Menstrual Blood as a Non-Invasive Alternative for Monitoring Vitamin Levels
Abstract
1. Introduction
2. Materials and Methods
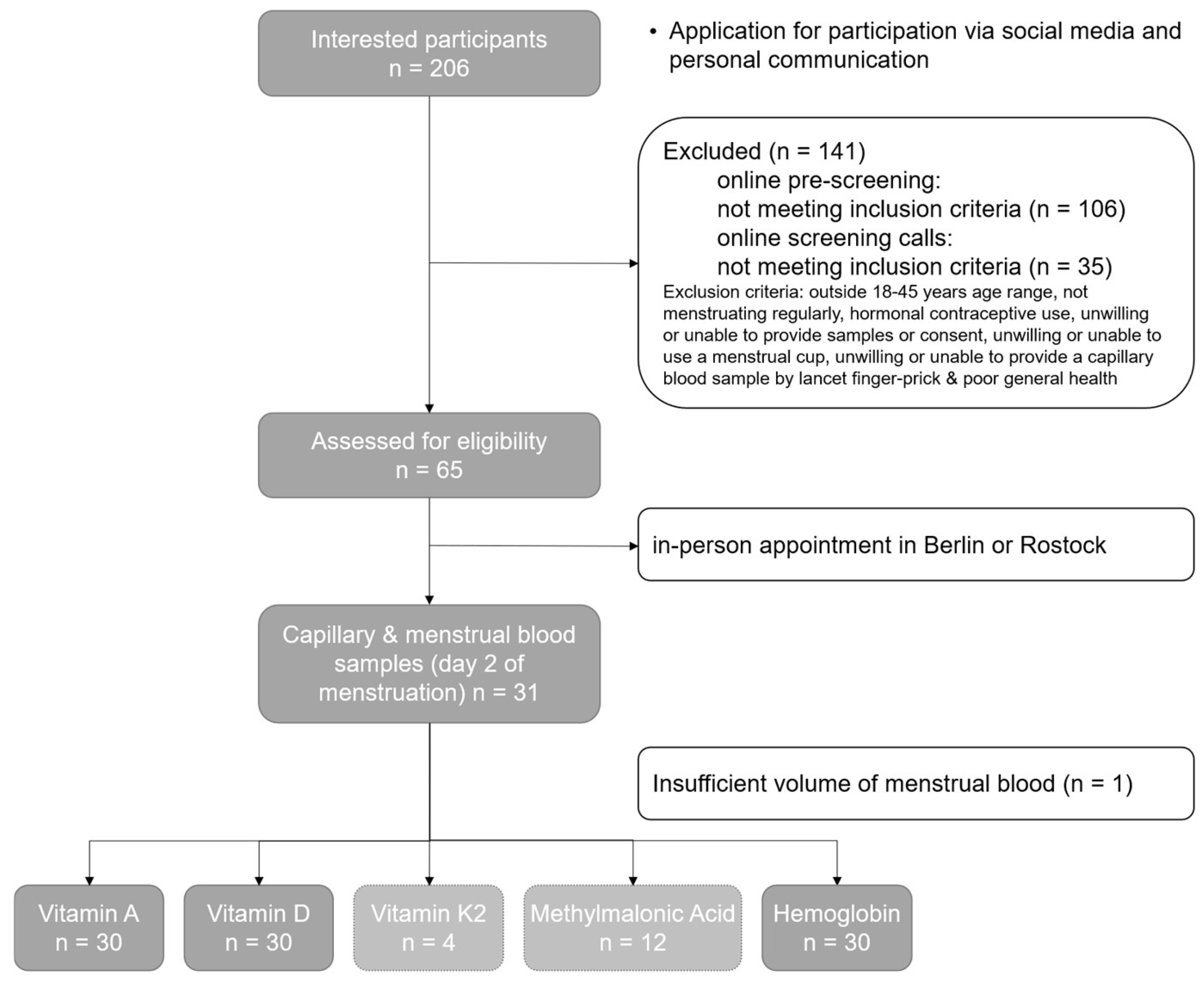
2.1. Inclusion Criteria as Well as Time and Organizational Procedures
2.2. Analysis of the Samples
2.3. Statistical Analysis
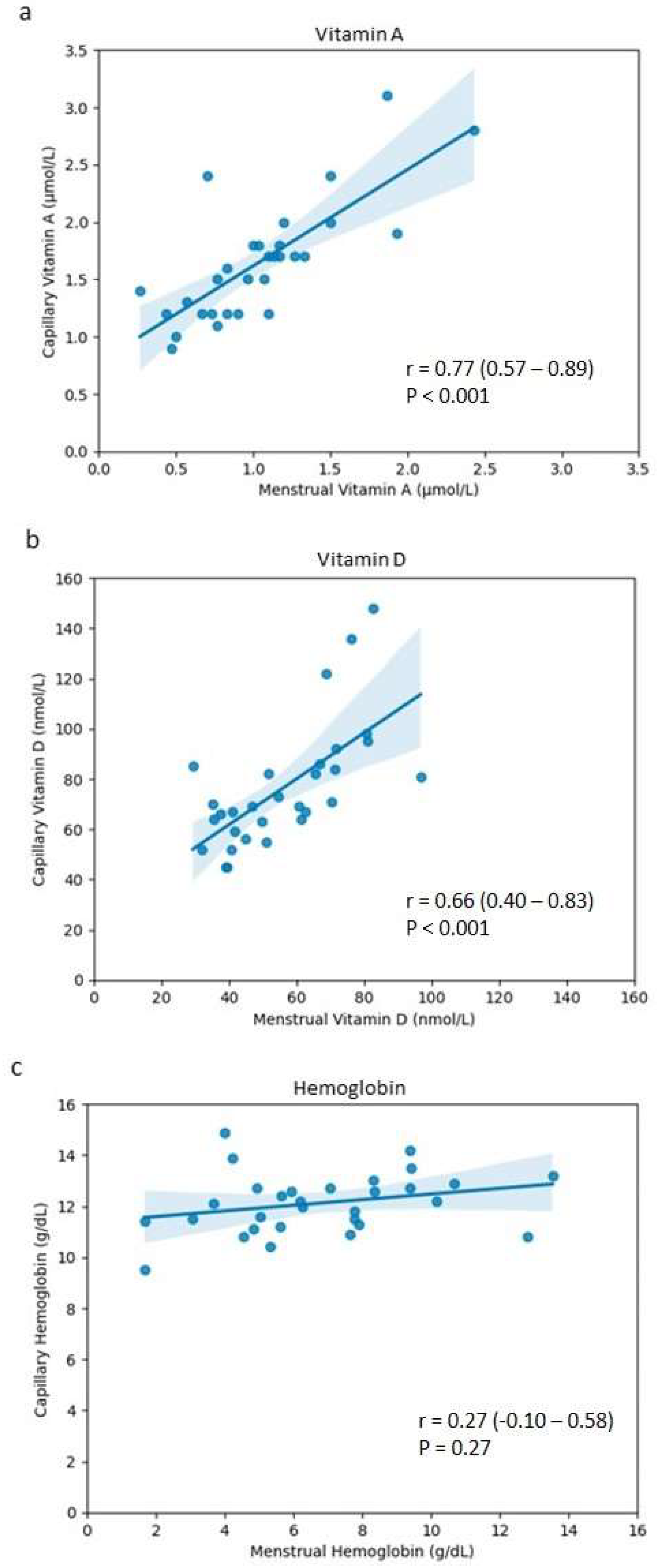
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nur, S.M.; Rath, S.; Ahmad, V.; Ahmad, A.; Ateeq, B.; Khan, M.I. Nutritive Vitamins as Epidrugs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D. The Discovery of the Vitamins. Int. J. Vitam. Nutr. Res. 2012, 82, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-W.; Lee, H.-C. Vitamin D and Health—The Missing Vitamin in Humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Gooch, H.; Groves, N.J.; Sah, P.; Burne, T.H.; Eyles, D.W.; McGrath, J.J. Vitamin D and the Brain: Key Questions for Future Research. J. Steroid Biochem. Mol. Biol. 2015, 148, 305–309. [Google Scholar] [CrossRef]
- Kang, J.; Park, M.; Lee, E.; Jung, J.; Kim, T. The Role of Vitamin D in Alzheimer’s Disease: A Transcriptional Regulator of Amyloidopathy and Gliopathy. Biomedicines 2022, 10, 1824. [Google Scholar] [CrossRef]
- Bahrami, A.; Bahrami-Taghanaki, H.; Khorasanchi, Z.; Timar, A.; Jaberi, N.; Azaryan, E.; Tayefi, M.; Ferns, G.A.; Sadeghnia, H.R.; Ghayour-Mobarhan, M. Menstrual Problems in Adolescence: Relationship to Serum Vitamins A and E, and Systemic Inflammation. Arch. Gynecol. Obstet. 2020, 301, 189–197. [Google Scholar] [CrossRef]
- Clagett-Dame, M.; Knutson, D. Vitamin A in Reproduction and Development. Nutrients 2011, 3, 385–428. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, J.; Jiang, X.; Wu, J.; Wu, X. Serum Fat-Soluble Vitamins and the Menstrual Cycle in Women of Childbearing Age. Food Funct. 2023, 14, 231–239. [Google Scholar] [CrossRef]
