The Role of Immunotherapy in MMR-Deficient Endometrial Carcinoma: State of the Art and Future Perspectives
Abstract
1. Introduction
- ▪
- POLE-ultramutated: These tumors exhibit mutations in the POLE gene, leading to an exceptionally high mutation rate. This results in a high neoantigen load, making these tumors highly immunogenic and generally responsive to immunotherapy, with a favorable prognosis.
- ▪
- MSI-H: Tumors in this category result from deficiencies in the mismatch repair system, leading to high levels of microsatellite instability and a high mutational burden. This makes them excellent candidates for immunotherapy due to their enhanced immune system visibility.
- ▪
- Copy-number low: These tumors are often hormone receptor-positive with fewer genetic alterations, generally following a more indolent clinical course compared to other subtypes.
- ▪
- Copy-number high: Associated with serous histology and TP53 mutations, these tumors have numerous chromosomal alterations and a poor prognosis.
2. Background
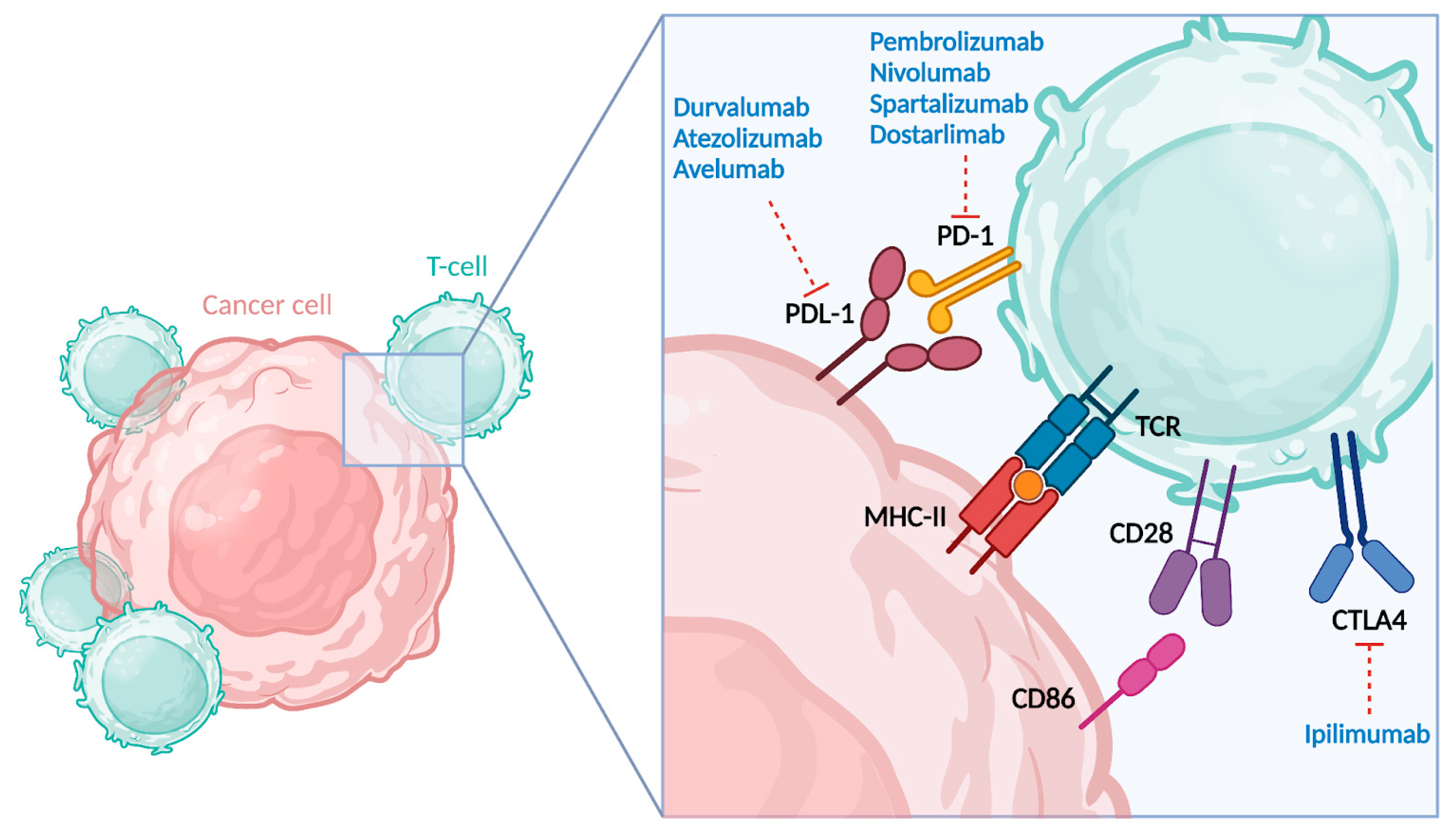
2.1. Immunogenicity and Pathogenic Basis of PD-1 and PD-L1 Overexpression in MMRd Endometrial Cancer
2.2. Anti-PD-1 and Anti-PD-L1 Agents
2.3. Anti-CTLA-4
3. Clinical Evidence and Current Indications—Advanced and Recurrent Disease
Randomized Clinical Trials on Immunotherapy
4. Ongoing Trials Investigating Immunotherapy in First Line as Monotherapy
5. Combined Therapies
| Trial Name (NCT ID) | Study Design | Drugs | Drug Class | Population | Treatment | Primary Endpoint | Estimated Study Completion and Status |
|---|---|---|---|---|---|---|---|
| ENGOT-en9/ LEAP-001 trial [43] (NCT03884101) | Phase III RCT | Pembrolizumab (MK3475) Lenvatinib (E7080) | TKI anti-VEGF | Newly diagnosed stage III/IV or first recurrent endometrial cancer (stratified for MMR status) | Experimental: Lenvatinib daily and Pembrolizumab once at the start of each 3-week treatment cycle; active comparator: Paclitaxel + Carboplatin | PFS, OS | 01/2025 Active, not recruiting |
| ATAPEMBRO trial [49] (NCT04014530) | Phase I/II | Ataluren (DB05016); Pembrolizumab (MK3475) | DMD drug, binding ribosomes | Metastatic MMRd EC (and MMRd and MMRp colorectal adenocarcinoma) | Phase I: 200 mg i.v. Pembrolizumab q3w and dose escalation of Ataluren. Phase II: 200 mg i.v. pembrolizumab q3w and Ataluren at MTD (maximum tolerated dose) | Toxicities and side effects | 3/2023 Recruiting |
| ABILITY-1 trial [50] (NCT05086692) | Phase I/II | MDNA11 (C187359); Pembrolizumab (MK3475) | rIL-2 | Advanced solid tumors (MSI-H EC) | Intervention I: MDNA11 i.v. q2w dose escalation until mRDE. Intervention II: MDNA11 + Pembrolizumab until cRDE | Tolerability; TRAEs; TEAEs | 12/2026 Recruiting |
| EndoBARR trial [39] (NCT03694262) | Phase II | Bevacizumab (J9035); Atezolizumab (MPDL-3280A); Rucaparib (L01XK03) | Anti-VEGF; PARPi | Patients must have recurrent or persistent/progressive endometrial carcinoma, which is refractory to curative therapy or established treatments | Atezolizumab i.v. 1200 mg, first day of 21-day cycle; Bevacizumab i.v. 15 mg/kg, first day of 21-day cycle and Rucaparib 600 mg orally twice daily by continuous dosing | ORR | 04/2023Completed |
| [51] (NCT03526432) | Phase II | Atezolizumab (MPDL-3280A); Bevacizumab (J9035) | Anti-VEGF | Advanced, recurrent, or persistent ECs that have relapsed or are refractory to established treatments | Bevacizumab i.v. 15 mg/kg, first day of 21-day cycle and Atezolizumab i.v. 1200 mg, first day of 21-day cycle | ORR | 05/2025Active, not recruiting |
