Decoding Chemotherapy Resistance of Undifferentiated Pleomorphic Sarcoma at the Single Cell Resolution: A Case Report
Abstract
:1. Introduction
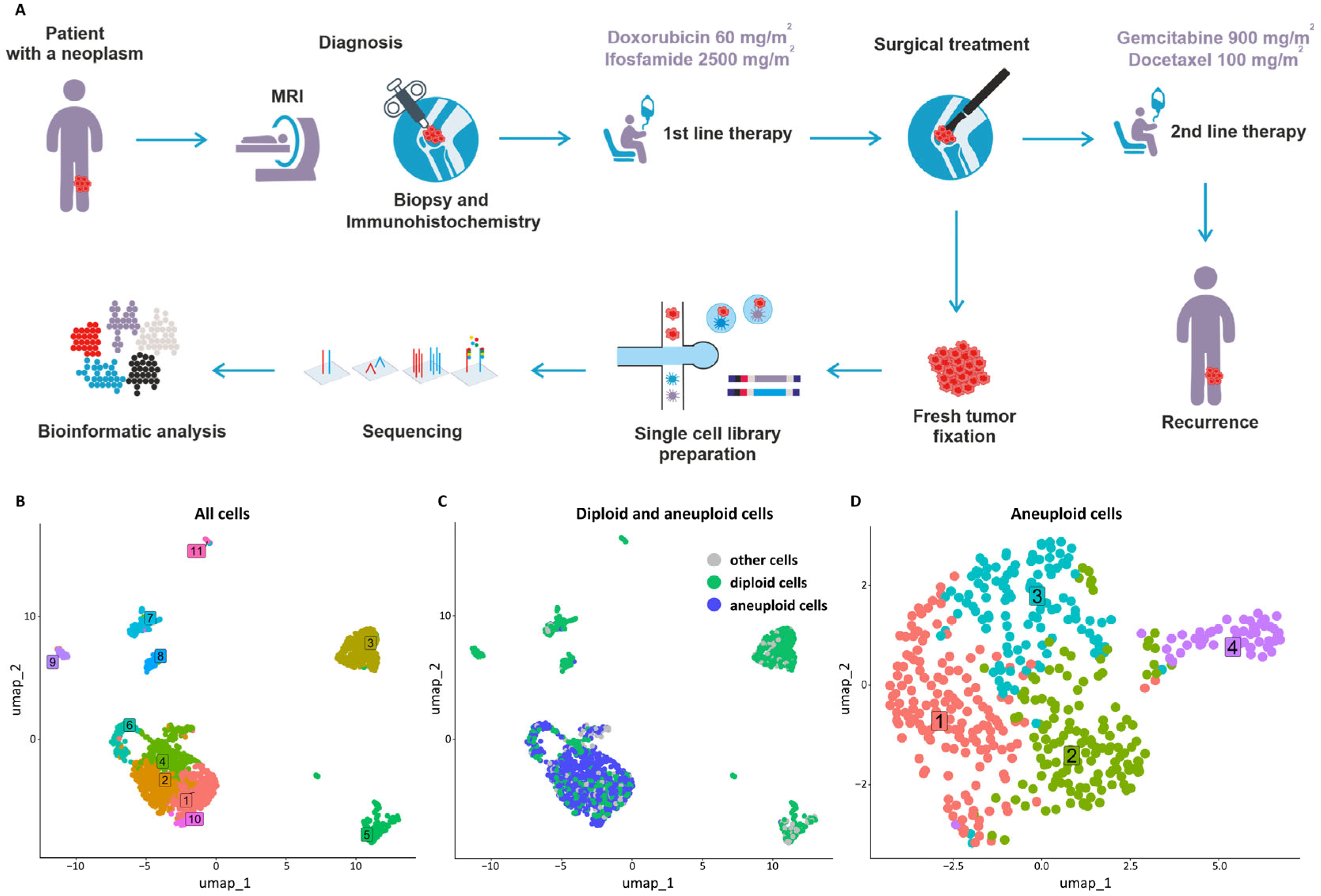
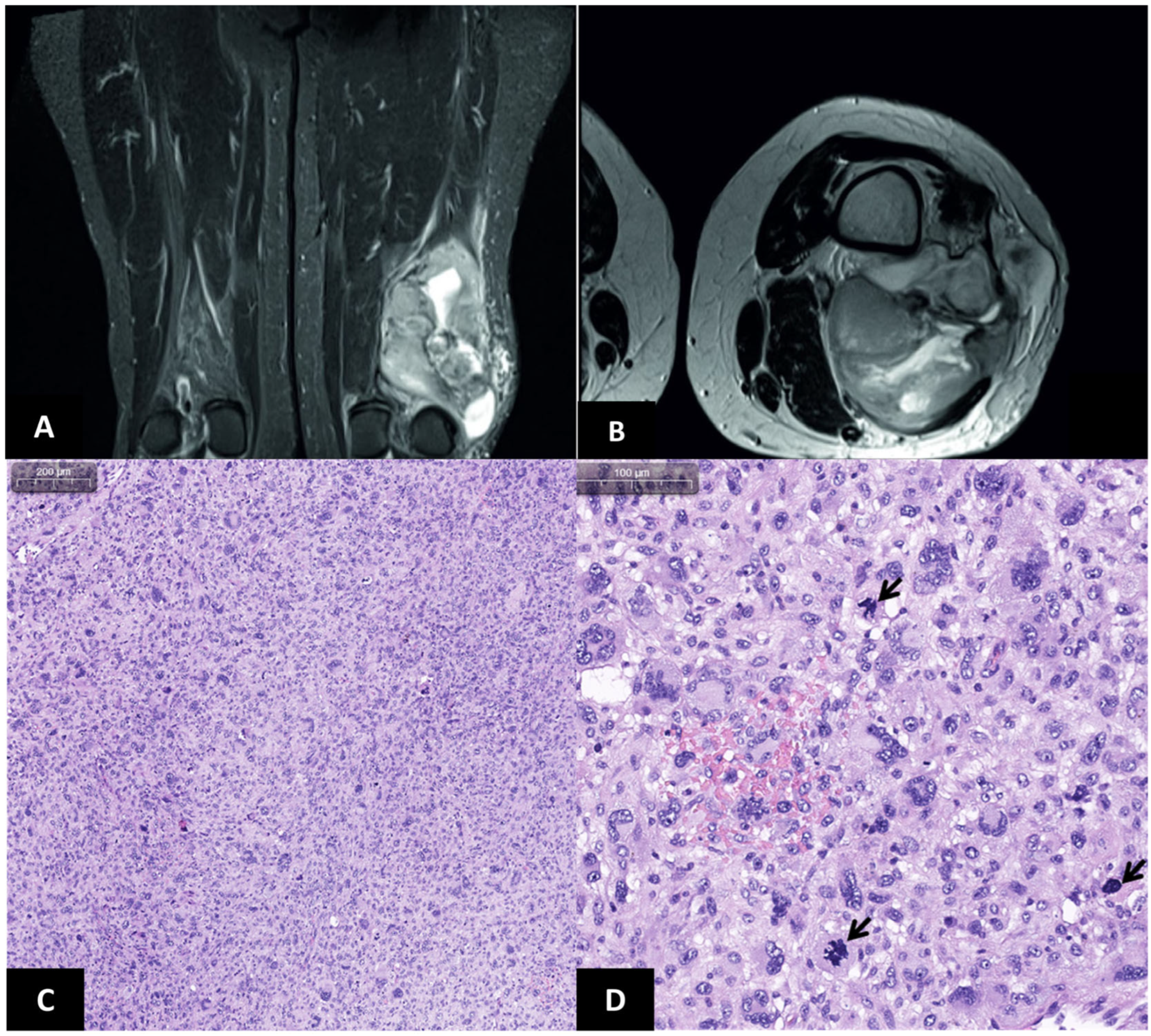
2. Case Report
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldblum, J.R. An approach to pleomorphic sarcomas: Can we subclassify, and does it matter? Mod. Pathol. 2014, 27, S39–S46. [Google Scholar] [CrossRef] [PubMed]
- Toro, J.R.; Travis, L.B.; Wu, H.J.; Zhu, K.; Fletcher, C.D.; Devesa, S.S. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int. J. Cancer 2006, 119, 2922–2930. [Google Scholar] [CrossRef] [PubMed]
- Widemann, B.C.; Italiano, A. Biology and Management of Undifferentiated Pleomorphic Sarcoma, Myxofibrosarcoma, and Malignant Peripheral Nerve Sheath Tumors: State of the Art and Perspectives. J. Clin. Oncol. 2018, 36, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, W.; Luo, P.; Cai, W.; Yang, L.; Sun, Z.; Zheng, B.; Yan, W.; Wang, C. Undifferentiated Pleomorphic Sarcoma: Long-Term Follow-Up from a Large Institution. Cancer Manag. Res. 2019, 11, 10001–10009. [Google Scholar] [CrossRef] [PubMed]
- Vodanovich, D.A.; Spelman, T.; May, D.; Slavin, J.; Choong, P.F.M. Predicting the prognosis of undifferentiated pleomorphic soft tissue sarcoma: A 20-year experience of 266 cases. ANZ J. Surg. 2019, 89, 1045–1050. [Google Scholar] [CrossRef]
- Peiper, M.; Zurakowski, D.; Knoefel, W.T.; Izbicki, J.R. Malignant fibrous histiocytoma of the extremities and trunk: An institutional review. Surgery 2004, 135, 59–66. [Google Scholar] [CrossRef]
- Gonzalez, M.R.; Clunk, M.J.; Bedi, A.D.S.; Werenski, J.O.; Lang, J.H.; Karczewski, D.; Sodhi, A.; Lozano-Calderon, S.A. Prognostic and predictive factors in undifferentiated pleomorphic sarcoma: A long-term study from a large tertiary care urban center. J. Surg. Oncol. 2023, 128, 322–331. [Google Scholar] [CrossRef]
- Kobayashi, H.; Zhang, L.; Hirai, T.; Tsuda, Y.; Ikegami, M.; Tanaka, S. Role of adjuvant chemotherapy in patients with localized, undifferentiated pleomorphic sarcoma of soft tissue: A population-based cohort study. Int. J. Clin. Oncol. 2022, 27, 802–810. [Google Scholar] [CrossRef]
