factor for the development of retinopathy of prematurity. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht. Graefe’s Arch. Klin. Exp. Ophthalmol. 2015, 253, 151–156. [Google Scholar] [CrossRef]
- El Kateb, A.; Ville, Y. Update on twin-to-twin transfusion syndrome. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 63–75. [Google Scholar] [CrossRef]
- Saugstad, O.D.; Aune, D. Optimal Oxygenation of Extremely Low Birth Weight Infants: A Meta-Analysis and Systematic Review of the Oxygen Saturation Target Studies. Neonatology 2013, 105, 55–63. [Google Scholar] [CrossRef]
- Graziosi, A.; Perrotta, M.; Russo, D.; Gasparroni, G.; D’Egidio, C.; Marinelli, B.; Di Marzio, G.; Falconio, G.; Mastropasqua, L.; Li Volti, G.; et al. Oxidative Stress Markers and the Retinopathy of Prematurity. J. Clin. Med. 2020, 9, 2711. [Google Scholar] [CrossRef]
| ROP 1 N (%) or Median (IQR) 2 | NON-ROP N (%) or Median (IQR) 2 | p-Value | |
|---|---|---|---|
| Birth weight (grams) Gestational age (weeks) | 711 (326.3) | 1125 (427.5) | p < 0.001 |
| 26 (3) | 29 (4) | p < 0.001 | |
| Gender Female Male | 69 (47.9%) | 82 (39.4%) | p = 0.113 |
| 75 (52.1%) | 126 (60.6%) | ||
| Pregnancy type Singleton birth Twin Triplet | p = 0.042 | ||
| 104 (72.2%) | 128 (61.5%) | ||
| 37 (25.7%) | 66 (31.7%) | ||
| 3 (2.1%) | |||
| Total | 144 (100%) | 208 (100%) |
| ROP Stage | Gestational Age (Weeks) | Treated Patients (N = 17) | Type of Treatment | Initial Success 1 N (%) | Type of Follow-Up Treatment |
|---|---|---|---|---|---|
| Stage 3 (N = 16) | ≤24 | 8 | Laser (N = 5) Anti-VEGF (N = 3) | 5 (100%) 2 (66.7%) | Laser (N = 1) |
25 ≥26 | 5 3 | Laser (N = 2) Anti-VEGF (N = 3) Laser (N = 3) Anti-VEGF (N = 0) | 2 (100%) 2 (66.7%) 3 (100%) | Anti-VEGF (N = 1) - | |
| Stage 4 (N = 1) | ≤24 25 ≥26 | 1 0 0 | Vitrectomy (N = 1) - - | 0 (0%) - - | Vitrectomy and cerclage (N = 1) - - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blazon, M.N.; Rezar-Dreindl, S.; Wassermann, L.; Neumayer, T.; Berger, A.; Stifter, E. Retinopathy of Prematurity: Incidence, Risk Factors, and Treatment Outcomes in a Tertiary Care Center. J. Clin. Med. 2024, 13, 6926. https://doi.org/10.3390/jcm13226926
Blazon MN, Rezar-Dreindl S, Wassermann L, Neumayer T, Berger A, Stifter E. Retinopathy of Prematurity: Incidence, Risk Factors, and Treatment Outcomes in a Tertiary Care Center. Journal of Clinical Medicine. 2024; 13(22):6926. https://doi.org/10.3390/jcm13226926
Chicago/Turabian StyleBlazon, Mara Nike, Sandra Rezar-Dreindl, Lorenz Wassermann, Thomas Neumayer, Angelika Berger, and Eva Stifter. 2024. "Retinopathy of Prematurity: Incidence, Risk Factors, and Treatment Outcomes in a Tertiary Care Center" Journal of Clinical Medicine 13, no. 22: 6926. https://doi.org/10.3390/jcm13226926
APA StyleBlazon, M. N., Rezar-Dreindl, S., Wassermann, L., Neumayer, T., Berger, A., & Stifter, E. (2024). Retinopathy of Prematurity: Incidence, Risk Factors, and Treatment Outcomes in a Tertiary Care Center. Journal of Clinical Medicine, 13(22), 6926. https://doi.org/10.3390/jcm13226926