| NRG-GY025 [47] (NCT05112601) | Phase II | Nivolumab(MDX-1106) Ipilimumab (MDX-010) | Anti-CTLA4 | Recurrent MMRd ECs | Arm I: Nivolumab i.v. on day 1 of each cycle and Ipilimumab i.v. on day 1 of every other cycle, q3w for up to 8 cycles (in the absence of disease progression, unacceptable toxicity, or CR). Arm II: Nivolumab | PFS | 04/2026 Recruiting |
| [52] (NCT03367741) | Phase II | Cabozantinib S-malate (XL184); Nivolumab (MDX-1: 106) | TKI | Advanced, recurrent, or metastatic endometrial cancer previously treated with at least one line of platinum-based chemotherapy | Arm I: Cabozantinib S-malate orally on days 1–28 and Nivolumab i.v. on days 1 and 15 Arm II: Nivolumab | PFS | 12/2024 Active, not recruiting |
Immunotherapy Combination with Novel Therapies
6. Future Applications: Early-Stage Disease Setting
| Trial Name (NCT ID) | Study Design | Drugs | Population | Treatment | Primary Endpoint | Estimated Study Completion and Status |
|---|---|---|---|---|---|---|
| NRG-GY020 trial [57] (NCT04214067) | Phase III RCT | Pembrolizumab (MK3475) | High-/intermediate-risk stage I/II MMRd EC | Experimental arm II: EBRT, VBRT + pembrolizumab IV within 7 days prior to the start of radiation therapy. Treatment with pembrolizumab repeats every 6 weeks for 9 cycles. Control arm: EBRT + VBRT. | 3-year RFS | 2/2025 Recruiting |
| RAINBO MMRd-GREEN trial GCIG/DGOG/ENGOT-EN142 (NCT05255653) | Phase III RCT | Durvalumab | Stage III EC (MMRd) or stage IB/II EC with substantial LVSI | Experimental arm: adjuvant radiotherapy (45.0–48.6 Gy; 1.8–2.0 Gy per fraction, 5 fractions a week) combined with and followed by durvalumab, 1500 mg intravenous once every 4 weeks for, in total, 1 year (13 cycles) starting in the first week of radiotherapy. Control arm: adjuvant pelvic EBRT (45.0–48.6 Gy; 1.8–2.0 Gy per fraction, 5 fractions a week). | RFS | 01/2031 Recruiting |
| SATELLITE trial (NCT06278857) | Phase IIb | Dostarlimab (TSR-042) | Stage I, FIGO G1-2, MMRd, endometrioid EC, and wish to preserve the uterus or not a suitable candidate for hysterectomy. | Dostarlimab 4 cycles q3w, 34w rest period, 3 cycles q6w (total 7 cycles). | pCR | 01/06/2028 Not yet recruiting |
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Monk, B.; Powell, M.; Westin, S.; Slomovitz, B.; Moore, K.; Eskander, R.N.; Raspagliesi, F.; Barretina-Ginesta, M.-P.; Colombo, N.; et al. Adding immunotherapy to first-line treatment of advanced and metastatic endometrial cancer. Ann. Oncol. 2024, 35, 414–428. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network; Levine, D.A. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73, Correction in Nature 2013, 500, 242. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Spagnol, G.; Vezzaro, T.; Bigardi, S.; De Tommasi, O.; Facchetti, E.; Tripepi, M.; Costeniero, D.; Munerol, C.; Maggino, T.; et al. Low-Risk and High-Risk NSMPs: A Prognostic Subclassification of No Specific Molecular Profile Subtype of Endometrial Carcinomas. Cancers 2024, 16, 3221. [Google Scholar] [CrossRef]
- Jamieson, A.; Huvila, J.; Chiu, D.; Thompson, E.F.; Scott, S.; Salvador, S.; Vicus, D.; Helpman, L.; Gotlieb, W.; Kean, S.; et al. Grade and Estrogen Receptor Expression Identify a Subset of No Specific Molecular Profile Endometrial Carcinomas at a Very Low Risk of Disease-Specific Death. Mod. Pathol. 2023, 36, 100085, Correction in Mod. Pathol. 2023, 36, 100212. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Campbell, B.B.; Light, N.; Fabrizio, D.; Zatzman, M.; Fuligni, F.; De Borja, R.; Davidson, S.; Edwards, M.; Elvin, J.A.; Hodel, K.P.; et al. Comprehensive Analysis of Hypermutation in Human Cancer. Cell 2017, 171, 1042–1056.e10. [Google Scholar] [CrossRef]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Grevenkamp, F.; Karnezis, A.; Yang, W.; et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef]