- Youness, R.A.; Dawoud, A.; ElTahtawy, O.; Farag, M.A. Fat-Soluble Vitamins: Updated Review of Their Role and Orchestration in Human Nutrition throughout Life Cycle with Sex Differences. Nutr. Metab. 2022, 19, 60. [Google Scholar] [CrossRef]
- Bendik, I.; Friedel, A.; Roos, F.F.; Weber, P.; Eggersdorfer, M. Vitamin D: A Critical and Essential Micronutrient for Human Health. Front. Physiol. 2014, 5, 248. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.; Stähelin, H.B.; Walter, P. Vitamin D Effects on Bone and Muscle. Int. J. Vitam. Nutr. Res. 2011, 81, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.B.J.; Barros, W.M.A.; da Silva, M.L.; Silva, J.M.L.; Souza, A.P.d.S.; da Silva, K.G.; de Sousa Fernandes, M.S.; Carneiro, A.C.B.d.F.; Toscano, A.E.; Lagranha, C.J. Impact of Vitamin D on Cognitive Functions in Healthy Individuals: A Systematic Review in Randomized Controlled Clinical Trials. Front. Psychol. 2022, 13, 987203. [Google Scholar] [CrossRef] [PubMed]
- Farghali, M.; Ruga, S.; Morsanuto, V.; Uberti, F. Can Brain Health Be Supported by Vitamin D-Based Supplements? A Critical Review. Brain Sci. 2020, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, R.E.; Toader, O.D.; Mutu, D.E.G.; Stănculescu, R.V.; Dragomir, R.E.; Toader, O.D.; Mutu, D.E.G.; Stănculescu, R.V. The Key Role of Vitamin D in Female Reproductive Health: A Narrative Review. Cureus 2024, 16, e65560. [Google Scholar] [CrossRef] [PubMed]
- Boisen, I.M.; Holt, R.; Kooij, I.; Yahyavi, S.K.; Mortensen, L.J.; Blomberg Jensen, M. Chapter 40—Vitamin D, Reproductive Endocrinology, and Male Reproductive Organ Function in Health and Disease. In Feldman and Pike’ s Vitamin D, 5th ed.; Hewison, M., Bouillon, R., Giovannucci, E., Goltzman, D., Meyer, M., Welsh, J., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 889–924. ISBN 978-0-323-91386-7. [Google Scholar]
