Abstract
Background: Infective endocarditis (IE) is associated with significant neurological complications (NCs). The impact of neurological sequelae due to IE, however, is not well characterized. Thus, the aim of this systematic review and meta-analysis is to determine whether patients who experienced NCs from IE had worse outcomes compared to those without neurological complications. Methods: We conducted a systematic and comprehensive literature search of MEDLINE, Cochrane Library, Google Scholar, and BioMed Central (PROSPERO registration ID: CRD42024518651). Data on the primary outcome of all-cause mortality and the secondary outcome of surgical timing were extracted from 25 observational studies on patients with confirmed IE, both with and without NC. Results: In the pooled total of patients with IE, NCs were present in 23.7% (60.8% ischaemic stroke and 16.4% haemorrhagic stroke). All-cause mortality was significantly higher in patients with IE and NCs (OR 1.78, CI 1.47–2.17, p < 0.0001) compared to those without, particularly in those with major neurological events (OR 2.18, CI 1.53–3.10, p < 0.0001). Conversely, minor or asymptomatic strokes showed no significant correlation with mortality (OR 1.10, CI 0.82–1.47, p = 0.543). There was no significant difference in the timing of surgical intervention (standardized mean difference −0.53, CI −1.67 to 0.61, p = 0.359) between the two patient groups. Conclusions: Major NCs due to infective endocarditis were associated with a significantly increased all-cause mortality. This underscores the critical importance of early recognition and management strategies tailored to the severity of neurological events.
1. Introduction
Infective endocarditis (IE) is a serious condition which can carry high morbidity and mortality rates. There has been a steady increase in the prevalence of IE in recent years, due in part to the aging population, an increase in the rates of the implantation of devices, such as pacemakers and defibrillators, and prosthetic valves, and the increased use of indwelling intravenous catheters [1,2,3].
Neurological complications (NCs) can often complicate the course of IE and contribute significantly to the mortality and morbidity associated with IE [4]. These include embolic cerebrovascular complications, intracranial haemorrhage, ruptured mycotic aneurysms, transient ischaemic attacks, meningitis, encephalopathy, and brain abscess, with up to 40% reported to be symptomatic and up to 80% asymptomatic [5,6]. NCs are also one of the reasons for delayed cardiac surgical interventions and can represent a significant management challenge in a patient with IE [4]. Delaying surgical intervention in patients with IE can lead to clinical deterioration and potentially death. There is a paucity of high level evidence for surgery in the treatment of IE in the current guideline recommendations by the American College of Cardiology (ACC), American Heart Association (AHA), and European Society of Cardiology (ESC). However, current guidelines do support urgent surgical intervention in the case of uncontrolled infection, persistent sepsis, acute heart failure, or a high risk of embolic recurrence [7], especially in the case of ischaemic or haemorrhagic stroke with favourable characteristics. Otherwise, the recommendation is that surgery should be deferred for up to 4 weeks where possible [7].
The decision to proceed with surgical intervention can be complicated as the need for surgery has to frequently be balanced with the increased risk of a poorer outcome associated with the need for required anticoagulation to prevent thrombosis within the extracorporeal circuit used for cardiopulmonary bypass. In patients who have recently experienced a stroke, anticoagulation also poses a significantly increased risk of a haemorrhagic transformation of the ischaemic brain tissue, particularly since the inflammatory and septic milieu of IE also carries an added risk of potential lethal intracranial bleeding [8].
Finally, the long-term prognosis of the different neurological complications (NCs) in the context of infective endocarditis is not well characterised. While there is agreement among studies that major NCs carry a significant risk of mortality, there is a paucity of evidence with regards to minor or asymptomatic strokes due to the heterogeneity in reported patient outcomes and variability in the definitions and reporting of neurological events. Hence the aims of this review are to (i) evaluate the association between NCs in patients with IE and mortality, focusing particularly on those with minor or asymptomatic strokes, and (ii) to determine if the occurrence of NCs in patients with IE is associated with delay in the time to surgical intervention.
2. Materials and Methods
We performed a systematic review and meta-analysis according to the principles outlined in the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA), Quality of Reporting of Meta-analyses (QUOROM) statement, and recommendations from The Cochrane Collaboration and Meta-analysis of Observational Studies in Epidemiology (MOOSE) [9]. The study protocol of the present meta-analysis has been registered with Prospero (ID: CRD42024518651).
2.1. Search Strategy
Appropriate articles were identified following MESH strategy in MEDLINE, Cochrane Library, Google Scholar, and BioMed Central databases. The following terms were searched: “endocarditis” AND “neurological complication” AND “mortality”. The search strategy was carried out in February 2023 and updated in June 2024. Only full-text articles, published in English and in peer review journals were selected. Two expert cardiologists (RP and FM) independently reviewed databases looking for studies regarding outcomes of patients with IE and NCs. Disagreements were resolved through discussion and consensus. In the case of unresolved disagreement, a third reviewer (GC) intervened to reach consensus. The reference list of included studies and the reference lists of relevant reviews were also reviewed for additional studies which were missed in the initial search.
2.1.1. Inclusion Criteria
Studies with the following characteristics were included in the meta-analysis: (a) studies that compared adult patients (age of ≥18 years) with a confirmed diagnosis of IE with and without an NC; (b) with data on mortality in the case of NCs; (c) reporting data on the timing regarding the delay in cardiac surgery in the case of NCs; (d) reporting on the effect size of data regarding mortality as a hazard ratio (HR) or an odds ratio (OR) with 95% confidence interval (CI); or (e) the absolute number of events in each study group (patients with versus without NC).
2.1.2. Exclusion Criteria
Studies were excluded based on the following criteria: (a) they were only in abstract or poster form; (b) studies on children (aged < 18 years old); (c) reviews, meta-analyses, or editorials; (d) rationale and study protocols; (e) the full-text article was not available; (f) they did not compare patients with and without NCs; or (g) they had missing data.
2.2. Data Extraction
Three independent reviewers (FS, FF, and LZ) extracted data from the full texts and published appendixes. The following information was collected for every included study: the year of publication, journal, number of patients included, time of the enrolment, follow-up length, source for follow-up, age, sex, cardiovascular risk factors, history of drug affection, site affected by the endocarditic process (native valve, prosthetic valve, intra-cardiac device, and catheters), presence of endocarditis complications (abscess, fistula, severe valve disease, or heart failure), surgery performed, timing of surgery (defined also as early or not early), and blood culture that was positive for staphylococcus aureus or fungi.