- Du, X.H.; Wei, H.; Zhang, P.; Yao, W.T.; Cai, Q.Q. Heterogeneity of Soft Tissue Sarcomas and Its Implications in Targeted Therapy. Front. Oncol. 2020, 10, 564852. [Google Scholar] [CrossRef]
- Tang, Y.J.; Puviindran, V.; Xiang, Y.; Yahara, Y.; Zhang, H.; Nadesan, P.; Diao, Y.; Kirsch, D.G.; Alman, B.A. Tumor-propagating side population cells are a dynamic subpopulation in undifferentiated pleomorphic sarcoma. JCI Insight 2021, 6, e148768. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, D.; Wang, B.; Chai, W.; Yan, M.; Chen, Y.; Zhan, Y.; Yang, R.; Zhou, E.; Dai, S.; et al. Single-cell landscape of undifferentiated pleomorphic sarcoma. Oncogene 2024, 43, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- Tessaro, F.H.G.; Ko, E.Y.; De Simone, M.; Piras, R.; Broz, M.T.; Goodridge, H.S.; Balzer, B.; Shiao, S.L.; Guarnerio, J. Single-cell RNA-seq of a soft-tissue sarcoma model reveals the critical role of tumor-expressed MIF in shaping macrophage heterogeneity. Cell Rep. 2022, 39, 110977. [Google Scholar] [CrossRef] [PubMed]
- Berlow, N.E.; Grasso, C.S.; Quist, M.J.; Cheng, M.; Gandour-Edwards, R.; Hernandez, B.S.; Michalek, J.E.; Ryan, C.; Spellman, P.; Pal, R.; et al. Deep Functional and Molecular Characterization of a High-Risk Undifferentiated Pleomorphic Sarcoma. Sarcoma 2020, 2020, 6312480. [Google Scholar] [CrossRef] [PubMed]
- Fetisov, T.I.; Khazanova, S.A.; Shtompel, P.A.; Trapeznikova, E.S.; Zinovieva, V.Y.; Marshall, V.I.; Lovenger, A.A.; Rogozhin, D.V.; Anastasia, T.A.; Bokhyan, B.Y.; et al. Perspectives of Cell Sensitivity/Resistance Assay in Soft Tissue Sarcomas Chemotherapy. Int. J. Mol. Sci. 2023, 24, 12292. [Google Scholar] [CrossRef] [PubMed]
- De Vita, A.; Recine, F.; Mercatali, L.; Miserocchi, G.; Spadazzi, C.; Liverani, C.; Bongiovanni, A.; Pieri, F.; Casadei, R.; Riva, N.; et al. Primary Culture of Undifferentiated Pleomorphic Sarcoma: Molecular Characterization and Response to Anticancer Agents. Int. J. Mol. Sci. 2017, 18, 2662. [Google Scholar] [CrossRef]