- Dennis, N.A.; Houghton, L.A.; Pankhurst, M.W.; Harper, M.J.; McLennan, I.S. Acute Supplementation with High Dose Vitamin D3 Increases Serum Anti-Müllerian Hormone in Young Women. Nutrients 2017, 9, 719. [Google Scholar] [CrossRef]
- Meliana, A.; Salsabila, H.; Witarto, B.S.; Wahyunitisari, M.R. The Role of Adequate Vitamin D Levels in the Menstrual Cycle of Reproductive-Age Women. Maj. Obstet. Ginekol. 2022, 30, 154–160. [Google Scholar] [CrossRef]
- Morgante, G.; Darino, I.; Spanò, A.; Luisi, S.; Luddi, A.; Piomboni, P.; Governini, L.; De Leo, V. PCOS Physiopathology and Vitamin D Deficiency: Biological Insights and Perspectives for Treatment. J. Clin. Med. 2022, 11, 4509. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, J.; Wan, Q.; Huang, J.; Han, T.; Qu, T.; Yu, L. Influence of Vitamin D Supplementation on Reproductive Outcomes of Infertile Patients: A Systematic Review and Meta-Analysis. Reprod. Biol. Endocrinol. 2023, 21, 17. [Google Scholar] [CrossRef]
- Godswill, A.G.; Somtochukwu, I.V.; Ikechukwu, A.O.; Kate, E.C. Health Benefits of Micronutrients (Vitamins and Minerals) and Their Associated Deficiency Diseases: A Systematic Review. Int. J. Food Sci. 2020, 3, 1–32. [Google Scholar] [CrossRef]
- Rosenfeld, L. A golden age of clinical chemistry: 1948-1960. Clin Chem. 2000, 46, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- WHO. Best Practices in Phlebotomy. In WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy; World Health Organization: Geneva, Switzerland, 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK138665/ (accessed on 31 July 2024).