The primary outcome of interest was all-cause mortality at the longest available follow-up in patients with NCs and a diagnosis of IE. The secondary outcome was the standardized mean difference in time to cardiac surgery among patients with and without NCs. A sub-analysis which examined the impact of major and minor NCs on mortality and time to surgery was also performed, where a minor NC was defined as a minor stroke with limited residual neurological deficit, transient ischaemic accident, or asymptomatic stroke and a major NC was defined as a stroke leading to significant neurological deficit and disability, territorial stroke, or haemorrhagic stroke. Table S2 (Supplementary Materials) reports the definitions used by each single study to determine major or minor NCs with the corresponding odds ratio.
2.3. Internal Validity and Quality Appraisal
The quality of the included studies was appraised by two unblinded reviewers (FS and FM) and tested using pre-specified electronic forms of MINORS criteria [10]. Twelve methodological items were given a score between 0 and 2. “Not reported (0 points)”, “Reported but inadequate (1 point)”, or “Reported and adequate (2 points)”. The maximum score achievable was 24 points. Among studies included in the meta-analysis, the minimum score was 14 and the maximum score was 21 (Supplementary Table S1).
2.4. Data Analysis and Synthesis
Values for continuous variables were expressed as a mean and standard deviation (SD). For the studies which reported values as a median and interquartile range, values were converted to mean (standard deviation) values to allow for meta-analysis using a previously published formula [11]. Categorical variables were expressed as absolute numbers and a percentage of the total pooled population.
Considering the high likelihood of between-study variance, we used a random effect model. Statistical heterogeneity was assessed using the Cochran’s Q test and I2 statistic with a value of I2 of 0 to 25% considered as insignificant heterogeneity, 26 to 50% low heterogeneity, 51 to 75% moderate heterogeneity, and >75% high heterogeneity. The mean value of the time to surgery for patients with neurological complication was also expressed in terms of the standardized mean. The impact of the severity of the neurological event (minor versus major) on the primary endpoint was tested with sub-group analysis, using the ANOVA test. Finally, random effect meta-regression analysis was performed to assess the effect of some potential confounding factors including device involvement, gender (male), prosthesis endocarditis, right heart valve endocarditis, positive blood culture for Staphylococcus aureus, positive blood culture for fungi, the presence of endocarditis anatomical complications, to be treated with cardiac surgery, aortic valve endocarditis, mitral valve endocarditis, intravenous drug users, early surgery, heart failure, and the mean age of the population. Meta-regression was considered for the variable with data sourced from at least 10 studies. Publication bias was appraised by a graphical valuation of funnel plots and through Egger’s linear regression test, and Duval and Tweedie trim and fill. Prometa software 3 (Internovi, Cesena, Itay) and RevMan 5 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) were the software used for statistical analyses.
3. Results
3.1. Search Results, Study Selection, and Patient Characteristics
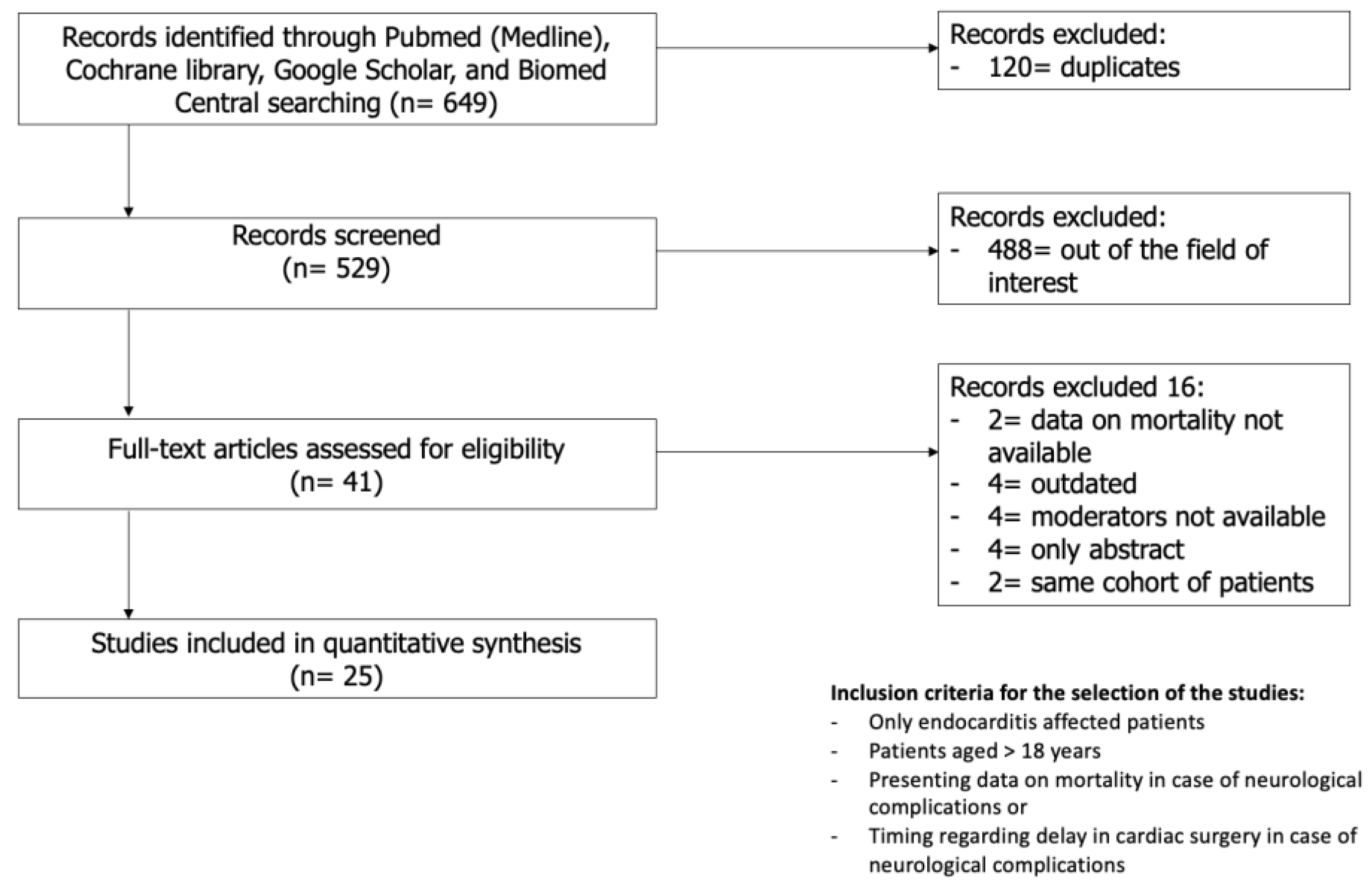
A total of 649 studies were identified in the initial search. After the removal of duplicates and screening of the title and abstract, 27 of the 41 full-text articles were included based on the inclusion and exclusion criteria. Of the 27 studies included, two pairs of studies (by Heiro et al. [12,13] and Ruttmann et al. [14,15]) were conducted on the same cohort spanning through different periods of time; hence, the studies from each pair with lesser patients were excluded [13,15]. The final 25 studies were quantitatively analysed [5,12,14,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] (Figure 1). Of note, the study of Okazaki et al. [26] was included only for the analyses of the secondary outcome. There was a pooled total of 14509 patients, of which 3439 had at least one NC (23.7%). The mean age of the population was 61.9 ± 9.9 years, with a prevalence of male patients accounting for 64% of the total number of patients. Baseline data and patient characteristics are reported in Table 1.