- Piano, M.A.; Brunello, A.; Cappellesso, R.; Del Bianco, P.; Mattiolo, A.; Fritegotto, C.; Montini, B.; Zamuner, C.; Del Fiore, P.; Rastrelli, M.; et al. Periostin and Epithelial-Mesenchymal Transition Score as Novel Prognostic Markers for Leiomyosarcoma, Myxofibrosarcoma, and Undifferentiated Pleomorphic Sarcoma. Clin. Cancer Res. 2020, 26, 2921–2931. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Zhao, Y.; Xiong, H.; Sneh, T.; Fan, Y.; Wang, J.; Zhou, X.; Gong, C. Complement and coagulation cascades pathway correlates with chemosensitivity and overall survival in patients with soft tissue sarcoma. Eur. J. Pharmacol. 2020, 879, 173121. [Google Scholar] [CrossRef]
- Toulmonde, M.; Lucchesi, C.; Verbeke, S.; Crombe, A.; Adam, J.; Geneste, D.; Chaire, V.; Laroche-Clary, A.; Perret, R.; Bertucci, F.; et al. High throughput profiling of undifferentiated pleomorphic sarcomas identifies two main subgroups with distinct immune profile, clinical outcome and sensitivity to targeted therapies. EBioMedicine 2020, 62, 103131. [Google Scholar] [CrossRef]
- Egorenkov, V.; Bokhyan, A.; Konev, A.; Nesterova, A.; Tararykova, A.; Tjuladina, A.; Sharabura, T.; Shelekhova, K.; Fedenko, A. Soft Tissue Sarcoma. Malig. Tumours 2023, 13, 356–374. (In Russia) [Google Scholar] [CrossRef]
- von Mehren, M.; Kane, J.M.; Agulnik, M.; Bui, M.M.; Carr-Ascher, J.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; Gonzalez, R.J.; et al. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 815–833. [Google Scholar] [CrossRef]
- Hayes, A.J.; Nixon, I.F.; Strauss, D.C.; Seddon, B.M.; Desai, A.; Benson, C.; Judson, I.R.; Dangoor, A. UK guidelines for the management of soft tissue sarcomas. Br. J. Cancer 2024, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C.; et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 2024, 42, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Xi, N.M.; Li, J.J. Protocol for executing and benchmarking eight computational doublet-detection methods in single-cell RNA sequencing data analysis. STAR Protoc. 2021, 2, 100699. [Google Scholar] [CrossRef] [PubMed]
- Finak, G.; McDavid, A.; Yajima, M.; Deng, J.; Gersuk, V.; Shalek, A.K.; Slichter, C.K.; Miller, H.W.; McElrath, M.J.; Prlic, M.; et al. MAST: A flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 2015, 16, 278. [Google Scholar] [CrossRef] [PubMed]