- Maroto-García, J.; Deza, S.; Fuentes-Bullejos, P.; Fernández-Tomás, P.; Martínez-Espartosa, D.; Marcos-Jubilar, M.; Varo, N.; González, Á. Analysis of Common Biomarkers in Capillary Blood in Routine Clinical Laboratory. Preanalytical and Analytical Comparison with Venous Blood. Diagnosis 2023, 10, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.S.F.; McKeage, J.W.; Xu, J.; Ruddy, B.P.; Nielsen, P.M.F.; Taberner, A.J. Minimally Invasive Capillary Blood Sampling Methods. Expert Rev. Med. Devices 2023, 20, 5–16. [Google Scholar] [CrossRef]
- Scriver, C.R.; Scriver, C.R. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants, by Robert Guthrie and Ada Susi, Pediatrics. Pediatrics 1998, 102, 236–237. [Google Scholar] [CrossRef]
- Lehmann, S.; Delaby, C.; Vialaret, J.; Ducos, J.; Hirtz, C. Current and Future Use of “Dried Blood Spot” Analyses in Clinical Chemistry. Clin. Chem. Lab. Med. 2013, 51, 1897–1909. [Google Scholar] [CrossRef]
- Lorenz, T.K. Autonomic, Endocrine, and Psychological Stress Responses to Different Forms of Blood Draw. PLoS ONE 2021, 16, e0257110. [Google Scholar] [CrossRef] [PubMed]
- Naseri, S.; Lerma, K.; Blumenthal, P.D. Comparative Assessment of Serum versus Menstrual Blood for Diagnostic Purposes: A Pilot Study. J. Clin. Lab. Med. 2019, 4, 10–16966. [Google Scholar] [CrossRef]
- Warren, L.A.; Shih, A.; Renteira, S.M.; Seckin, T.; Blau, B.; Simpfendorfer, K.; Lee, A.; Metz, C.N.; Gregersen, P.K. Analysis of Menstrual Effluent: Diagnostic Potential for Endometriosis. Mol. Med. Camb. Mass 2018, 24, 1. [Google Scholar] [CrossRef]
- Zaheer, A.; Komel, A.; Abu Bakr, M.B.; Singh, A.K.; Saji, A.S.; Kharal, M.M.; Ahsan, A.; Khan, M.H.; Akbar, A. Potential for and Challenges of Menstrual Blood as a Non-Invasive Diagnostic Specimen: Current Status and Future Directions. Ann. Med. Surg. 2024, 86, 4591–4600. [Google Scholar] [CrossRef]
- Critchley, H.O.D.; Babayev, E.; Bulun, S.E.; Clark, S.; Garcia-Grau, I.; Gregersen, P.K.; Kilcoyne, A.; Kim, J.-Y.J.; Lavender, M.; Marsh, E.E.; et al. Menstruation: Science and Society. Am. J. Obstet. Gynecol. 2020, 223, 624–664. [Google Scholar] [CrossRef]
- Wyatt, K.A.; Filby, C.E.; Davies-Tuck, M.L.; Suke, S.G.; Evans, J.; Gargett, C.E. Menstrual Fluid Endometrial Stem/Progenitor Cell and Supernatant Protein Content: Cyclical Variation and Indicative Range. Hum. Reprod. 2021, 36, 2215–2229. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, B.; Prinz, M.; Siegel, D. Proteomic Analysis of Menstrual Blood. Mol. Cell. Proteom. 2012, 11, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Liu, Y.; Yan, L.; Zhang, Y.; Li, Y.; Zhu, Q.; Xia, W.; Ge, S.; Zhang, J. DIA-Based Analysis of the Menstrual Blood Proteome Identifies Association between CXCL5 and IL1RN and Endometriosis. J. Proteom. 2023, 289, 104995. [Google Scholar] [CrossRef]
- Naseri, S.; Cruz, G.I.; Young, S.; Blumenthal, P.D. Screening for High Risk Human Papillomavirus Using Passively Self-Collected Menstrual Blood [A91]. Obstet. Gynecol. 2022, 139, 27S. [Google Scholar] [CrossRef]