- Spranger, S.; Gajewski, T.F. Tumor-intrinsic oncogene pathways mediating immune avoidance. OncoImmunology 2015, 5, e1086862. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oaknin, A.; Tinker, A.V.; Gilbert, L.; Samouëlian, V.; Mathews, C.; Brown, J.; Barretina-Ginesta, M.-P.; Moreno, V.; Gravina, A.; Abdeddaim, C.; et al. Clinical Activity and Safety of the Anti–Programmed Death 1 Monoclonal Antibody Dostarlimab for Patients With Recurrent or Advanced Mismatch Repair–Deficient Endometrial Cancer: A Nonrandomized Phase 1 Clinical Trial. JAMA Oncol. 2020, 6, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357, Correction in Lancet 2021, 398, 1686. [Google Scholar] [CrossRef]
- Loibl, S.; Schneeweiss, A.; Huober, J.; Braun, M.; Rey, J.; Blohmer, J.-U.; Furlanetto, J.; Zahm, D.-M.; Hanusch, C.; Thomalla, J.; et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann. Oncol. 2022, 33, 1149–1158. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Tivol, E.A.; Borriello, F.; Schweitzer, A.N.; Lynch, W.P.; Bluestone, J.A.; Sharpe, A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995, 3, 541–547. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Oaknin, A.; Pothuri, B.; Gilbert, L.; Sabatier, R.; Brown, J.; Ghamande, S.; Mathews, C.; O’Malley, D.M.; Kristeleit, R.; Boni, V.; et al. Safety, Efficacy, and Biomarker Analyses of Dostarlimab in Patients with Endometrial Cancer: Interim Results of the Phase I GARNET Study. Clin. Cancer Res. 2023, 29, 4564–4574. [Google Scholar] [CrossRef] [PubMed]
- Oaknin, A.; Bosse, T.; Creutzberg, C.; Giornelli, G.; Harter, P.; Joly, F.; Lorusso, D.; Marth, C.; Makker, V.; Mirza, M.; et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, M.; Montico, M.; Lorusso, D.; Mazzeo, R.; Oaknin, A.; Musacchio, L.; Scambia, G.; Puglisi, F.; Pignata, S. Incorporation of anti-PD1 or anti PD-L1 agents to platinum-based chemotherapy for the primary treatment of advanced or recurrent endometrial cancer. A meta-analysis. Cancer Treat. Rev. 2024, 125, 102701. [Google Scholar] [CrossRef] [PubMed]
- Pignata, S.; Califano, D.; Lorusso, D.; Arenare, L.; Bartoletti, M.; De Giorgi, U.; Andreetta, C.; Pisano, C.; Scambia, G.; Lombardi, D.; et al. MITO END-3: Efficacy of avelumab immunotherapy according to molecular profiling in first-line endometrial cancer therapy. Ann. Oncol. 2024, 35, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; Christensen, R.D.; Novák, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef]
- Westin, S.N.; Moore, K.; Chon, H.S.; Lee, J.Y.; Thomes Pepin, J.; Sundborg, M.; Shai, A.; de la Garza, J.; Nishio, S.; Gold, M.A.; et al. Durvalumab Plus Carboplatin/Paclitaxel Followed by Maintenance Durvalumab With or Without Olaparib as First-Line Treatment for Advanced Endometrial Cancer: The Phase III DUO-E Trial. J. Clin. Oncol. 2024, 42, 283–299, Correction in J. Clin. Oncol. 2024, 42, 3262. [Google Scholar] [CrossRef]
- Colombo, N.; Biagioli, E.; Harano, K.; Galli, F.; Hudson, E.; Antill, Y.; Choi, C.H.; Rabaglio, M.; Marmé, F.; Marth, C.; et al. Atezolizumab and chemotherapy for advanced or recurrent endometrial cancer (AtTEnd): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2024, 25, 1135–1146. [Google Scholar] [CrossRef]