- De Falco, A.; Caruso, F.; Su, X.D.; Iavarone, A.; Ceccarelli, M. A variational algorithm to detect the clonal copy number substructure of tumors from scRNA-seq data. Nat. Commun. 2023, 14, 1074. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Ju, J.A.; Godet, I.; DiGiacomo, J.W.; Gilkes, D.M. RhoB is regulated by hypoxia and modulates metastasis in breast cancer. Cancer Rep. 2020, 3, e1164. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Xu, Y.; Chen, L.; Ding, P.; Chen, J.; Hu, W. An Integrated Analysis of C5AR2 Related to Malignant Properties and Immune Infiltration of Breast Cancer. Front. Oncol. 2021, 11, 736725. [Google Scholar] [CrossRef]
- Zhang, S.; Jia, X.; Dai, H.; Zhu, X.; Song, W.; Bian, S.; Wu, H.; Chen, S.; Tang, Y.; Chen, J.; et al. SERPINE2 promotes liver cancer metastasis by inhibiting c-Cbl-mediated EGFR ubiquitination and degradation. Cancer Commun. 2024, 44, 384–407. [Google Scholar] [CrossRef]
- Song, Y.; Fu, Y.; Wang, J.; Tang, J.; Yin, J.; Zhang, Z.; Song, Q.; Zhang, B. Complement C1q induces the M2-polarization of tumor-associated macrophages in lung adenocarcinoma. Genes Dis. 2024, 11, 101093. [Google Scholar] [CrossRef]
- Ni, B.; Huang, G.; Yang, R.; Wang, Z.; Song, H.; Li, K.; Zhang, Y.; Wu, K.; Shi, G.; Wang, X.; et al. The short isoform of MS4A7 is a novel player in glioblastoma microenvironment, M2 macrophage polarization, and tumor progression. J. Neuroinflamm. 2023, 20, 80. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.M.; Liu, K.; Liu, J.H.; Jiang, X.L.; Wang, X.L.; Chen, Y.Z.; Li, S.G.; Zou, H.; Pang, L.J.; Liu, C.X.; et al. CD163 as a marker of M2 macrophage, contribute to predicte aggressiveness and prognosis of Kazakh esophageal squamous cell carcinoma. Oncotarget 2017, 8, 21526–21538. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Jeon, Y.K.; Jeong, D.H.; Byun, J.M.; Bogen, B.; Choi, I. VSIG4-expressing tumor-associated macrophages impair anti-tumor immunity. Biochem. Biophys. Res. Commun. 2022, 628, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Zhang, M.; Shang, Z.; Chen, S.; Zhao, J.; Ding, B.; Jiang, D.; Zhu, Q.; Teng, H.; Zhu, L.; et al. Single-cell profiling reveals the trajectory of FOLR2-expressing tumor-associated macrophages to regulatory T cells in the progression of lung adenocarcinoma. Cell Death Dis. 2023, 14, 493. [Google Scholar] [CrossRef] [PubMed]