- Naseri, S.; Rosenberg-Hasson, Y.; Maecker, H.T.; Avrutsky, M.I.; Blumenthal, P.D. A Cross-Sectional Study Comparing the Inflammatory Profile of Menstrual Effluent vs. Peripheral Blood. Health Sci. Rep. 2023, 6, e1038. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.C.; Au, T.C.C.; Chan, S.C.S.; Ng, L.P.W.; Tsang, H.F. Menstrual Blood Human Papillomavirus DNA and TAP1 Gene Polymorphisms as Potential Biomarkers for Screening and Monitoring of Cervical Squamous Intraepithelial Lesion. J. Infect. Dis. 2018, 218, 1739–1745. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, X.; Chen, Y.; Huang, S.; Cui, Z.; Tian, R.; Zeng, Z.; Liang, W.; Gong, Q.; Shang, R.; et al. Feasibility and Accuracy of Menstrual Blood Testing for High-Risk Human Papillomavirus Detection with Capture Sequencing. JAMA Netw. Open 2021, 4, e2140644. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.P.; Fraser, I.S.; Caterson, I.; Grivas, A.; McCarron, G.; Norman, T.; Tan, K. Reproductive Hormones in Menstrual Blood. J. Clin. Endocrinol. Metab. 1989, 69, 338–342. [Google Scholar] [CrossRef]
- Naseri, S.; Avrutsky, M.I.; Capati, C.; Desai, K.; Alvero, R.; Blumenthal, P.D. Concordance of Hemoglobin A1c and Reproductive Hormone Levels in Menstrual and Venous Blood. FS Rep. 2024, 5, 33–39. [Google Scholar] [CrossRef]
- Naseri, S.; Brewster, R.C.L.; Blumenthal, P.D. Novel Use of Menstrual Blood for Monitoring Glycaemic Control in Patients with Diabetes: A Proof-of-Concept Study. BMJ Sex. Reprod. Health 2022, 48, 123–127. [Google Scholar] [CrossRef]
- Qvin—Empowering Women. Period. Available online: https://qvin.com/ (accessed on 2 July 2024).
- Crona Guterstam, Y.; Strunz, B.; Ivarsson, M.A.; Zimmer, C.; Melin, A.; Jonasson, A.F.; Björkström, N.K.; Gidlöf, S.B. The Cytokine Profile of Menstrual Blood. Acta Obstet. Gynecol. Scand. 2021, 100, 339–346. [Google Scholar] [CrossRef]
- Burnhill, M.S.; Birnberg, C.H. The contents of menstrual fluid: An analysis of 260 samples from human females. Am. J. Obstet. Gynecol. 1965, 92, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Fraser, I.S.; Warner, P.; Marantos, P.A. Estimating Menstrual Blood Loss in Women with Normal and Excessive Menstrual Fluid Volume. Obstet. Gynecol. 2001, 98, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Fraser, I.S.; McCarron, G.; Markham, R.; Resta, T. Blood and Total Fluid Content of Menstrual Discharge. Obstet. Gynecol. 1985, 65, 194–198. [Google Scholar] [PubMed]
- Van der Molen, R.G.; Schutten, J.H.F.; van Cranenbroek, B.; ter Meer, M.; Donckers, J.; Scholten, R.R.; van der Heijden, O.W.H.; Spaanderman, M.E.A.; Joosten, I. Menstrual Blood Closely Resembles the Uterine Immune Micro-Environment and Is Clearly Distinct from Peripheral Blood. Hum. Reprod. 2014, 29, 303–314. [Google Scholar] [CrossRef]
- Craft, N.E.; Bulux, J.; Valdez, C.; Li, Y.; Solomons, N.W. Retinol Concentrations in Capillary Dried Blood Spots from Healthy Volunteers: Method Validation123. Am. J. Clin. Nutr. 2000, 72, 450–454. [Google Scholar] [CrossRef]
- Hocher, B.; Grön, H.J.; Schumann, C.; Tsuprykov, O.; Seifert, S.; Hitzler, W.E.; Armbruster, F.P. Vitamin D Status from Dried Capillary Blood Samples. Clin. Lab. 2013, 59, 851. [Google Scholar] [CrossRef]