- Joly, F.; Ray-Coquard, I.L.; Rubio, M.J.; Paoletti, X.; Davis, A.J.; Hudson, E.; Lorusso, D.; Tognon, G.; Hasler-Strub, U.; Choi, C.H.; et al. Randomized phase III trial in MMR deficient (MMRd) endometrial cancer (EC) patients comparing chemotherapy (CT) alone versus dostarlimab in first line advanced/metastatic setting: DOMENICA study (GINECO-EN105b/ENGOT-en13 study). J. Clin. Oncol. 2023, 41 (Suppl. S16), TPS5630. [Google Scholar] [CrossRef]
- Slomovitz, B.M.; Cibula, D.; Simsek, T.; Mirza, M.R.; Maćkowiak-Matejczk, B.; Hudson, E.; Romero, I.; Colombo, N.; Korach, J.; Yin, R.; et al. KEYNOTE-C93/GOG-3064/ENGOT-en15: A phase 3, randomized, open-label study of first-line pembrolizumab versus platinum-doublet chemotherapy in mismatch repair deficient advanced or recurrent endo-metrial carcinoma. J. Clin. Oncol. 2022, 40 (Suppl. S16), TPS5623. [Google Scholar] [CrossRef]
- Ciciola, P.; Cascetta, P.; Bianco, C.; Formisano, L.; Bianco, R. Combining Immune Checkpoint Inhibitors with Anti-Angiogenic Agents. J. Clin. Med. 2020, 9, 675. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wan, Y.; Zhang, L.; Meng, H.; Yuan, L.; Zhou, S.; Cheng, W.; Jiang, Y. Beyond monotherapy: An era ushering in combinations of PARP inhibitors with immune checkpoint inhibitors for solid tumors. Biomed. Pharmacother. 2024, 175, 116733. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.S.; Da Cruz Paula, A.; Cadoo, K.A.; Abu-Rustum, N.R.; Pei, X.; Brown, D.N.; Ferrando, L.; Sebastiao, A.P.M.; Riaz, N.; Robson, M.E.; et al. Endometrial Cancers in BRCA1 or BRCA2 Germline Mutation Carriers: Assessment of Homologous Recombination DNA Repair Defects. JCO Precis. Oncol. 2019, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, L.; Cong, Z.; Amoozgar, Z.; Kiner, E.; Xing, D.; Orsulic, S.; Matulonis, U.; Goldberg, M.S. The PARP1 inhibitor BMN 673 exhibits immunoregulatory effects in a Brca1 −/− murine model of ovarian cancer. Biochem. Biophys. Res. Commun. 2015, 463, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Yélamos, J.; Monreal, Y.; Saenz, L.; Aguado, E.; Schreiber, V.; Mota, R.; Fuente, T.; Minguela, A.; Parrilla, P.; de Murcia, G.; et al. PARP-2 deficiency affects the survival of CD4+CD8+ double-positive thymocytes. EMBO J. 2006, 25, 4350–4360. [Google Scholar] [CrossRef]
- Samstein, R.M.; Krishna, C.; Ma, X.; Pei, X.; Lee, K.-W.; Makarov, V.; Kuo, F.; Chung, J.; Srivastava, R.M.; Purohit, T.A.; et al. Mutations in BRCA1 and BRCA2 differentially affect the tumor microenvironment and response to checkpoint blockade immunotherapy. Nat. Cancer 2020, 1, 1188–1203. [Google Scholar] [CrossRef]
- Rimel, B.J.; Enserro, D.; Bender, D.P.; Jackson, C.G.; Tan, A.; Alluri, N.; Borowsky, M.; Moroney, J.; Hendrickson, A.W.; Backes, F.; et al. NRG-GY012: Randomized phase 2 study comparing olaparib, cediranib, and the combination of cediranib/olaparib in women with recurrent, persistent, or metastatic endometrial cancer. Cancer 2023, 130, 1234–1245. [Google Scholar] [CrossRef]
- Skopelja-Gardner, S.; An, J.; Elkon, K.B. Role of the cGAS–STING pathway in systemic and organ-specific diseases. Nat. Rev. Nephrol. 2022, 18, 558–572. [Google Scholar] [CrossRef]
- Bradley, W.H.; Hayes, M.P.; Taylor, N.; Rader, J.S.; Bishop, E.; Hopp, E.; McAlarnen, L.A.; Liegl, M.; Simpson, P.; Uyar, D. An open label, nonrandomized, multisite phase II trial combining bevacizumab, atezolizumab, and rucaparib for the treatment of previously treated recurrent and progressive endometrial cancer. J. Clin. Oncol. 2022, 40, 5510. [Google Scholar] [CrossRef]
- Makker, V.; Colombo, N.; Herráez, A.C.; Santin, A.D.; Colomba, E.; Miller, D.S.; Fujiwara, K.; Pignata, S.; Baron-Hay, S.; Ray-Coquard, I.; et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N. Engl. J. Med. 2022, 386, 437–448. [Google Scholar] [CrossRef]