- Luchtel, R.A.; Zhao, Y.; Aggarwal, R.K.; Pradhan, K.; Maqbool, S.B. ETS1 is a novel transcriptional regulator of adult T-cell leukemia/lymphoma of North American descent. Blood Adv. 2022, 6, 5613–5624. [Google Scholar] [CrossRef]
- Zhang, C.; Dang, D.; Cong, L.; Sun, H.; Cong, X. Pivotal factors associated with the immunosuppressive tumor microenvironment and melanoma metastasis. Cancer Med. 2021, 10, 4710–4720. [Google Scholar] [CrossRef]
- Cords, L.; Tietscher, S.; Anzeneder, T.; Langwieder, C.; Rees, M.; de Souza, N.; Bodenmiller, B. Cancer-associated fibroblast classification in single-cell and spatial proteomics data. Nat. Commun. 2023, 14, 4294. [Google Scholar] [CrossRef]
- Xie, Z.; Niu, L.; Zheng, G.; Du, K.; Dai, S.; Li, R.; Dan, H.; Duan, L.; Wu, H.; Ren, G.; et al. Single-cell analysis unveils activation of mast cells in colorectal cancer microenvironment. Cell Biosci. 2023, 13, 217. [Google Scholar] [CrossRef]
- Ye, Z.; Yang, Y.; Wei, Y.; Li, L.; Wang, X.; Zhang, J. Correction: PCDH1 promotes progression of pancreatic ductal adenocarcinoma via activation of NF-κB signalling by interacting with KPNB1. Cell Death Dis. 2024, 15, 22. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Yue, Y.; Zhang, L.; Liu, T.; Jing, M.; Liang, X.; Ma, M.; Xu, S.; Wang, K.; et al. ITPR3 facilitates tumor growth, metastasis and stemness by inducing the NF-ĸB/CD44 pathway in urinary bladder carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 65. [Google Scholar] [CrossRef]
- Huang, J.; Liu, K.; Song, D.; Ding, M.; Wang, J.; Jin, Q.; Ni, J. Krüppel-like factor 4 promotes high-mobility group box 1-induced chemotherapy resistance in osteosarcoma cells. Cancer Sci. 2016, 107, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xian, M.; Yang, B.; Ying, M.; He, Q. Inhibition of KLF4 by Statins Reverses Adriamycin-Induced Metastasis and Cancer Stemness in Osteosarcoma Cells. Stem Cell Rep. 2017, 8, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Shi, Q.; Jin, Y.; Liu, Z.; Li, J.; Sun, W. Blockage of AMPK-ULK1 pathway mediated autophagy promotes cell apoptosis to increase doxorubicin sensitivity in breast cancer (BC) cells: An in vitro study. BMC Cancer 2021, 21, 195. [Google Scholar] [CrossRef] [PubMed]