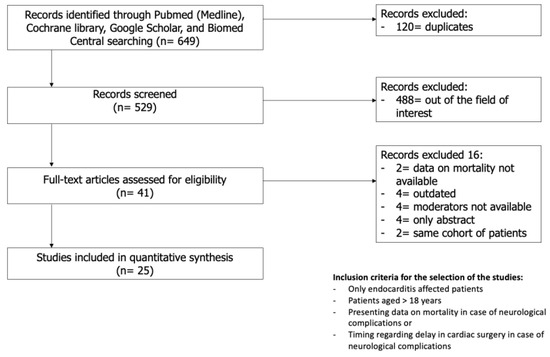
Figure 1.
Search strategy.

Table 1.
Studies included in the meta-analysis.
Cardiac surgery was performed in 68.5% (2004) of patients with NCs and in 65.8% (6592) of patients without an NC (p < 0.0001). The heart valve most commonly involved in patients with an NC was the mitral valve (47.2%), followed by the aortic valve (36.9%) and then the right heart valves (4.3%). Prosthetic valve endocarditis was present in 23.2% of NC patients. Abscess or local valvular complications (namely abscess, fistula, or new severe valve disease) were present in 26.2% of the patients with NCs. There was no significant difference in the rates of heart failure on admission to hospital in those with an NC vs. those without (32.5% vs. 33.4%; p = 0.590). Staphylococcus aureus was the most frequently identified organism. Interestingly, Staphylococcus aureus was also found to be more prevalent in patients with an NC vs. those without (31.4% of patients with NC vs. 21.7% of those without; p < 0.0001).
Twenty-one out of twenty-seven studies reported on the different types of NCs (Supplementary Materials Table S2). The majority of NCs were ischaemic strokes (60.8%; 2091/3439 events), followed by subclinical brain embolization or transient ischaemic attacks (4.1%; 140/3439 events), intracranial haemorrhages (16.4%; 564/3439 events), brain abscesses (1.7%; 60/3439 events), meningitis (5%; 171/3439 events), and mycotic aneurysm (0.7%; 25/3439 events). Some patients experienced two concurrent complications: 0.6% (19/3439) had an ischaemic stroke and brain abscess or meningitis, 1.4% (51/3439) had an ischaemic stroke and an intracranial haemorrhage, and 0.4% (15/3439) had a brain abscess and meningitis.