- Makker, V.; Rasco, D.; Vogelzang, N.J.; Brose, M.S.; Cohn, A.L.; Mier, J.; Di Simone, C.; Hyman, D.M.; E Stepan, D.; E Dutcus, C.; et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: An interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Balasubramaniam, S.; Zhang, W.; Zhang, L.; Sridhara, R.; Spillman, D.; Mathai, J.P.; Scott, B.; Golding, S.J.; Coory, M.; et al. FDA Approval Summary: Pembrolizumab plus Lenvatinib for Endometrial Carcinoma, a Collaborative International Review under Project Orbis. Clin. Cancer Res. 2020, 26, 5062–5067. [Google Scholar] [CrossRef] [PubMed]
- Marth, C.; Tarnawski, R.; Tyulyandina, A.; Pignata, S.; Gilbert, L.; Kaen, D.; Rubio, M.J.; Frentzas, S.; Beiner, M.; Magallanes-Maciel, M.; et al. Phase 3, randomized, open-label study of pembrolizumab plus lenvatinib versus chemotherapy for first-line treatment of advanced or recurrent endometrial cancer: ENGOT-en9/LEAP-001. Int. J. Gynecol. Cancer 2022, 32, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ban, X.; Yang, F.; Li, J.; Cheng, X.; Zhang, R.; Huang, X.; Huang, Y.; Li, Q.; Qiu, Y.; et al. Phase II trial of efficacy, safety and biomarker analysis of sintilimab plus anlotinib for patients with recurrent or advanced endometrial cancer. J. Immunother. Cancer 2022, 10, e004338. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; A Rizvi, N.; Goldman, J.W.; Gettinger, S.N.; Borghaei, H.; Brahmer, J.R.; E Ready, N.; E Gerber, D.; Chow, L.Q.; A Juergens, R.; et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): Results of an open-label, phase 1, multicohort study. Lancet Oncol. 2016, 18, 31–41. [Google Scholar] [CrossRef]
- Rubinstein, M.M.; Doria, E.R.; Konner, J.; Lichtman, S.; Zhou, Q.; Iasonos, A.; Sarasohn, D.; Troso-Sandoval, T.; Friedman, C.; O’Cearbhaill, R.; et al. Durvalumab with or without tremelimumab in patients with persistent or recurrent endometrial cancer or endometrial carcinosarcoma: A randomized open-label phase 2 study. Gynecol. Oncol. 2023, 169, 64–69. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Testing Nivolumab With or Without Ipilimumab in Deficient Mismatch Repair System (dMMR) Recurrent Endometrial Carcinoma (NRG-GY025). NIH National Library of Medicine. 2024. Available online: www.clinicaltrials.gov (accessed on 9 October 2024).
- Knisely, A.; Hinchcliff, E.; Fellman, B.; Mosley, A.; Lito, K.; Hull, S.; Westin, S.N.; Sood, A.K.; Schmeler, K.M.; Taylor, J.S.; et al. Phase 1b study of intraperitoneal ipilimumab and nivolumab in patients with recurrent gynecologic malignancies with peritoneal carcinomatosis. Med 2024, 5, 311–320.e3. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Study of Pembrolizumab Combined with Ataluren in Patients with Metastatic pMMR and dMMR Colorectal Cancer Adenocarcinomas or Metastatic dMMR Endometrial Carcinoma: The ATAPEMBRO Study (ATAPEMBRO). NIH National Library of Medicine. 2024. Available online: www.clinicaltrials.gov (accessed on 9 October 2024).
- ClinicalTrials.gov. A Beta-only IL-2 ImmunoTherapY Study (ABILITY-1). NIH National Library of Medicine. 2024. Available online: www.clinicaltrials.gov (accessed on 9 October 2024).
- ClinicalTrials.gov. Phase II Study of Atezolizumab plus Bevacizumab in Endometrial Cancer. NIH National Library of Medicine. 2024. Available online: www.clinicaltrials.gov (accessed on 9 October 2024).
- ClinicalTrials.gov. Cabozantinib S-malate and Nivolumab in Treating Patients with Advanced, Recurrent, or Metastatic En-dometrial Cancer. NIH National Library of Medicine. 2024. Available online: www.clinicaltrials.gov (accessed on 9 October 2024).
