The Role of the Endometrial Microbiota in Endometrial Cancer: A Systematic Review of the Literature
Abstract
1. Introduction
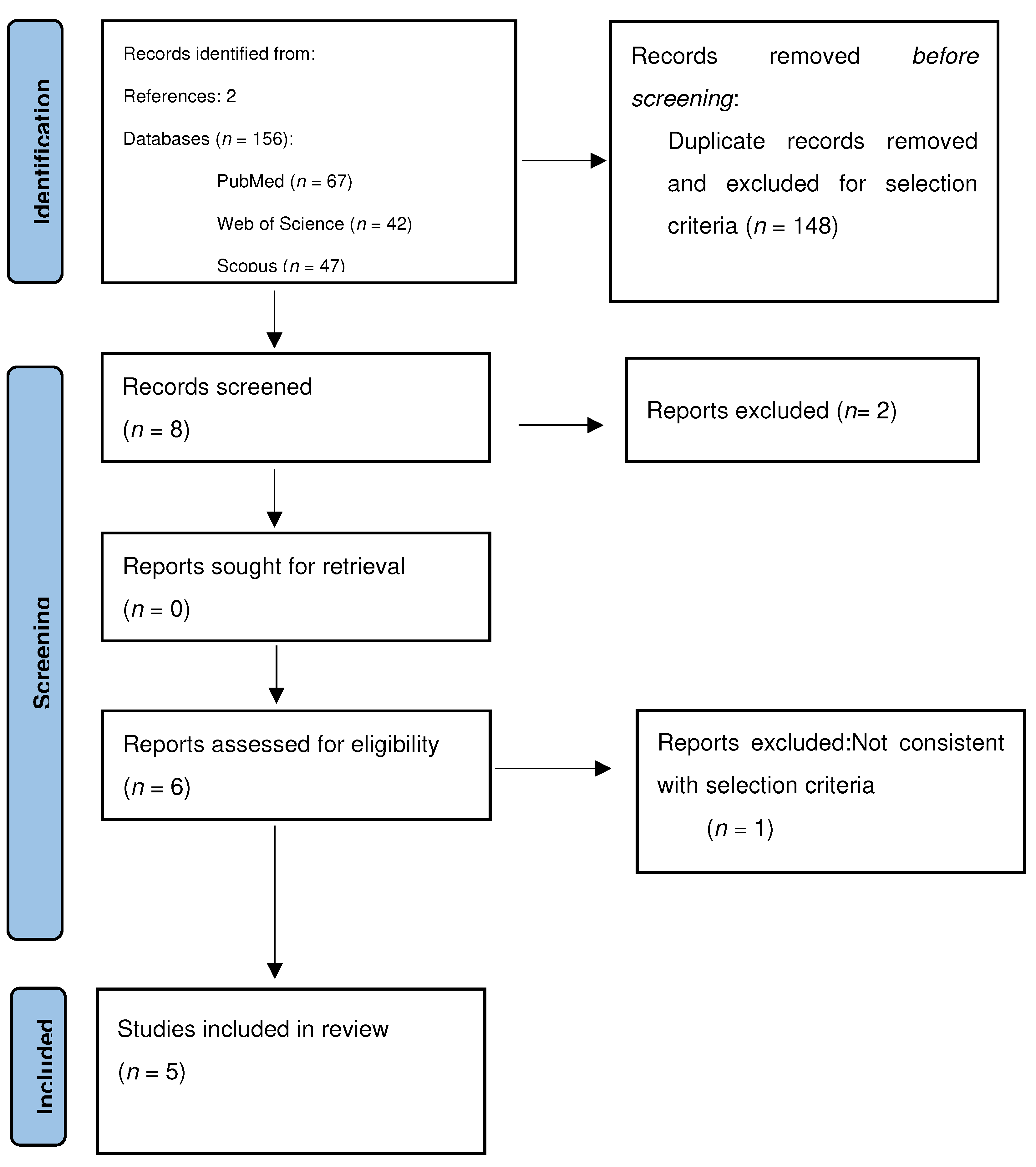
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Guidelines Aiom Neoplasms of the Uterus: Endometrium and Cervix Ed. 2022 Updated to September 2022. Available online: https://www.aiom.it/linee-guida-aiom-2022-neoplasie-dellutero-endometrio-e-cervice/ (accessed on 14 January 2024).