Twelve studies reported on mean surgical times defined as the time between the time of diagnosis of the onset of NCs and cardiac surgery [5,14,17,18,21,26,31,32,33,34,35,36]. In eight of these studies, the mean surgical waiting time of the control group was also reported (Table 2).

Table 2.
Studies reporting time between neurological complications and cardiac surgery.
3.2. Primary Endpoint
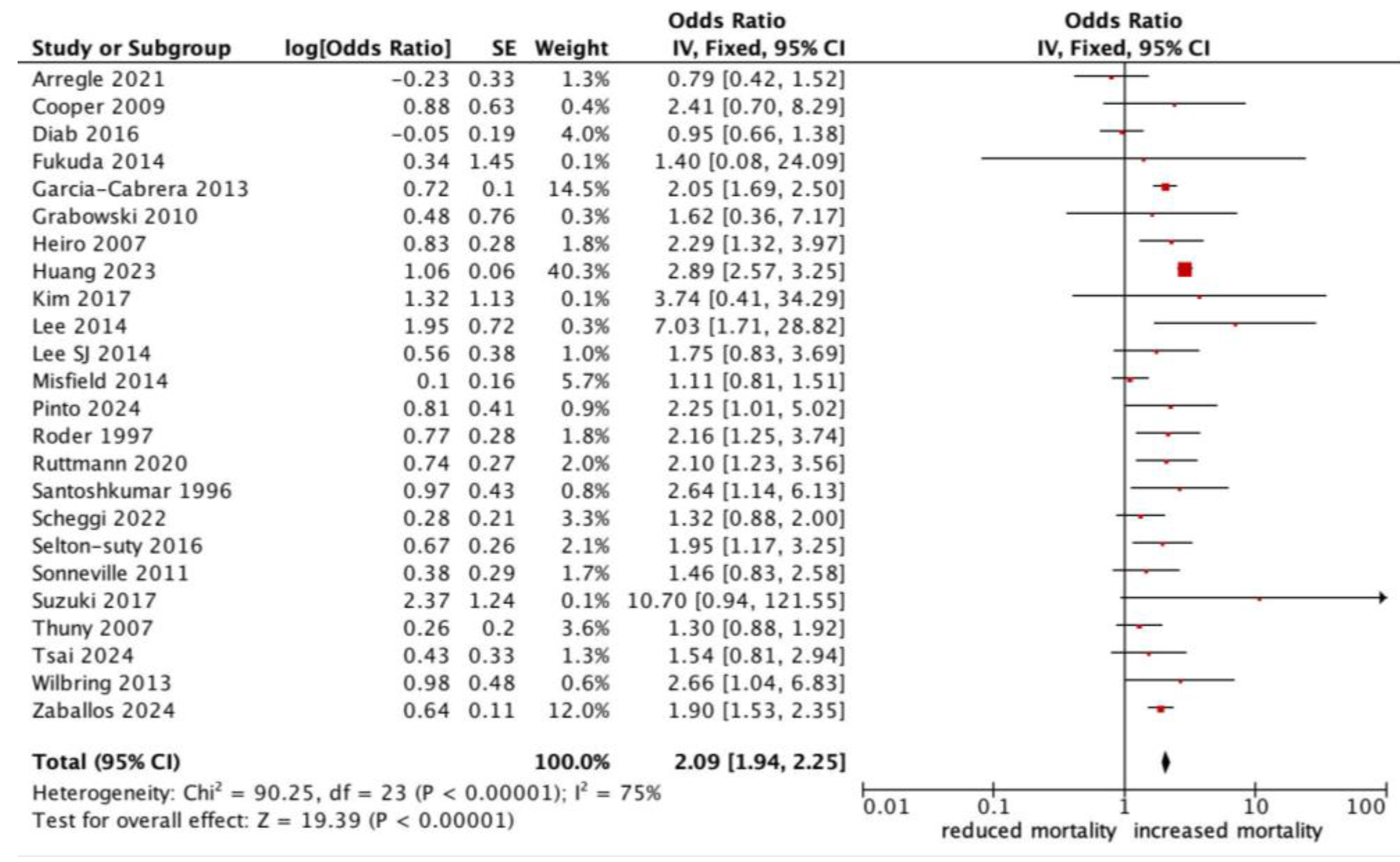
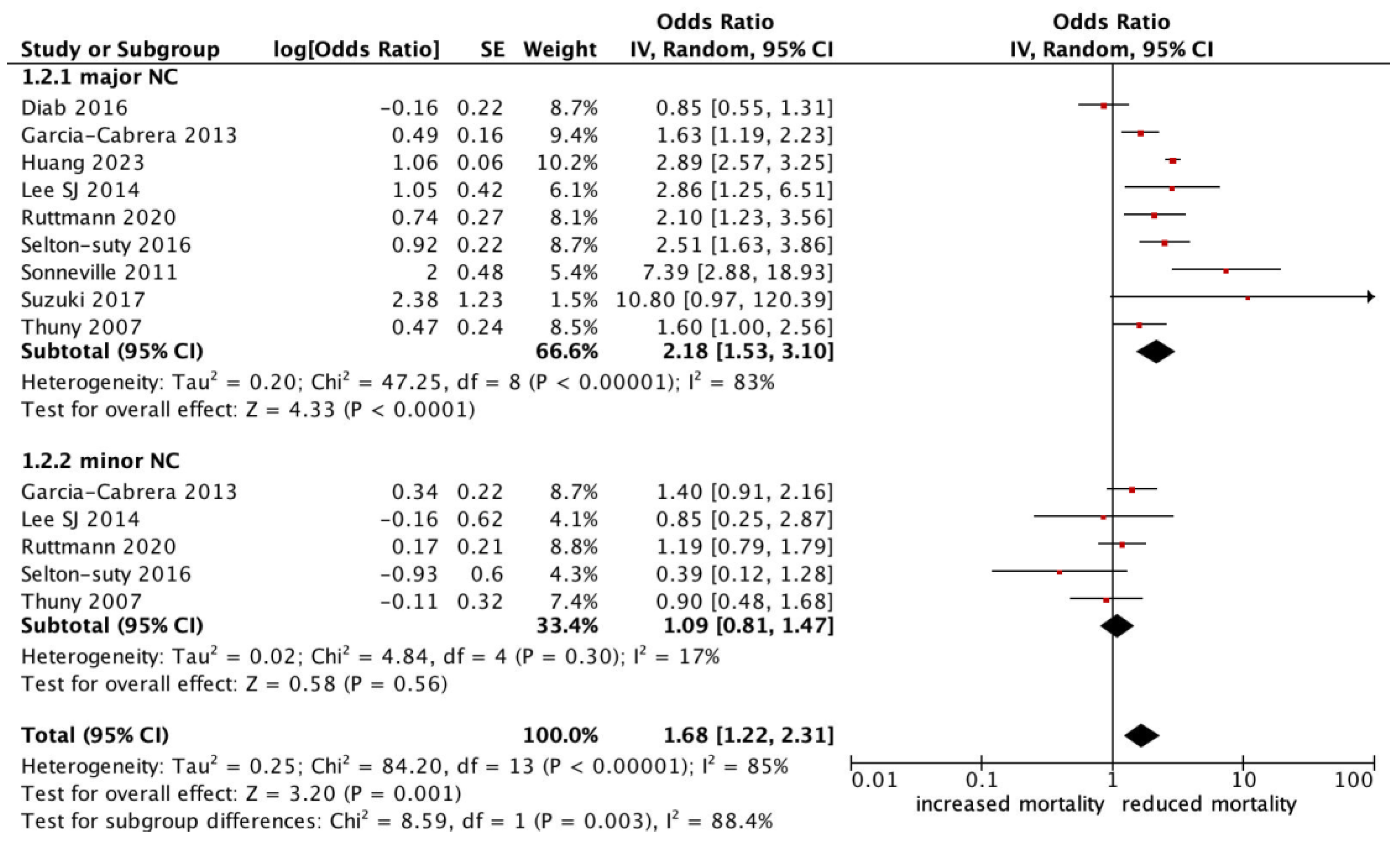
All-cause mortality was significantly higher in patients with infective endocarditis with an NC (OR 1.78, 95% CI 1.47–2.17, p < 0.0001, I2: 75%) (Figure 2). Those who experienced a major neurological event, defined as a territorial infarct, haemorrhagic stroke, or a stroke with impaired neurological status, had an even stronger correlation with all-cause mortality (OR 2.18, 95% CI 1.53–3.10, p < 0.0001, I2: 83%) (Figure 3), compared to a silent or minor stroke that was not correlated with all-cause mortality (OR 1.09, 95% CI 0.81–1.47, p = 0.56, I2: 17%). ANOVA testing revealed a significant difference in mortality among subgroups of minor versus major NCs (p = 0.003) (Figure 3).
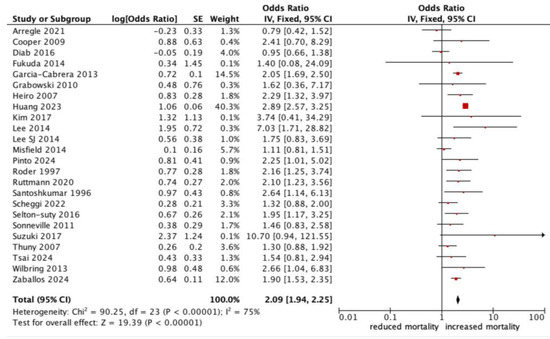
Figure 2.
Forest plot for the primary outcome: neurological complications and all-cause mortality.
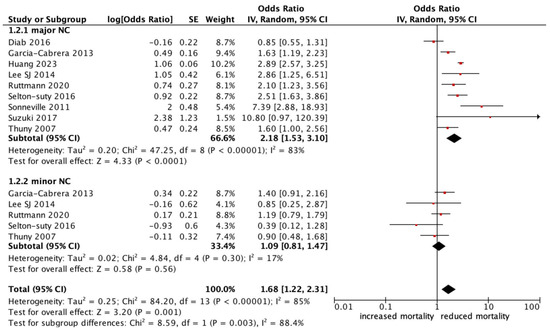
Figure 3.
Forest plot for the primary outcomes according to subgroup analysis of major vs. minor neurological complications.