- Ou, G.; Tian, Z.; Su, M.; Yu, M.; Gong, J.; Chen, Y. Identification of gemcitabine resistance-related AHNAK2 gene associated with prognosis and immune infiltration in pancreatic cancer. Heliyon 2024, 10, e33687. [Google Scholar] [CrossRef]
- Klejewski, A.; Sterzyńska, K.; Wojtowicz, K.; Świerczewska, M.; Partyka, M.; Brązert, M.; Nowicki, M.; Zabel, M.; Januchowski, R. The significance of lumican expression in ovarian cancer drug-resistant cell lines. Oncotarget 2017, 8, 74466–74478. [Google Scholar] [CrossRef]
- Biondini, M.; Kiepas, A.; El-Houjeiri, L.; Annis, M.G.; Hsu, B.E.; Fortier, A.M.; Morin, G.; Martina, J.A.; Sirois, I.; Aguilar-Mahecha, A.; et al. HSP90 inhibitors induce GPNMB cell-surface expression by modulating lysosomal positioning and sensitize breast cancer cells to glembatumumab vedotin. Oncogene 2022, 41, 1701–1717. [Google Scholar] [CrossRef]
- Yi, J.S.; Mun, D.G.; Lee, H.; Park, J.S.; Lee, J.W.; Lee, J.S.; Kim, S.J.; Cho, B.R.; Lee, S.W.; Ko, Y.G. PTRF/cavin-1 is essential for multidrug resistance in cancer cells. J. Proteome Res. 2013, 12, 605–614. [Google Scholar] [CrossRef]
- Papoutsidakis, A.; Giatagana, E.M.; Berdiaki, A.; Spyridaki, I.; Spandidos, D.A.; Tsatsakis, A.; Tzanakakis, G.N.; Nikitovic, D. Lumican mediates HTB94 chondrosarcoma cell growth via an IGF-IR/Erk1/2 axis. Int. J. Oncol. 2020, 57, 791–803. [Google Scholar] [CrossRef]
- Kwon, R.; Hong, B.K.; Lee, K.G.; Choi, E.; Sabbagh, L.; Cho, C.S.; Lee, N.; Kim, W.U. Regulation of tumor growth by leukocyte-specific protein 1 in T cells. J. Immunother. Cancer 2020, 8, e001180. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, Z.; Liang, W.; Zhu, Q.; Wang, C.; Wang, H.; Zhang, J.; Li, P.; Gao, Y. ECM2, a prognostic biomarker for lower grade glioma, serves as a potential novel target for immunotherapy. Int. J. Biochem. Cell Biol. 2023, 158, 106409. [Google Scholar] [CrossRef]