- Jiao, X.; Yuan, Y.; Wang, X.; Li, J.; Liu, B.; Yuan, T.; Yu, X. Development of a Sensitive HPLC-MS/MS Method for 25-Hydroxyvitamin D2 and D3 Measurement in Capillary Blood. J. Clin. Lab. Anal. 2020, 34, e23451. [Google Scholar] [CrossRef]
- Jensen, M.E.; Ducharme, F.M.; Théorêt, Y.; Bélanger, A.-S.; Delvin, E. Assessing Vitamin D Nutritional Status: Is Capillary Blood Adequate? Clin. Chim. Acta Int. J. Clin. Chem. 2016, 457, 59–62. [Google Scholar] [CrossRef]
- Dayre McNally, J.; Matheson, L.A.; Sankaran, K.; Rosenberg, A.M. Capillary Blood Sampling as an Alternative to Venipuncture in the Assessment of Serum 25 Hydroxyvitamin D Levels. J. Steroid Biochem. Mol. Biol. 2008, 112, 164–168. [Google Scholar] [CrossRef]
- Srinivasan, L.; Khilnani, R.; Fung, P.; Low, M.; Esquivel, U.; White, B.; Sidhu, G.; Rangel, J.; Lim, S.A.; Sudhof, L.S.; et al. Population-Scale Prediabetic Assessment Using HbA1c from Menstrual Blood. bioRxiv 2019, 758805. [Google Scholar] [CrossRef]
- American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.; Ozgoli, G.; Rahnemaie, F.S. A Systematic Review of the Role of Vitamin D and Calcium in Premenstrual Syndrome. Obstet. Gynecol. Sci. 2019, 62, 73–86. [Google Scholar] [CrossRef]
- Amzajerdi, A.; Keshavarz, M.; Ghorbali, E.; Pezaro, S.; Sarvi, F. The Effect of Vitamin D on the Severity of Dysmenorrhea and Menstrual Blood Loss: A Randomized Clinical Trial. BMC Womens Health 2023, 23, 138. [Google Scholar] [CrossRef]
- Krul-Poel, Y.H.M.; Koenders, P.P.; Steegers-Theunissen, R.P.; Ten Boekel, E.; ter Wee, M.M.; Louwers, Y.; Lips, P.; Laven, J.S.E.; Simsek, S. Vitamin D and Metabolic Disturbances in Polycystic Ovary Syndrome (PCOS): A Cross-Sectional Study. PLoS ONE 2018, 13, e0204748. [Google Scholar] [CrossRef]
- Kowalówka, M.; Główka, A.K.; Karaźniewicz-Łada, M.; Kosewski, G. Clinical Significance of Analysis of Vitamin D Status in Various Diseases. Nutrients 2020, 12, 2788. [Google Scholar] [CrossRef]
- Sinbad, O.O.; Folorunsho, A.A.; Olabisi, O.L.; Ayoola, O.A.; Temitope, E.J. Vitamins as Antioxidants. J. Food Sci. Nutr. Res. 2019, 2, 214–235. [Google Scholar] [CrossRef]
- Shimizu, H.; Tsubota, T.; Kanki, K.; Shiota, G. All-Trans Retinoic Acid Ameliorates Hepatic Stellate Cell Activation via Suppression of Thioredoxin Interacting Protein Expression. J. Cell. Physiol. 2018, 233, 607–616. [Google Scholar] [CrossRef]
- Gad, A.; Abu Hamed, S.; Khalifa, M.; Amin, A.; El-Sayed, A.; Swiefy, S.A.; El-Assal, S. Retinoic Acid Improves Maturation Rate and Upregulates the Expression of Antioxidant-Related Genes in in Vitro Matured Buffalo (Bubalus bubalis) Oocytes. Int. J. Vet. Sci. Med. 2018, 6, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H. Vitamin D Is a Membrane Antioxidant. Ability to Inhibit Iron-Dependent Lipid Peroxidation in Liposomes Compared to Cholesterol, Ergosterol and Tamoxifen and Relevance to Anticancer Action. FEBS Lett. 1993, 326, 285–288. [Google Scholar] [CrossRef]