Finally, random effect meta-regression demonstrated no significant interaction between the increased risk of all-cause mortality associated with NCs and the following variables: male sex (β = −0.01, p = 0.450), prosthesis involvement (β = −0.00, p = 0.854), right heart valve involvement (β = 0.02, p = 0.645), S. aureus positive blood cultures (β = −0.00, p = 0.675), the presence of abscess (β = 0.01, p = 0.629), having cardiac surgery performed (β = −0.01, p = 0.330), aortic valve involvement (β = −0.01, p = 0.073), mitral valve involvement (β = 0.01, p = 0.124), and heart failure at admission (β = 0.00, p = 0.937) (Supplementary Table S3). On the contrary, a significant interaction was found with mean age (β = −0.02, p = 0.003). Due to the low number of studies reporting data for those with fungus infection and intravenous drug use, meta-regression analyses were not performed for these patients (Supplementary Table S3).
3.3. Secondary Endpoint
The meta-analysis did not demonstrate a significant standardized mean difference (SMD) in the time interval to cardiac surgery between patients with and without NCs during infective endocarditis (−0.53, 95% CI interval −1.67 to −0.61, p value = 0.359). The non-standardized mean difference expressed in days was −1.86 (95% CI interval −6.44–2.72, p = 0.426).
3.4. Publication Bias of the Primary Endpoint
The publication bias analysis evaluated using Egger’s linear regression test was negative (p = 0.101). Trim and Fill analysis indicated the potential absence of six studies; however, even with the virtual addition of these studies, the estimated effect size remained significant (OR 1.59, 95% CI 1.24–2.04, p < 0.0001) (Supplementary Figure S1).
4. Discussion
NCs are the most common extracardiac complications of IE and include a heterogeneous group of conditions, the most common of which is ischaemic stroke, followed by haemorrhagic stroke, mycotic aneurysm, cerebral abscess, or encephalitis. A significant number of these NCs may occur sub-clinically or are asymptomatic [5].
The main findings of the present systematic review and meta-analysis are as follows:
- (i)
- A rate of NCs in patients with endocarditis of 24%;
- (ii)
- All-cause mortality was significantly higher in the patients with IE experiencing any NC vs. those without an NC (OR 1.78, 95% CI 1.47–2.17, p < 0.0001);
- (iii)
- Major stroke or haemorrhagic stroke were related to all-cause mortality, whereas minor strokes/TIA or asymptomatic strokes were not;
- (iv)
- The presence of an NC was not significantly associated with the time to surgery among patients with and without NCs.
The rates of NCs in patients with IE in our study are consistent with the rates previously reported [8,15], although the prevalence of asymptomatic complications or transient ischaemic attacks was significantly lower in our study (4.1%). This may be an underestimation of the true prevalence of minor NCs as no distinction was made between symptomatic and asymptomatic ischaemic strokes in a significant number of studies as in many studies the diagnosis of an NC was made clinically with no brain imaging performed (Table 2).
The high prevalence of mitral valve endocarditis observed in the meta-analysis is also consistent with the reported prevalence for mitral valve endocarditis, which identifies mitral valve endocarditis as a risk factor for stroke [38,39]. Similarly, we found that Staphylococcus aureus was not only the most frequently isolated pathogen in subjects with infective endocarditis but also more prevalent in those with NCs. Several studies have reported a significant correlation between Staphylococcus aureus endocarditis and NCs [40].
The significant association between NCs and all-cause mortality aligns with multiple studies in the literature, both retrospective and prospective, where NCs have emerged as independent risk factors for short- and medium-term all-cause mortality [13,19,41,42,43]. In the European registry EURO-ENDO by Habib et al. [41], a cerebral complication was reported to have a HR of 2.21 (95% CI 1.61–3.04, p < 0.0001). It is important to note that the study by Habib and colleagues aimed to provide predictors of in-hospital or short-term mortality related to IE rather than focusing solely on NCs.
On the other hand, our study is unique since we have focused only on studies reporting on patients with NCs, which allowed us to identify independent risk factors associated with NCs and mortality in these patients. Our meta-regression analysis identified age as the significant primary risk factor with mortality. This inverse association between age and mortality is likely to be explained by the selection bias characteristic of this type of study, where very elderly patients with multiple comorbidities are usually excluded as inferred by the mean age of our pooled cohort that was only 61.9 ± 9.9 years. The other key question relates to the relationship between all-cause mortality and the severity of the NC. The cohort that was the focus of our study comprises a heterogeneous group of clinical conditions classified predominantly as an ischaemic stroke, which included functionally debilitating major strokes to clinically mild/minor strokes, TIAs, completely asymptomatic strokes, and other secondary complications with distinct prognostic implications such as intraparenchymal haemorrhage or cerebral abscess, which can occur independently or exacerbate the severity of an ischaemic stroke. Major or haemorrhagic strokes have been documented to confer a worse prognosis [44] and are frequently the main reason for exclusion from surgery. In the study by Garcia-Cabrera [19], patients with NCs had a mortality rate almost doubled in comparison to those without NCs (45% vs. 24%), with a cerebral haemorrhage and/or moderate–severe ischaemic event found to be independently associated with mortality. Likewise, in this study, fewer patients with NCs underwent surgery compared to patients without NCs (32% vs. 41%, p < 0.01) with the reasons for exclusion from surgery attributed to deteriorating neurological conditions or an unacceptably high risk of a poor outcome.
However, the impact of minor or asymptomatic strokes on the overall prognosis remains unclear. The definition of major versus minor stroke varied across studies (Table 2 and Table S2). Our meta-analysis did not demonstrate any significant association between minor strokes/TIA or asymptomatic strokes and all-cause mortality, while major and haemorrhagic stroke were highly associated with an increased risk of all-cause mortality. This finding was consistent with the results reported in the study by Lee and colleagues [24], where major cerebrovascular events were associated with a high risk of mortality although total cerebrovascular events were not significantly related to the risk of death. In contrast, the study by Misfeld and colleagues [25], which was a surgical series of consecutive patients with endocarditis and neurological complications, found that long-term survival after surgery was not influenced by pre-operative symptomatology, but by the presence of cerebral embolism as a whole. In the study, both patients with silent or symptomatic cerebral embolism had an equally reduced long-term mortality [25].
From the published studies that we had included in our systematic review, we identified that the main surgery contraindications were haemorrhagic stroke, poor functional capacity after ischaemic stroke, or major prior comorbidities.