- Cantillo, E.; Zemla, T.J.; Moroney, J.W.; A Alvarez, E.; Yauch, L.; Lin, Y.G.; Brockman, J.M.; DiLallo, G.; Liu, J.F.; Secord, A.A.; et al. AFT-50 EndoMAP: A phase IB/II multi-cohort study of targeted agents for patients with recurrent or persistent endometrial cancer. J. Clin. Oncol. 2023, 41, TPS5634. [Google Scholar] [CrossRef]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8+ T cell effector function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef]
- Van Gorp, T.; Cibula, D.; Lv, W.; Backes, F.; Ortaç, F.; Hasegawa, K.; Lindemann, K.; Savarese, A.; Laenen, A.; Kim, Y.; et al. ENGOT-en11/GOG-3053/KEYNOTE-B21: A randomised, double-blind, phase III study of pembrolizumab or placebo plus adjuvant chemotherapy with or without radiotherapy in patients with newly diagnosed, high-risk endometrial cancer. Ann. Oncol. 2024, 35, 968–980. [Google Scholar] [CrossRef]
- RAINBO Research Consortium. Refining adjuvant treatment in endometrial cancer based on molecular features: The RAINBO clinical trial program. Int. J. Gynecol. Cancer 2022, 33, 109–117. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Phase III Randomized Trial of Radiation +/- Pembrolizumab (MK-3475) for Newly Diagnosed Early Stage High Intermediate Risk Mismatch Repair Deficient (dMMR) Endometrioid Endometrial Cancer (NRG-GY020). NIH National Library of Medicine. 2024. Available online: www.clinicaltrials.gov (accessed on 9 October 2024).
- linicalTrials.gov. SATELLITE Study (feaSibility sAfeTy Efficacy dostarLimab earLy-stage defIcient endomeTrial cancEr). NIH National Library of Medicine. 2024. Available online: www.clinicaltrials.gov (accessed on 9 October 2024).
- Eerkens, A.L.; Brummel, K.; Vledder, A.; Paijens, S.T.; Requesens, M.; Loiero, D.; van Rooij, N.; Plat, A.; Haan, F.-J.; Klok, P.; et al. Neoadjuvant immune checkpoint blockade in women with mismatch repair deficient endometrial cancer: A phase I study. Nat. Commun. 2024, 15, 7695. [Google Scholar] [CrossRef] [PubMed]
| Trial Name (NCT ID) | Author | Design of the Study | Population | Treatment | Maintenance | Primary Endpoint | Secondary Endpoint | ||
|---|---|---|---|---|---|---|---|---|---|
| Experimental Arm | Control Arm | Experimental Arm | Control Arm | ||||||
| MITO END-3 trial (2024) [24] (NCT03503786) | Pignata, S., et al. | RC phase II | FIGO stage III and IV or recurrent EC with no previous systemic CHT as primary treatment for advanced or metastatic disease | Carboplatin and Paclitaxel + Avelumab every 3 weeks for 6 cycles | Carboplatin and Paclitaxel + Placebo every 3 weeks for 6 to 8 cycles | Avelumab every 2 weeks | Placebo | PFS (in the ITT population) | OS; QoL; objective response rate; duration of response; safety and tolerability |
| RUBY trial (2023) [25] (NCT03981796) | Mirza, M.R., et al. | RC phase III | Primary treatment for advanced or metastatic disease (FIGO stage III or IV) or metastatic disease not amenable to curative therapy | Carboplatin and Paclitaxel + Dostarlimab every 3 weeks for 6 cycles | Carboplatin and Paclitaxel + Placebo every 3 weeks for 6 cycles | Dostarlimab every 6 weeks | Placebo every 6 weeks | PFS; OS (in the overall population) | Objective response; safety; disease control; response duration; time to second PD; PROs; pharmacokinetic and immunogenicity analyses |
| NRG GY018 trial (2023) [26] (NCT03914612) | Eskander, R.N., et al. | RC phase III | Advanced (FIGO stage III-IV) or recurrent EC of any histologic subtype (except for carcinosarcoma) | Carboplatin and Paclitaxel + Pembrolizumab for 6 cycles | Carboplatin and Paclitaxel + Placebo for 6 cycles | Pembrolizumab every 6 weeks for 14 cycles | Placebo every 6 weeks for 14 cycles | PFS (in two cohorts based on MMR status) | Safety; OS; QoL |