- Salum, E.; Kals, J.; Kampus, P.; Salum, T.; Zilmer, K.; Aunapuu, M.; Arend, A.; Eha, J.; Zilmer, M. Vitamin D Reduces Deposition of Advanced Glycation End-Products in the Aortic Wall and Systemic Oxidative Stress in Diabetic Rats. Diabetes Res. Clin. Pract. 2013, 100, 243–249. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and Human Health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Wang, Q.; He, C. Dietary Vitamin A Intake and the Risk of Ovarian Cancer: A Meta-Analysis. Biosci. Rep. 2020, 40, BSR20193979. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Minisola, S.; Colangelo, L.; Pepe, J.; Diacinti, D.; Cipriani, C.; Rao, S.D. Osteomalacia and Vitamin D Status: A Clinical Update 2020. JBMR Plus 2021, 5, e10447. [Google Scholar] [CrossRef] [PubMed]
- Rabelink, N.M.; Westgeest, H.M.; Bravenboer, N.; Jacobs, M.A.J.M.; Lips, P. Bone Pain and Extremely Low Bone Mineral Density Due to Severe Vitamin D Deficiency in Celiac Disease. Arch. Osteoporos. 2011, 6, 209–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harrison, S.R.; Li, D.; Jeffery, L.E.; Raza, K.; Hewison, M. Vitamin D, Autoimmune Disease and Rheumatoid Arthritis. Calcif. Tissue Int. 2020, 106, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Arayici, M.E.; Basbinar, Y.; Ellidokuz, H. Vitamin D Intake, Serum 25-Hydroxyvitamin-D (25(OH)D) Levels, and Cancer Risk: A Comprehensive Meta-Meta-Analysis Including Meta-Analyses of Randomized Controlled Trials and Observational Epidemiological Studies. Nutrients 2023, 15, 2722. [Google Scholar] [CrossRef]
- Deuster, E.; Jeschke, U.; Ye, Y.; Mahner, S.; Czogalla, B. Vitamin D and VDR in Gynecological Cancers—A Systematic Review. Int. J. Mol. Sci. 2017, 18, 2328. [Google Scholar] [CrossRef]
- Chen, Y.; Zhi, X. Roles of Vitamin D in Reproductive Systems and Assisted Reproductive Technology. Endocrinology 2020, 161, bqaa023. [Google Scholar] [CrossRef]
- Várbíró, S.; Takács, I.; Tűű, L.; Nas, K.; Sziva, R.E.; Hetthéssy, J.R.; Török, M. Effects of Vitamin D on Fertility, Pregnancy and Polycystic Ovary Syndrome—A Review. Nutrients 2022, 14, 1649. [Google Scholar] [CrossRef]
- Mohan, A.; Haider, R.; Fakhor, H.; Hina, F.; Kumar, V.; Jawed, A.; Majumder, K.; Ayaz, A.; Lal, P.M.; Tejwaney, U.; et al. Vitamin D and Polycystic Ovary Syndrome (PCOS): A Review. Ann. Med. Surg. 2023, 85, 3506. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, B.K.; Simon-Collins, M.; Nylander, E.; Segars, J.; Singh, B. A Systematic Review of Vitamin D and Endometriosis: Role in Pathophysiology, Diagnosis, Treatment, and Prevention. FS Rev. 2023, 4, 1–14. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, C.; Wang, D.; Li, B.; Shan, Z.; Teng, W.; Li, J. Causal Association between Low Vitamin D and Polycystic Ovary Syndrome: A Bidirectional Mendelian Randomization Study. J. Ovarian Res. 2024, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Ursache, A.; Lozneanu, L.; Bujor, I.E.; Mandici, C.E.; Boiculese, L.V.; Bausic, A.I.G.; Grigore, M.; Socolov, D.; Matasariu, D.R. Vitamin D—The Iceberg in Endometriosis—Review and Meta-Analysis. J. Pers. Med. 2024, 14, 119. [Google Scholar] [CrossRef]
- Qureshi, G.A. Effects of 1,25-Dihydroxy Vitamin D3 on Endometriosis. J. Clin. Endocrinol. Metab. 2016, 101, 2371–2379. [Google Scholar] [CrossRef]