In these instances, excluding them from surgery effectively denies them a potentially curative therapy, resulting in the progression of infections, a worsening of local complications, and a higher risk of systemic embolisms. In the study by Arregle et al. [16], 94 out of 351 patients with IE had NCs. Even though 6-month mortality rates did not differ between the two groups, the 40 patients in which cardiac surgery was postponed due to surgical contraindication had the worst prognosis (death, new neurological event, or cardiac or septic deterioration). Among predictive factors of death in patients presenting with NCs, there was temporary surgical neurological contraindication. In the study by Thuny et al. [34], 59% of patients treated with medical therapy had a theoretical indication for surgery, whereby almost 50% died at the 1-year follow-up mark. However, when compared with patients who underwent surgery, those who had an indication for surgery but did not have surgery had more comorbidities (p = 0.007) and a lower Glasgow Coma Scale (14.6 ± 1 vs. 12 ± 4, p = 0.0001) when compared to those who underwent surgery.
Nonetheless, stroke remains a reason for delaying surgery in clinical practice, although not an absolute contraindication unless the neurological prognosis is very poor or in the case of a recent cerebral haemorrhage [6]. In our study, mean surgical waiting times varied greatly between the included studies. Most recent studies and meta-analyses in patients with IE have focused on determining whether early surgery is feasible and safe in cases of ischaemic stroke and on identifying the most appropriate waiting times for haemorrhagic stroke [40,44,45]. In our study, we showed that there was no significant difference in the mean time to surgery in patients with infective endocarditis complicated by an NC compared to those without NCs. However, several factors have to be noted: (i) Although our analysis included all NCs, a selection bias is inherent in the retrospective nature of most of the studies we considered. Patients excluded from surgery, namely the most critically ill or those with worse complications, were not likely to have been included in the analysis. (ii) Ischaemic stroke, particularly according to recent evidence, is also a factor that favours early surgery, as these patients are at an increased risk of systemic embolism and early mortality. (iii) Some of the studies considered [14,35] were specifically designed to demonstrate the safety of early surgery in these patients. Given the limited number of cases available for analysis, new studies focusing on this specific question are clearly still needed.
Finally, although cardiac surgery can be lifesaving, it also carries significant risks, which are mainly related to the need for high-dose systemic anticoagulation during cardiopulmonary bypass, which carries the risks of further bleeding, particularly in patients with intracranial haemorrhage, and potential further neurologic deterioration in those with an ischaemic stroke [44,45,46]. In our study, it is worth noting that the mean peri-operative mortality in patients with NCs undergoing cardiac surgery was 17.1% in the studies we evaluated. In one study [14], early surgery seemed safe, without a worsening of neurological symptoms in patients with ischaemic stroke. However, there was a trend towards higher perioperative mortality in patients with complicated neurological injuries (nine patients, 21.4%) compared to patients with uncomplicated ischaemic lesions (six patients, 6.5%; p = 0.063).
Study Limitations
This meta-analysis has intrinsic limitations inherent to study-level meta-analyses of observational series. One major limitation is the risk of methodological heterogeneity among the included studies, which can arise from differences in study design, patient populations, diagnostic criteria, treatment protocols, and regarding the outcomes and analysis performed in particular, and as already pointed out, a great source of heterogeneity is present in the definition of major versus minor NCs as well as in mean surgical waiting times across the studies included. Additionally, treatment allocation bias or selection bias is likely present, particularly when comparing patients with different operative risks. Patients with NCs who underwent surgery (particularly early surgery) often had stronger surgical indications, representing a higher-risk population. Survivor bias is another concern. Patients who survived long enough to receive surgery might inherently have had better prognoses, which could have influenced the observed outcomes. The retrospective nature of many included studies may have led to incomplete data collection and reporting, further complicating the analysis. This is particularly relevant when assessing the primary outcome of mortality and the secondary outcome of waiting times for surgery. The variability in reporting NCs and the lack of standardized definitions across studies also pose challenges, as these factors can introduce inconsistencies and reduce the comparability of the included studies. Since no study included in the meta-analysis was specifically designed to evaluate the impact of NCs in patients with IE in TAVI, it was not possible to perform subgroup analyses regarding this specific population that is now rapidly growing due to population aging. Furthermore, the analysis may be limited by the lack of individual patient data, which restricts the ability to perform more granular subgroup analyses and adjust for patient-level covariates. Future research should aim to address these limitations by incorporating individual patient data and employing more rigorous and standardized methodologies to enhance the validity and reliability of the findings.
5. Conclusions
Our meta-analysis specifically investigated the impact of neurological complications on the outcomes of patients with IE, with a primary focus on all-cause mortality and a secondary focus on the impact of the NC on the timing to cardiac surgery. Neurological complications during IE were significantly associated with increased all-cause mortality. This association was especially pronounced for major NCs, such as ischaemic stroke with severe functional impairment and haemorrhagic stroke, while minor or asymptomatic strokes did not show a significant association with mortality. Regarding the timing of surgery, we did not find a statistically significant delay for patients with NCs compared to those without.
Prospective studies designed to evaluate the optimal timing of surgery for these patients, considering the severity and type of neurological event and incorporating standardized definitions and imaging protocols, are still needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13237053/s1, Table S1: MINORS criteria for the selected studies included in the meta-analysis; Table S2: Subtypes of neurological complications and their relation to the primary outcome; Table S3: Meta-regression analysis on the primary outcome; Figure S1: Funnel plot.