- Khanna, A.; Mahalingam, K.; Chakrabarti, D.; Periyasamy, G. Ets-1 expression and gemcitabine chemoresistance in pancreatic cancer cells. Cell. Mol. Biol. Lett. 2011, 16, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Li, F.; Zhao, Z.; Zhang, Z.; Hu, J.; Zhang, Y. Tumor-Associated Macrophage Promotes the Survival of Cancer Cells upon Docetaxel Chemotherapy via the CSF1/CSF1R-CXCL12/CXCR4 Axis in Castration-Resistant Prostate Cancer. Genes 2021, 12, 773. [Google Scholar] [CrossRef] [PubMed]
- Weizman, N.; Krelin, Y.; Shabtay-Orbach, A.; Amit, M.; Binenbaum, Y.; Wong, R.J.; Gil, Z. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene 2014, 33, 3812–3819. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Ma, J.; Cheng, Y.; Zheng, P. Exosomal Transfer of Macrophage-Derived miR-223 Confers Doxorubicin Resistance in Gastric Cancer. OncoTargets Ther. 2020, 13, 12169–12179. [Google Scholar] [CrossRef]
- Tang, M.; Chen, B.; Xia, H.; Pan, M.; Zhao, R.; Zhou, J.; Yin, Q.; Wan, F.; Yan, Y.; Fu, C.; et al. pH-gated nanoparticles selectively regulate lysosomal function of tumour-associated macrophages for cancer immunotherapy. Nat. Commun. 2023, 14, 5888. [Google Scholar] [CrossRef]
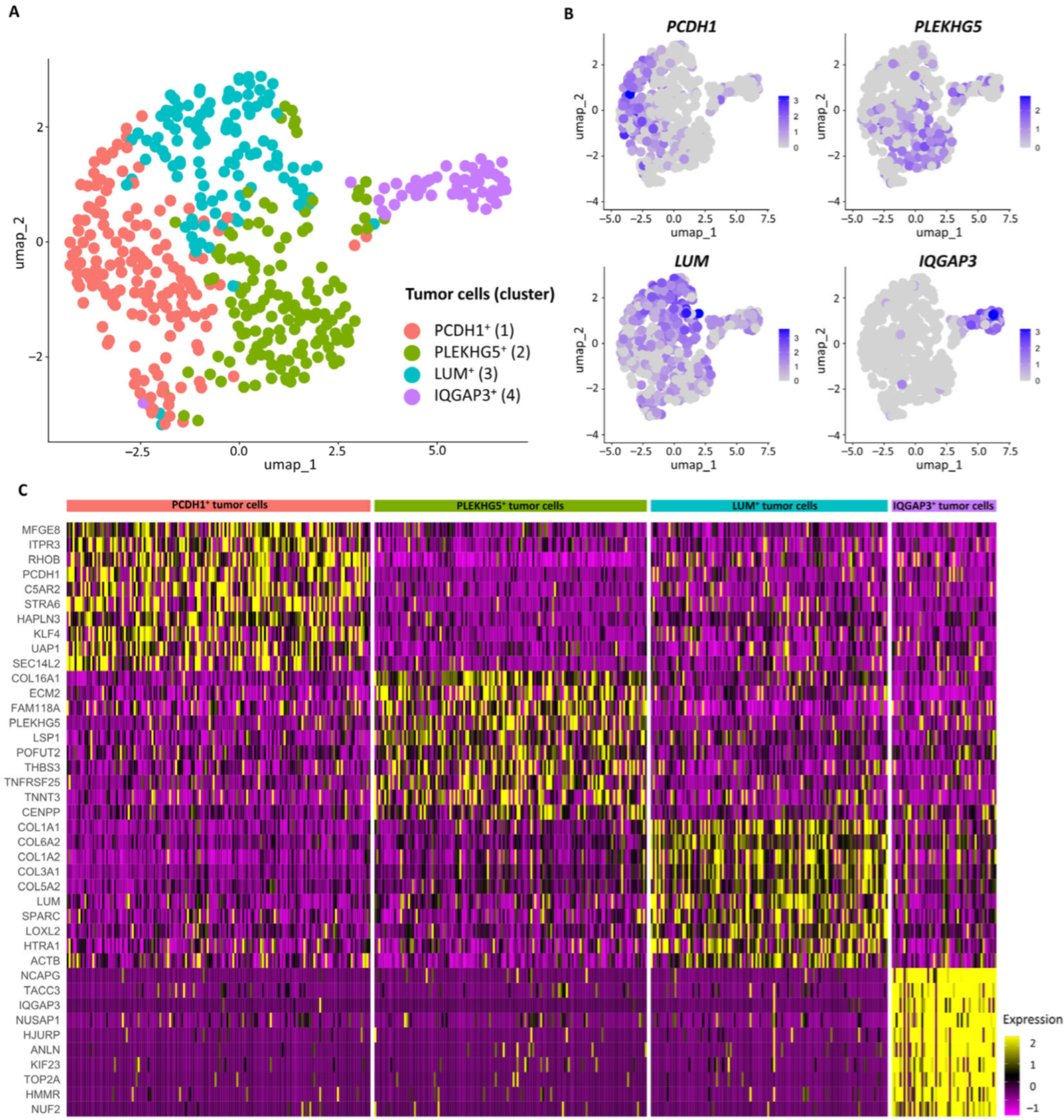
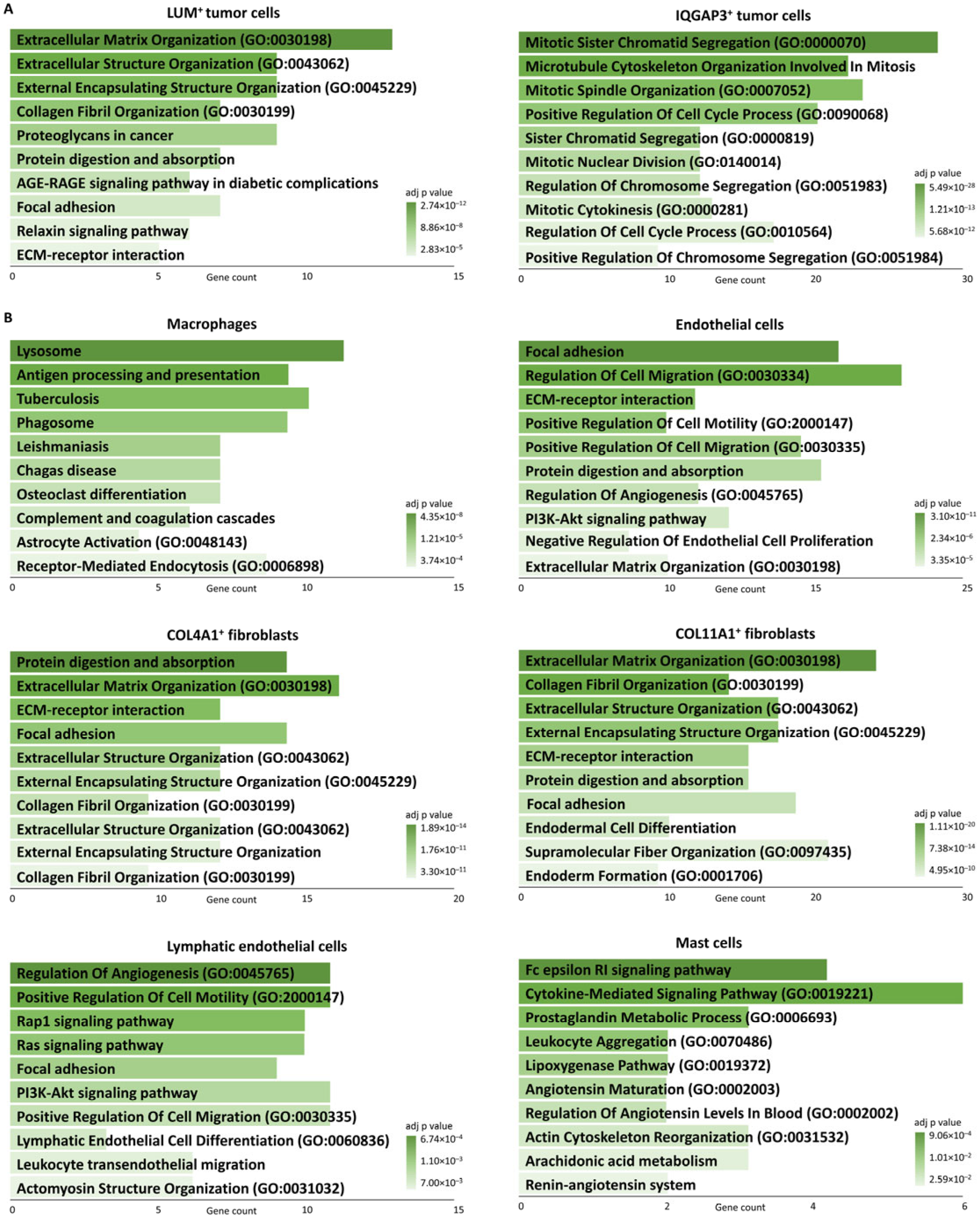
| Subpopulations | Marker Genes | Functionally Enriched Terms |
|---|---|---|
| PCDH1+ | PCDH1, STRA6, SEC14L2, C5AR2, KLF4 | TNF signaling pathway, Extracellular matrix-receptor interaction, Integrated stress response |
| PLEKHG5+ | PLEKHG5, TNFRSF25, TNNT3, ECM2, THBS3 | Pluripotency of stem cells, Circadian rhythm |
| LUM+ | LUM, C1R, GPNMB, COL1A1, PRSS23 | Proteoglycans in cancer, Protein digestion and absorption, Extracellular matrix organization |
| IQGAP3+ | IQGAP3, TROAP, DLGAP5, AURKB, KIF20A | Cell cycle, Mitotic sister chromatid segregation |
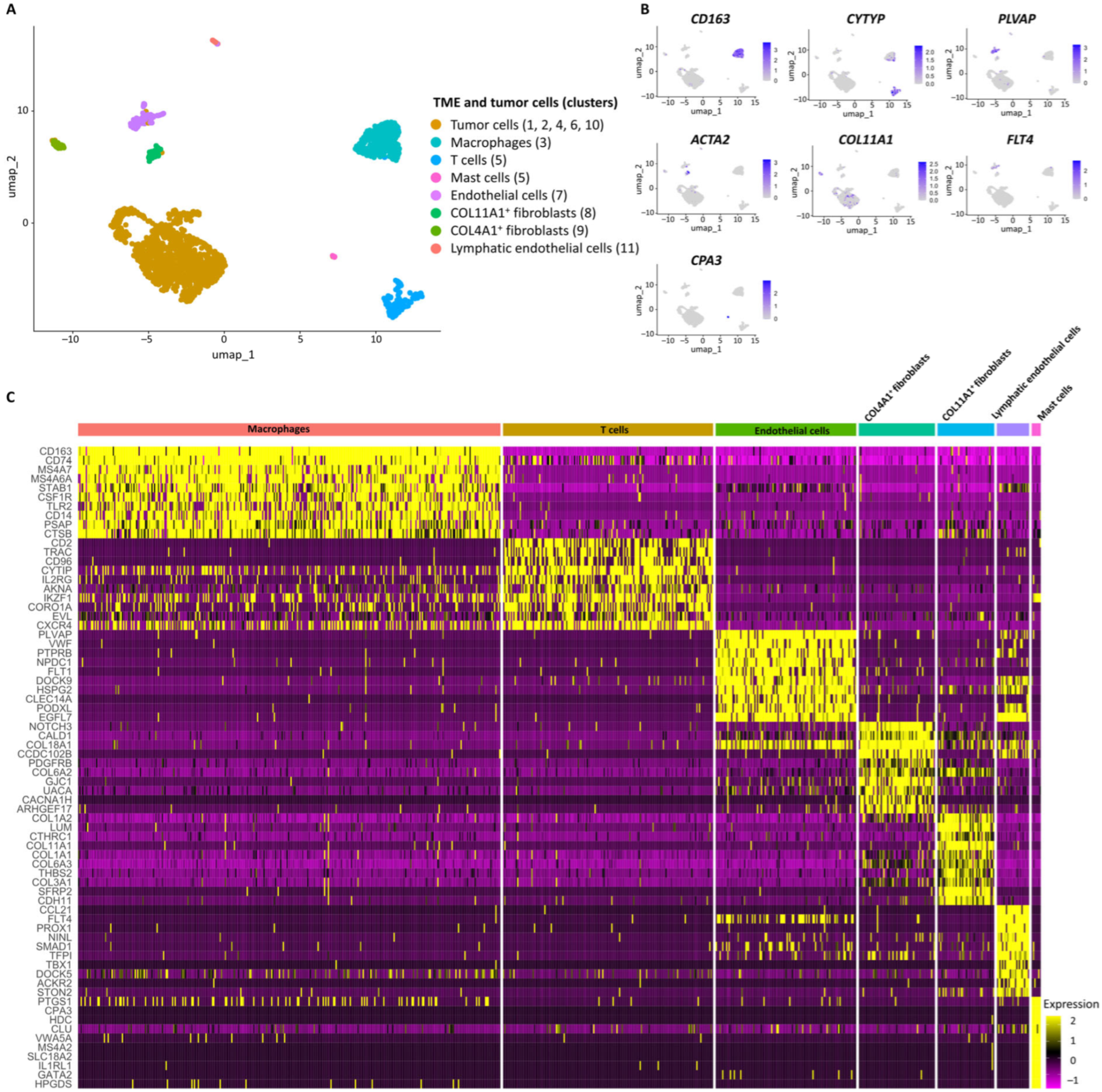
| Subpopulations | Marker Genes | Functionally Enriched Terms |
|---|---|---|
| Macrophages | CD163, CD74, MS4A7, MS4A6A, PSAP | Antigen processing and presentation, phagosome, inflammatory response |
| T cells | CD2, CD96, CYTIP, TRAC, TMSB4X | Regulation of actin cytoskeleton, negative regulation of NF-kappaB transcription factor activity, antigen processing and presentation of peptide antigen via MHC Class I, regulation of lymphocyte differentiation |
| Endothelial cells | PLVAP, HSPG2, VWF, PECAM1, CLEC14A | Extracellular matrix-receptor interaction, focal adhesion, Regulation of angiogenesis, regulation of cell migration |