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef]
- Esposito, G.; Bravi, F.; Serraino, D.; Parazzini, F.; Crispo, A.; Augustin, L.S.A.; Negri, E.; La Vecchia, C.; Turati, F. Diabetes Risk Reduction Diet and Endometrial Cancer Risk. Nutrients 2021, 13, 2630. [Google Scholar] [CrossRef]
- Katagiri, R.; Iwasaki, M.; Abe, S.K.; Islam, R.; Rahman, S.; Saito, E.; Merritt, M.A.; Choi, J.-Y.; Shin, A.; Sawada, N.; et al. Reproductive Factors and Endometrial Cancer Risk Among Women. JAMA Netw. Open 2023, 6, e2332296. [Google Scholar] [CrossRef]
- Lee, M.; Piao, J.; Jeon, M.J. Risk Factors Associated with Endometrial Pathology in Premenopausal Breast Cancer Patients Treated with Tamoxifen. Yonsei Med. J. 2020, 61, 317. [Google Scholar] [CrossRef]
- Morańska, K.; Englert-Golon, M.; Durda-Masny, M.; Sajdak, S.; Grabowska, M.; Szwed, A. Why Does Your Uterus Become Malignant? The Impact of the Microbiome on Endometrial Carcinogenesis. Life 2023, 13, 2269. [Google Scholar] [CrossRef]
- Giudice, L.C. Challenging dogma: The endometrium has a microbiome with functional consequences! Am. J. Obstet. Gynecol. 2016, 215, 682–683. [Google Scholar] [CrossRef][Green Version]
- Ventolini, G.; Vieira-Baptista, P.; De Seta, F.; Verstraelen, H.; Lonnee-Hoffmann, R.; Lev-Sagie, A. The Vaginal Microbiome: IV. The Role of Vaginal Microbiome in Reproduction and in Gynecologic Cancers. J. Low. Genit. Tract. Dis. 2022, 26, 93–98. [Google Scholar] [CrossRef]
- Barczyński, B.; Frąszczak, K.; Grywalska, E.; Kotarski, J.; Korona-Głowniak, I. Vaginal and Cervical Microbiota Composition in Patients with Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 8266. [Google Scholar] [CrossRef]
- Istrate-Ofiţeru, A.M.; Mogoantă, C.A.; Zorilă, G.L.; Roşu, G.C.; Drăguşin, R.C.; Berbecaru, E.I.; Zorilă, M.V.; Comănescu, C.M.; Mogoantă, S.Ș.; Vaduva, C.C.; et al. Clinical Characteristics and Local Histopathological Modulators of Endometriosis and Its Progression. Int. J. Mol. Sci. 2024, 25, 1789. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Walther-António, M.R.S.; Chen, J.; Multinu, F.; Hokenstad, A.; Distad, T.J.; Cheek, E.H.; Keeney, G.L.; Creedon, D.J.; Nelson, H.; Mariani, A.; et al. Potential Contribution of the Uterine Microbiome in the Development of Endometrial Cancer. Genome Med. 2016, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; He, F.; Lin, Z.; Liu, S.; Tang, L.; Huang, Y.; Hu, Z. Dysbiosis of the endometrial microbiota and its association with inflammatory cytokines in endometrial cancer. Int. J. Cancer 2021, 148, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Gressel, G.M.; Usyk, M.; Frimer, M.; Kuo, D.Y.S.; Burk, R.D. Characterization of the endometrial, cervicovaginal and anorectal microbiota in post-menopausal women with endometrioid and serous endometrial cancers. PLoS ONE 2021, 16, e0259188. [Google Scholar] [CrossRef]
- Hawkins, G.M.; Burkett, W.C.; McCoy, A.N.; Nichols, H.B.; Olshan, A.F.; Broaddus, R.; Merker, J.D.; Weissman, B.; Brewster, W.R.; Roach, J.; et al. Differences in the microbial profiles of early stage endometrial cancers between Black and White women. Gynecol. Oncol. 2022, 165, 248–256. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Su, H.; Shi, L.; Chen, B.; Zhang, S. Endometrial microbiota from endometrial cancer and paired pericancer tissues in postmenopausal women: Differences and clinical relevance. Menopause 2022, 29, 1168–1175. [Google Scholar] [CrossRef]