Author Contributions
R.P., T.C.T. and C.S.: design, conception and interpretation of the data. F.S., F.F. and L.Z.: data collection, analysis and interpretation of data. F.S., F.M. and G.C.: drafting of the manuscript and revising it critically for important intellectual content. G.C., R.P., T.C.T. and C.S.: drafting of the manuscript and revising it critically for important intellectual content, data collection, verification of data. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the fact that this is a meta-analysis of data already published.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Talha, K.M.; Baddour, L.M.; Thornhill, M.H.; Arshad, V.; Tariq, W.; Tleyjeh, I.M.; Scott, C.G.; Hyun, M.C.; Bailey, K.R.; Anavekar, N.S.; et al. Escalating incidence of infective endocarditis in Europe in the 21st century. Open Heart 2021, 8, e001846. [Google Scholar] [CrossRef] [PubMed]
- Pavasini, R.; Sinning, C.; Campo, G.; Tan, T.C. ObsErvatioNal prospective multicenter stuDy tO characterize the cLinical ANd DiagnoStiC feAtures of endocarditis in the contemPorary Era (ENDO-LANDSCAPE study): Rationale and design. J. Cardiovasc. Med. 2023, 24, 354–360. [Google Scholar] [CrossRef]
- Momtazmanesh, S.; Saeedi Moghaddam, S.; Malakan Rad, E.; Azadnajafabad, S.; Ebrahimi, N.; Mohammadi, E.; Rouhifard, M.; Rezaei, N.; Masinaei, M.; Rezaei, N.; et al. Global, regional, and national burden and quality of care index of endocarditis: The global burden of disease study 1990–2019. Eur. J. Prev. Cardiol. 2022, 29, 1287–1297. [Google Scholar] [CrossRef]
- Pizzino, F.; Paradossi, U.; Trimarchi, G.; Benedetti, G.; Marchi, F.; Chiappino, S.; Conti, M.; Di Bella, G.; Murzi, M.; Di Sibio, S.; et al. Clinical Features and Patient Outcomes in Infective Endocarditis with Surgical Indication: A Single-Centre Experience. J. Cardiovasc. Dev. Dis. 2024, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.A.; Thompson, E.C.; Laureno, R.; Fuisz, A.; Mark, A.S.; Lin, M.; Goldstein, S.A. Subclinical brain embolization in left-sided infective endocarditis: Results from the evaluation by MRI of the brains of patients with left-sided intracardiac solid masses (EMBOLISM) pilot study. Circulation 2009, 120, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Hess, A.; Klein, I.; Iung, B.; Lavallée, P.; Ilic-Habensus, E.; Dornic, Q.; Arnoult, F.; Mimoun, L.; Wolff, M.; Duval, X.; et al. Brain MRI findings in neurologically asymptomatic patients with infective endocarditis. AJNR Am. J. Neuroradiol. 2013, 34, 1579–1584. [Google Scholar] [CrossRef]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doesn’t, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef]
- Tam, D.Y.; Yanagawa, B.; Verma, S.; Ruel, M.; Fremes, S.E.; Mazine, A.; Adams, S.; Friedrich, J.O. Early vs Late Surgery for Patients with Endocarditis and Neurological Injury: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2018, 34, 1185–1199. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Heiro, M.; Helenius, H.; Hurme, S.; Savunen, T.; Engblom, E.; Nikoskelainen, J.; Kotilainen, P. Short-term and one-year outcome of infective endocarditis in adult patients treated in a Finnish teaching hospital during 1980–2004. BMC Infect. Dis. 2007, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Heiro, M.; Nikoskelainen, J.; Engblom, E.; Kotilainen, E.; Marttila, R.; Kotilainen, P. Neurologic manifestations of infective endocarditis: A 17-year experience in a teaching hospital in Finland. Arch. Intern. Med. 2000, 160, 2781–2787. [Google Scholar] [CrossRef]
- Ruttmann, E.; Abfalterer, H.; Wagner, J.; Grimm, M.; Müller, L.; Bates, K.; Ulmer, H.; Bonaros, N. Endocarditis-related stroke is not a contraindication for early cardiac surgery: An investigation among 440 patients with left-sided endocarditis. Eur. J. Cardiothorac. Surg. 2020, 58, 1161–1167. [Google Scholar] [CrossRef]
- Ruttmann, E.; Willeit, J.; Ulmer, H.; Chevtchik, O.; Hofer, D.; Poewe, W.; Laufer, G.; Muller, L.C. Neurological outcome of septic cardioembolic stroke after infective endocarditis. Stroke 2006, 37, 2094–2099. [Google Scholar] [CrossRef]
- Arregle, F.; Martel, H.; Philip, M.; Gouriet, F.; Casalta, J.P.; Riberi, A.; Torras, O.; Casalta, A.-C.; Camoin-Jau, L.; Lavagna, F.; et al. Infective endocarditis with neurological complications: Delaying cardiac surgery is associated with worse outcome. Arch. Cardiovasc. Dis. 2021, 114, 527–536. [Google Scholar] [CrossRef]
- Diab, M.; Guenther, A.; Sponholz, C.; Lehmann, T.; Faerber, G.; Matz, A.; Franz, M.; Witte, O.W.; Pletz, M.W. Pre-operative stroke and neurological disability do not independently affect short- and long-term mortality in infective endocarditis patients. Clin. Res. Cardiol. 2016, 105, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, W.; Daitoku, K.; Minakawa, M.; Fukui, K.; Suzuki, Y.; Fukuda, I. Management of infective endocarditis with cerebral complications. Ann. Thorac. Cardiovasc. Surg. 2014, 20, 229–236. [Google Scholar] [CrossRef]
- García-Cabrera, E.; Fernández-Hidalgo, N.; Almirante, B.; Ivanova-Georgieva, R.; Noureddine, M.; Plata, A.; Lomas, J.M.; Gálvez-Acebal, J.; Hidalgo-Tenorio, C.; Ruíz-Morales, J.; et al. Neurological complications of infective endocarditis: Risk factors, outcome, and impact of cardiac surgery: A multicenter observational study. Circulation 2013, 127, 2272–2284. [Google Scholar] [CrossRef]
- Grabowski, M.; Hryniewiecki, T.; Janas, J.; Stępińska, J. Clinically overt and silent cerebral embolism in the course of infective endocarditis. J. Neurol. 2011, 258, 1133–1139. [Google Scholar] [CrossRef]
- Huang, J.B.; Lu, C.C.; Wen, Z.K.; Yang, J.R.; Li, J.J. Surgical treatment of left-sided infective endocarditis with symptomatic neurological complications before surgery in China. Front. Cardiovasc. Med. 2023, 10, 1217148. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kim, H.W.; Jo, K.H. Neurologic outcomes after early surgery for infective endocarditis in patients with combined cerebral septic embolism. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jeon, D.; Cho, W.H.; Kim, Y.S. The clinical impacts of apparent embolic event and the predictors of in-hospital mortality in patients with infective endocarditis. J. Korean Med. Sci. 2014, 29, 1646–1650. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Oh, S.S.; Lim, D.S.; Na, C.Y.; Kim, J.H. Clinical significance of cerebrovascular complications in patients with acute infective endocarditis: A retrospective analysis of a 12-year single-center experience. BMC Neurol. 2014, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Misfeld, M.; Girrbach, F.; Etz, C.D.; Binner, C.; Aspern, K.V.; Dohmen, P.M.; Davierwala, P.; Pfannmueller, B.; Borger, M.A.; Mohr, F.-W. Surgery for infective endocarditis complicated by cerebral embolism: A consecutive series of 375 patients. J. Thorac. Cardiovasc. Surg. 2014, 147, 1837–1844. [Google Scholar] [CrossRef]