| DUO-E trial (2024) [27] (NCT04269200) | Westin, S.N., et al. | RC phase III | Newly diagnosed advanced (FIGO stage III-IV) or recurrent EC of epithelial histology (excluding sarcomas) | 2 experimental arms: both receiving Carboplatin and Paclitaxel + Durvalumab every 3 weeks for 6 cycles | Carboplatin and Paclitaxel + Placebo every 3 weeks for 6 cycles | 2 arms: (i) Durvalumab every 4 weeks; (ii) Durvalumab and Olaparaib every 4 weeks | Placebo every 4 weeks | PFS (in the ITT population) | OS; QoL; safety |
| AtTEnd trial (2024) [28] (NCT03603184) | Colombo, N., et al. | RC phase III | EC with residual disease after surgery, or inoperable FIGO stage III–IV EC or carcinosarcoma with no previous systemic anticancer therapy, or recurrent disease if not previously treated with chemotherapy for recurrence | Carboplatin and Paclitaxel + Atezolizumab every 3 weeks for 6–8 cycles | Carboplatin and Paclitaxel + Placebo every 3 weeks for 6–8 cycles | Atezolizumab every 21 days | Placebo every 21 days | PFS, OS (in the ITT population) | Objective response rate; duration of response; second PFS; QoL; safety; pharmacokinetics; anti-drug antibodies |
| MITO END-3 Trial [24] (NCT03503786) | RUBY Trial [25] (NCT03981796) | NRG GY018 Trial [26] (NCT03914612) | DUO-E Trial [27] (NCT04269200) | AtTEnd Trial [28] (NCT03603184) | |||||||
| Experimental Arm | Control Arm | Experimental Arm | Control Arm | Experimental Arm | Control Arm | Experimental Arm 1 | Experimental Arm 2 | Control Arm | Experimental Arm | Control Arm | |
| Histology, n (%) | |||||||||||
| Endometrioid | 44 (70) | 46 (74) | 44 (83) § | 56 (86) § | 88 (78.5) § | 92 (81.4) § | 141 (59.2) | 152 (63.6) | 139 (57.7) | 74 (95) § | 38 (88) § |
| Serous | 10 (16) | 9 (15) | 1 (2) § | 1 (2) § | 4 (3.6) § | 1 (0.9) § | 58 (24.4) | 42 (17.6) | 54 (22.4) | 0 § | 0 § |
| Clear cells | 3 (5) | 1 (2) | 0 § | 0 § | 1 (0.9) § | 0 § | 4 (1.7) | 8 (3.3) | 7 (2.9) | 0 § | 0 § |
| Carcinosarcoma | 0 | 0 | 4 (8) § | 1 (2) § | Excluded | Excluded | 12 (5) | 18 (75) | 21 (8.7) | 3 (4) § | 1 (2) § |
| Undifferentiated | 4 (6) | 3 (5) | 0 § | 0 § | 4 (3.6) § | 4 (3.5) § | 4 (1.7) | 5 (2.1) | 3 (1.2) | 1 (1) § | 3 (7) § |
| Mucinous | 1 (2) | 0 | 0 § | 0 § | 0 § | 0 § | 1 (0.4) | 0 | 0 | 0 § | 0 § |
| Mixed | 1 (2) | 3 (5) | 2 (4) § | 4 (8) § | 3 (2.7) § | 2 (1.8) § | 9 (3.8) | 9 (3.8) | 11 (4.6) | 3 (4) § | 1 (2) § |
| Other | 0 | 0 | 2 (4) § | 3 (5) § | 12 (10.7) § | 14 (12.4) § | 9 (3.8%) | 5 (2.1) | 6 (2.5) | 0 § | 1 (2) § |
| FIGO stage at diagnosis (2009), n (%) | |||||||||||
| I-II | 20 (32) | 24 (39) | 21 (40) § | 27 (42) § | - | - | 0 | 1 (0.9) | 1 (0.4) | 39 (49) § | 18 (41) § |
| III | 23 (37) | 22 (35) | 14 (26) § | 20 (31) § | - | - | 17 (7.1) | 12 (5.0) | 12 (5.0) | 16 (20) § | 7 (16) § |
| IV | 20 (32) | 16 (26) | 14 (26) § | 15 (23) § | - | - | 96 (40.3) | 99 (41.4) | 101 (41.9) | 26 (32) § | 19 (43) § |
| Unknown | 0 | 0 | 4 (8) § | 3 (5) § | - | - | - | - | - | 0 § | 0 § |
| Status of the disease at study entry in MMRd pop, n (%) | |||||||||||
| New diagnosis FIGO stage III-IV | 30 (48) | 30 (48) | 26 (49) | 33 (51) | - | - | 113 (47.5) | 114 (47.7) | 115 (47.7) | 29 (36) | 16 (36) |
| Recurrence | 33 (52) | 32 (52) | 27 (51) | 32 (49) | - | - | 125 (52.5) | 125 (52.3) | 126 (52.3) | 52 (64) | 28 (64) |
| Population MMRd, n (%) | 31 | 26 | 53 (21.6) | 65 (26.1) | 112 (27.7) | 113 (27.7) | 46 (19.3) | 48 (20.1) | 49 (20.3) | 81 (22.5) | 44 (23) |
| Previous chemotherapy in MMRd pop, n (%) | - | - | 7 (13.2) | 10 (15.4) | 5 (4.5) | 8 (7.1) | - | - | - | 14 (17) | 11 (25) |
| Previous radiotherapy | 29 (46%) | 28 (45%) | 8 (15) | 13 (20) | 155 (38.3) | 174 (42.7) | 73 (30.7) | 85 (35.6) | 71 (29.5) | - | - |