- Moreno, I.; Cicinelli, E.; Garcia-Grau, I.; Gonzalez-Monfort, M.; Bau, D.; Vilella, F.; De Ziegler, D.; Resta, L.; Valbuena, D.; Simon, C. The diagnosis of chronic endometritis in infertile asymptomatic women: A comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am. J. Obstet. Gynecol. 2018, 218, e1–e602. [Google Scholar] [CrossRef]
- Benner, M.; Ferwerda, G.; Joosten, I.; van der Molen, R.G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Update 2018, 24, 393–415. [Google Scholar] [CrossRef]
- Peric, A.; Weiss, J.; Vulliemoz, N.; Baud, D.; Stojanov, M. Bacterial Colonization of the Female Upper Genital Tract. Int. J. Mol. Sci. 2019, 20, 3405. [Google Scholar] [CrossRef]
- Fiorentini, C.; Carlini, F.; Germinario, E.A.P.; Maroccia, Z.; Travaglione, S.; Fabbri, A. Gut Microbiota and Colon Cancer: A Role for Bacterial Protein Toxins? Int. J. Mol. Sci. 2020, 21, 6201. [Google Scholar] [CrossRef] [PubMed]
- Hakimjavadi, H.; George, S.H.; Taub, M.; Dodds, L.V.; Sanchez-Covarrubias, A.P.; Huang, M.; Pearson, J.M.; Slomovitz, B.M.; Kobetz, E.N.; Gharaibeh, R.; et al. The vaginal microbiome is associated with endometrial cancer grade and histology. Cancer Res. Commun. 2022, 2, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Sobstyl, M.; Brecht, P.; Sobstyl, A.; Mertowska, P.; Grywalska, E. The Role of Microbiota in the Immunopathogenesis of Endometrial Cancer. Int. J. Mol. Sci. 2022, 23, 5756. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Hokenstad, A.N.; Chen, J.; Sung, J.; Jenkins, G.D.; Chia, N.; Nelson, H.; Mariani, A.; Walther-Antonio, M.R.S. Postmenopause as a Key Factor in the Composition of the Endometrial Cancer Microbiome (ECbiome). Sci. Rep. 2019, 9, 19213. [Google Scholar] [CrossRef]
- Li, C.; Gu, Y.; He, Q.; Huang, J.; Song, Y.; Wan, X.; Li, Y. Integrated Analysis of Microbiome and Transcriptome Data Reveals the Interplay Between Commensal Bacteria and Fibrin Degradation in Endometrial Cancer. Front. Cell Infect. Microbiol. 2021, 11, 748558. [Google Scholar] [CrossRef]
- Richard, M.L.; Liguori, G.; Lamas, B.; Brandi, G.; da Costa, G.; Hoffmann, T.W.; Pierluigi Di Simone, M.; Calabrese, C.; Poggioli, G.; Langella, P.; et al. Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut Microbes. 2018, 9, 131–142. [Google Scholar] [CrossRef]
- De Seta, F.; Banco, R.; Turrisi, A.; Airoud, M.; De Leo, R.; Stabile, G.; Ceccarello, M.; Restaino, S.; De Santo, D. Pelvic inflammatory disease (PID) from Chlamydia trachomatis versus PID from Neisseria gonorrhea: From clinical suspicion to therapy. G. Ital. Dermatol. Venereol. 2012, 147, 423–430. [Google Scholar]
- Kyono, K.; Hashimoto, T.; Kikuchi, S.; Nagai, Y.; Sakuraba, Y. A pilot study and case reports on endometrial microbiota and pregnancy outcome: An analysis using 16S rRNA gene sequencing among IVF patients, and trial therapeutic intervention for dysbiotic endometrium. Reprod. Med. Biol. 2019, 18, 72–82. [Google Scholar] [CrossRef]
- Doerflinger, S.Y.; Throop, A.L.; Herbst-Kralovetz, M.M. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J. Infect. Dis. 2014, 209, 1989–1999. [Google Scholar] [CrossRef]