- Okazaki, S.; Yoshioka, D.; Sakaguchi, M.; Sawa, Y.; Mochizuki, H.; Kitagawa, K. Acute ischemic brain lesions in infective endocarditis: Incidence, related factors, and postoperative outcome. Cerebrovasc. Dis. 2013, 35, 155–162. [Google Scholar] [CrossRef]
- Pinto, P.H.O.M.; Fae, I.G.; Oliveira, G.B.; Duque, R.A.S.; Oliveira, M.V.M.; Barbalho, L.S.M.; Parreiras, A.O.; Gelape, F.A.; Cambraia, F.S.L.; Costa, G.L.; et al. Impact of Neurological Complications on Long-Term Outcomes in Patients with Infective Endocarditis. Trop Med. Infect. Dis. 2024, 9, 132. [Google Scholar] [CrossRef]
- Røder, B.L.; Wandall, D.A.; Espersen, F.; Frimodt-Møller, N.; Skinhøj, P.; Rosdahl, V.T. Neurologic manifestations in Staphylococcus aureus endocarditis: A review of 260 bacteremic cases in nondrug addicts. Am. J. Med. 1997, 102, 379–386. [Google Scholar] [CrossRef]
- Santoshkumar, B.; Radhakrishnan, K.; Balakrishnan, K.G.; Sarma, P.S. Neurologic complications of infective endocarditis observed in a south Indian referral hospital. J. Neurol. Sci. 1996, 137, 139–144. [Google Scholar] [CrossRef]
- Scheggi, V.; Menale, S.; Tonietti, B.; Bigiarini, C.; Giovacchini, J.; Del Pace, S.; Zoppetti, N.; Alterini, B.; Stefàno, P.L.; Marchionni, N. Impact of septic cerebral embolism on prognosis and therapeutic strategies of infective endocarditis: A retrospective study in a surgical centre. BMC Infect. Dis. 2022, 22, 554. [Google Scholar] [CrossRef]
- Selton-Suty, C.; Delahaye, F.; Tattevin, P.; Federspiel, C.; Le Moing, V.; Chirouze, C.; Nazeyrollas, P.; Vernet-Garnier, V.; Bernard, Y.; Chocron, S.; et al. Symptomatic and Asymptomatic Neurological Complications of Infective Endocarditis: Impact on Surgical Management and Prognosis. PLoS ONE. 2016, 11, e0158522. [Google Scholar] [CrossRef] [PubMed]
- Sonneville, R.; Mirabel, M.; Hajage, D.; Tubach, F.; Vignon, P.; Perez, P.; Lavoue, S.; Kouatchet, A.; Pajot, O.; Dessap, A.M.; et al. Neurologic complications and outcomes of infective endocarditis in critically ill patients: The ENDOcardite en REAnimation prospective multicenter study. Crit. Care Med. 2011, 39, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Takanashi, S.; Ohshima, Y.; Nagatomo, Y.; Seki, A.; Takamisawa, I.; Tobaru, T.; Naito, K.; Kin, H.; Umemura, J.; et al. Critical potential of early cardiac surgery for infective endocarditis with cardio-embolic strokes. Int. J. Cardiol. 2017, 227, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; Avierinos, J.F.; Tribouilloy, C.; Giorgi, R.; Casalta, J.P.; Milandre, L.; Brahim, A.; Nadji, G.; Riberi, A.; Collart, F.; et al. Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis: A prospective multicentre study. Eur. Heart J. 2007, 28, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- MD, S.-S.T.; Wu, V.C.-C.; Chan, Y.-H.; Chen, D.-Y.; Cheng, Y.-T.; Hung, K.-C.; Hsiao, F.-C.; Tung, Y.-C.; Lin, C.-P.; Chu, P.-H.; et al. Early Surgery for Infective Endocarditis Complicated with Neurologic Injury. J. Cardiothorac. Vasc. Anesth. 2024, 38, 1161–1168. [Google Scholar] [CrossRef]
- Wilbring, M.; Irmscher, L.; Alexiou, K.; Matschke, K.; Tugtekin, S.M. The impact of preoperative neurological events in patients suffering from native infective valve endocarditis. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 740–747. [Google Scholar] [CrossRef]
- Álvarez-Zaballos, S.; Vázquez-Alen, P.; Muñoz, P.; de Alarcón, A.; Carretero, E.G.; Álvarez-Uría, A.; Fariñas, C.; Rodríguez-García, R.; Goenaga, M.Á.; Cuervo, G.; et al. Prevalence and prognostic impact of stroke in a national cohort of infective endocarditis. Int. J. Stroke. 2024. [Google Scholar] [CrossRef]
- Das, A.S.; McKeown, M.; Jordan, S.A.; Li, K.; Regenhardt, R.W.; Feske, S.K. Neurological Complications and Clinical Outcomes of Infective Endocarditis. J. Stroke Cerebrovasc. Dis. 2022, 31, 106626. [Google Scholar] [CrossRef]
- Yanagawa, B.; Pettersson, G.B.; Habib, G.; Ruel, M.; Saposnik, G.; Latter, D.A.; Verma, S. Surgical Management of Infective Endocarditis Complicated by Embolic Stroke: Practical Recommendations for Clinicians. Circulation 2016, 134, 1280–1292. [Google Scholar] [CrossRef]
- Sambola, A.; Lozano-Torres, J.; Boersma, E.; Olmos, C.; Ternacle, J.; Calvo, F.; Tribouilloy, C.; Reskovic-Luksic, V.; Separovic-Hanzevacki, J.; Park, S.-W.; et al. Predictors of embolism and death in left-sided infective endocarditis: The European Society of Cardiology EURObservational Research Programme European Infective Endocarditis registry. Eur. Heart J. 2023, 44, 4566–4575. [Google Scholar] [CrossRef]
- Habib, G.; Erba, P.A.; Iung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A.; et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: A prospective cohort study. Eur. Heart J. 2019, 40, 3222–3232. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Wu, V.C.-C.; Chang, C.-H.; Chen, C.-T.; Hsieh, P.-C.; Liu, Z.-H.; Wong, H.-F.; Yang, C.-H.; Chou, A.-H.; Chu, P.-H.; et al. Long-term Outcome of Neurological Complications after Infective Endocarditis. Sci. Rep. 2020, 10, 3994. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R.; Jenks, J.D.; Baddley, J.W.; Lewis, J.S.; Egger, M.; Schwartz, I.S.; Boyer, J.; Patterson, T.F.; Chen, S.C.-A.; Pappas, P.G.; et al. Fungal Endocarditis: Pathophysiology, Epidemiology, Clinical Presentation, Diagnosis, and Management. Clin. Microbiol. Rev. 2023, 36, e0001923. [Google Scholar] [CrossRef] [PubMed]
- Musleh, R.; Schlattmann, P.; Caldonazo, T.; Kirov, H.; Witte, O.W.; Doenst, T.; Günther, A.; Diab, M. Surgical Timing in Patients with Infective Endocarditis and With Intracranial Hemorrhage: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2022, 11, e024401. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Goto, T. Midterm outcomes of early versus late surgery for infective endocarditis with neurologic complications: A meta-analysis. J. Cardiothorac. Surg. 2021, 16, 49. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).