| Mean follow-up, month | 23.3 | 23.5 | 24.8 | 24.8 | 12 | 12 | 15.4 | 15.4 | 12.6 | 28.3 | 28.3 |
| Objective response rate | 17% | 6% | 77.6% | 69% | 81.5% | 70.7% | - | - | - | 82.4% | 75.7% |
| Complete response rate | 41 (65%) | 31 (50%) | 53 (25.0) | 43 (19.6) | - | - | - | - | - | - | - |
| PFS in MMRd patients, months—median (95% CI) | 8.0 (4.8–12.3) | NE (8.9–NE) | NE (12.4–NE) | 6.9 (6.3–10.1) | NE (30.6–NE) | NE (30.6–NE) | NE (NE-NE) | 31.8 (12.4-NE) | 7.0( 6.7–14.8) | NE (12.4–NE) | 6.9 (6.3–10.1) |
| PFS in MMRd, HR (95% CI) | 0.46 (0.22–0.94) | 0.28 (0.16–0.50) | 0.30 (0.19–0.48) | 0.41 (0.21–0.75) *; 0.42 (0.22–0.80) **; 0.97 (0.49–1.98) *** | 0.36 (0.23–0.57) | ||||||
| OS in MMRd, months—median (95% CI) | NE (NE–NE) | 26.1 (13.0–NE) | 83.3 (66.8–92.0) | 58.7 (43.4–71.2) | - | - | - | - | - | NE (NE–NE) | 25.7(14.5–NE) |
| OS in MMRd, HR (95% CI) | 0.41 (0.14–1.18) | 0.30 (0.13–0.70) | - | - | 0.41 (0.22–0.76) | ||||||
| Adverse events in overall population (Clavien–Dindo classification), n (%) | 455 | 361 | 241 | 246 | 365 | 361 | 232 | 237 | 236 | 351 | 185 |
| Grade 1–2 | 350 | 289 | - | - | 144 | 187 | 103 | 77 | 103 | 113 (32) | 67 (36) |
| Grade 3 | 84 | 59 | - | - | 214 | 170 | 129 | 160 | 133 | 157 (44) | 86 (46) |
| Grade 4 | 19 | 13 | - | - | 70 (20) | 28 (15) | |||||
| Grade 5 | 2 | 0 | - | - | 7 | 4 | 11 (3) | 4 (2) | |||
| Trial Name (NCT ID) | Study Design and Status | Drugs | Population | Treatment | Primary Endpoint | Estimated Study Completion and Status |
|---|---|---|---|---|---|---|
| DOMENICA GINECO-EN105b/ENGOT-en13 [29] (NCT05201547) | RCT phase III | Dostarlimab (TSR-042) | First recurrent or primary advanced (stage III-IV) EC | Experimental arm: Dostarlimab 500 mg, every 3 weeks, four cycles and then 1000 mg every 6 weeks. Control arm: Carboplatin AUC 5 or 6 plus Paclitaxel 175 mg/m2, every 3 weeks, six cycles. | PFS | 10/2029 Recruiting |
| KEYNOTE-C93/ MK-3475-C93/ GOG-3064/ ENGOT-en15 [30] (NCT05173987) | RCT phase III | Pembrolizumab (MK3475) | Advanced or recurrent MMRd EC not previously treated with chemotherapy | Experimental arm: Pembrolizumab 400 mg i.v. every 6 weeks (q6w) for up to 18 cycles (up to approximately 2 years). Control arm: paclitaxel 175 mg/m2 and carboplatin AUC 5 or 6 every 3 weeks (q3w) for 6 cycles (up to approximately 4 months). | PFS, OS | 5/2027 Active, not recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, M.; Ferrari, J.; Vezzaro, T.; Masatti, L.; Tasca, G.; Maggino, T.; Tozzi, R.; Saccardi, C.; Noventa, M.; Spagnol, G. The Role of Immunotherapy in MMR-Deficient Endometrial Carcinoma: State of the Art and Future Perspectives. J. Clin. Med. 2024, 13, 7041. https://doi.org/10.3390/jcm13237041
Marchetti M, Ferrari J, Vezzaro T, Masatti L, Tasca G, Maggino T, Tozzi R, Saccardi C, Noventa M, Spagnol G. The Role of Immunotherapy in MMR-Deficient Endometrial Carcinoma: State of the Art and Future Perspectives. Journal of Clinical Medicine. 2024; 13(23):7041. https://doi.org/10.3390/jcm13237041
Chicago/Turabian StyleMarchetti, Matteo, Jacopo Ferrari, Tommaso Vezzaro, Laura Masatti, Giulia Tasca, Tiziano Maggino, Roberto Tozzi, Carlo Saccardi, Marco Noventa, and Giulia Spagnol. 2024. "The Role of Immunotherapy in MMR-Deficient Endometrial Carcinoma: State of the Art and Future Perspectives" Journal of Clinical Medicine 13, no. 23: 7041. https://doi.org/10.3390/jcm13237041
APA StyleMarchetti, M., Ferrari, J., Vezzaro, T., Masatti, L., Tasca, G., Maggino, T., Tozzi, R., Saccardi, C., Noventa, M., & Spagnol, G. (2024). The Role of Immunotherapy in MMR-Deficient Endometrial Carcinoma: State of the Art and Future Perspectives. Journal of Clinical Medicine, 13(23), 7041. https://doi.org/10.3390/jcm13237041