- Chen, P.; Guo, Y.; Jia, L.; Wan, J.; He, T.; Fang, C.; Li, T. Interaction between functionally activateendometrial microbiota and host gene regulation in endometrial cancer. Front. Cell Dev. Biol. 2021, 9, 727286. [Google Scholar] [CrossRef]
- Stabile, G.; Topouzova, G.A.; De Seta, F. The role of microbiota in the management of genitourinary syndrome of menopause. Climacteric 2023, 26, 353–360. [Google Scholar] [CrossRef]
- Stabile, G.; Gentile, R.M.; Carlucci, S.; Restaino, S.; De Seta, F. A New Therapy for Uncomplicated Vulvovaginal Candidiasis and Its Impact on Vaginal Flora. Healthcare 2021, 9, 1555. [Google Scholar] [CrossRef]
| Authors | N Patients with EC | Pre/Post-Menopause | Microbiota in EC—Phylum (Genus/Species) | Sampling Type |
|---|---|---|---|---|
| Walther-Antonio MRS et al. 2016 [14] | 17 | Post-menopause | Firmicutes (Anaerostipes, ph2, Dialister, Peptoniphilus, 1–68, Ruminococcus, Anaerotruncus), Spirochaetes (Treponema), Actinobacteria (Atopobium), Bacteroidetes (Bacteroides, Porphyromonas), Proteobacteria (Arthrospira) | Post-hysterectomy biopsy |
| Lu W et al. 2020 [15] | 25 | Pre- and post-menopause | Actinobacteria (Micrococcus), Firmicutes (Pseudoramibacter, Eubacterium, Megamonas), Proteobacteria (Rhodobacter, Vogesella, Bilophila, Rheinheimera) | Post-hysterectomy biopsy |
| Gressel GM et al. 2021 [16] | 25 | Post-menopause | Bacteroidetes (Flavobacterium) | Post-hysterectomy biopsy |
| Hawkins GM et al. 2022 [17] | 95 | Post-menopause | Bacteroidetes (Flavobacterium), Pseudomonadota (Pelomonas, Hyphomicrobium, Bradyrhizobium), Proteobacteria (Pseudomonas, Acidovorax) | Post-hysterectomy biopsy |
| Wang L et al. 2022 [18] | 28 | Post-menopause | Bacteroidetes (Prevotella, Porphyromonas), Actinobacteria (Atopobium), Firmicutes (Anaerococcus, Dialister, Peptoniphilus) | Post-hysterectomy biopsy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stabile, G.; Doria, A.; Bruno, M.; D’Indinosante, M.; Gallotta, V.; Fanfani, F.; Scambia, G.; Restaino, S.; Vizzielli, G.; Carlucci, S.; et al. The Role of the Endometrial Microbiota in Endometrial Cancer: A Systematic Review of the Literature. J. Clin. Med. 2024, 13, 7135. https://doi.org/10.3390/jcm13237135
Stabile G, Doria A, Bruno M, D’Indinosante M, Gallotta V, Fanfani F, Scambia G, Restaino S, Vizzielli G, Carlucci S, et al. The Role of the Endometrial Microbiota in Endometrial Cancer: A Systematic Review of the Literature. Journal of Clinical Medicine. 2024; 13(23):7135. https://doi.org/10.3390/jcm13237135
Chicago/Turabian StyleStabile, Guglielmo, Alessandra Doria, Matteo Bruno, Marco D’Indinosante, Valerio Gallotta, Francesco Fanfani, Giovanni Scambia, Stefano Restaino, Giuseppe Vizzielli, Stefania Carlucci, and et al. 2024. "The Role of the Endometrial Microbiota in Endometrial Cancer: A Systematic Review of the Literature" Journal of Clinical Medicine 13, no. 23: 7135. https://doi.org/10.3390/jcm13237135
APA StyleStabile, G., Doria, A., Bruno, M., D’Indinosante, M., Gallotta, V., Fanfani, F., Scambia, G., Restaino, S., Vizzielli, G., Carlucci, S., & Nappi, L. (2024). The Role of the Endometrial Microbiota in Endometrial Cancer: A Systematic Review of the Literature. Journal of Clinical Medicine, 13(23), 7135. https://doi.org/10.3390/jcm13237135