| COL4A1+ Fibroblasts | COL18A1, NOTCH3, COL4A2, ACTA2, COL4A1 | Extracellular matrix organization, basement membrane organization |
| COL11A1+ Fibroblasts | POSTN, COL11A1, CDH11, DCN, ASPN | Protein digestion and absorption, collagen fibril organization |
| Lymphatic endothelial cells | FLT4, PROX1, CCL21 | PI3K-Akt signaling pathway, regulation of endothelial cell proliferation, Lymphatic endothelial cell differentiation |
| Mast cells | CPA3, MS4A2, HDC | Mast cell mediated immunity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fetisov, T.I.; Menyailo, M.E.; Ikonnikov, A.V.; Khozyainova, A.A.; Tararykova, A.A.; Kopantseva, E.E.; Korobeynikova, A.A.; Senchenko, M.A.; Bokova, U.A.; Kirsanov, K.I.; et al. Decoding Chemotherapy Resistance of Undifferentiated Pleomorphic Sarcoma at the Single Cell Resolution: A Case Report. J. Clin. Med. 2024, 13, 7176. https://doi.org/10.3390/jcm13237176
Fetisov TI, Menyailo ME, Ikonnikov AV, Khozyainova AA, Tararykova AA, Kopantseva EE, Korobeynikova AA, Senchenko MA, Bokova UA, Kirsanov KI, et al. Decoding Chemotherapy Resistance of Undifferentiated Pleomorphic Sarcoma at the Single Cell Resolution: A Case Report. Journal of Clinical Medicine. 2024; 13(23):7176. https://doi.org/10.3390/jcm13237176
Chicago/Turabian StyleFetisov, Timur I., Maxim E. Menyailo, Alexander V. Ikonnikov, Anna A. Khozyainova, Anastasia A. Tararykova, Elena E. Kopantseva, Anastasia A. Korobeynikova, Maria A. Senchenko, Ustinia A. Bokova, Kirill I. Kirsanov, and et al. 2024. "Decoding Chemotherapy Resistance of Undifferentiated Pleomorphic Sarcoma at the Single Cell Resolution: A Case Report" Journal of Clinical Medicine 13, no. 23: 7176. https://doi.org/10.3390/jcm13237176
APA StyleFetisov, T. I., Menyailo, M. E., Ikonnikov, A. V., Khozyainova, A. A., Tararykova, A. A., Kopantseva, E. E., Korobeynikova, A. A., Senchenko, M. A., Bokova, U. A., Kirsanov, K. I., Yakubovskaya, M. G., & Denisov, E. V. (2024). Decoding Chemotherapy Resistance of Undifferentiated Pleomorphic Sarcoma at the Single Cell Resolution: A Case Report. Journal of Clinical Medicine, 13(23), 7176. https://doi.org/10.3390/jcm13237176