- Anastasi, E.; Fuggetta, E.; Vito, C.D.; Migliara, G.; Viggiani, V.; Manganaro, L.; Granato, T.; Panici, P.B.; Angeloni, A.; Porpora, M.G. Low Levels of 25-OH Vitamin D in Women with Endometriosis and Associated Pelvic Pain. Clin. Chem. Lab. Med. 2017, 55, e282–e284. [Google Scholar] [CrossRef]
- Baek, J.C.; Jo, J.Y.; Lee, S.M.; Cho, I.A.; Shin, J.K.; Lee, S.A.; Lee, J.H.; Cho, M.-C.; Choi, W.J. Differences in 25-Hydroxy Vitamin D and Vitamin D-Binding Protein Concentrations According to the Severity of Endometriosis. Clin. Exp. Reprod. Med. 2019, 46, 125–131. [Google Scholar] [CrossRef]
- Hager, M.; Wenzl, R.; Riesenhuber, S.; Marschalek, J.; Kuessel, L.; Mayrhofer, D.; Ristl, R.; Kurz, C.; Ott, J. The Prevalence of Incidental Endometriosis in Women Undergoing Laparoscopic Ovarian Drilling for Clomiphene-Resistant Polycystic Ovary Syndrome: A Retrospective Cohort Study and Meta-Analysis. J. Clin. Med. 2019, 8, 1210. [Google Scholar] [CrossRef]
- Delbandi, A.-A.; Torab, M.; Abdollahi, E.; Khodaverdi, S.; Rokhgireh, S.; Moradi, Z.; Heidari, S.; Mohammadi, T. Vitamin D Deficiency as a Risk Factor for Endometriosis in Iranian Women. J. Reprod. Immunol. 2021, 143, 103266. [Google Scholar] [CrossRef]
- Bouzid, K.; Bourdon, M.; Bartkowski, R.; Verbanck, M.; Chapron, C.; Marcellin, L.; Batteux, F.; Santulli, P.; Doridot, L. Menstrual Blood Donation for Endometriosis Research: A Cross-Sectional Survey on Women’s Willingness and Potential Barriers. Reprod. Sci. 2024, 31, 1617–1625. [Google Scholar] [CrossRef]
- Van Eijk, A.M.; Zulaika, G.; Lenchner, M.; Mason, L.; Sivakami, M.; Nyothach, E.; Unger, H.; Laserson, K.; Phillips-Howard, P.A. Menstrual Cup Use, Leakage, Acceptability, Safety, and Availability: A Systematic Review and Meta-Analysis. Lancet Public Health 2019, 4, e376–e393. [Google Scholar] [CrossRef] [PubMed]
- Menstrual Cup Market Analysis, Trends|Forecast—2032. Available online: https://www.gminsights.com/industry-analysis/menstrual-cup-market (accessed on 11 October 2024).
- Wilhelm, A.J.; den Burger, J.C.G.; Swart, E.L. Therapeutic Drug Monitoring by Dried Blood Spot: Progress to Date and Future Directions. Clin. Pharmacokinet. 2014, 53, 961–973. [Google Scholar] [CrossRef]
- Zhang, T.; O’Connor, C.; Sheridan, H.; Barlow, J.W. Vitamin K2 in Health and Disease: A Clinical Perspective. Foods 2024, 13, 1646. [Google Scholar] [CrossRef] [PubMed]
- Schroder, T.H.; Quay, T.A.W.; Lamers, Y. Methylmalonic Acid Quantified in Dried Blood Spots Provides a Precise, Valid, and Stable Measure of Functional Vitamin B-12 Status in Healthy Women. J. Nutr. 2014, 144, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liang, C.; Kong, Z.; Su, Y.; Ren, W.; Dong, H.; Wu, Y.; Yang, N.; Liu, R.; Wu, J.; et al. Determination of Vitamin K1, MK-4, MK-7, and D Levels in Human Serum of Postmenopausal Osteoporosis Women Based on High Stability LC-MS/MS: MK-7 May Be a New Marker of Bone Metabolism. Ann. Nutr. Metab. 2023, 79, 334–342. [Google Scholar] [CrossRef]
- Kaplan, P.; Ficicioglu, C.; Mazur, A.T.; Palmieri, M.J.; Berry, G.T. Liver Transplantation Is Not Curative for Methylmalonic Acidopathy Caused by Methylmalonyl-CoA Mutase Deficiency. Mol. Genet. Metab. 2006, 88, 322–326. [Google Scholar] [CrossRef]
- Schechter, A.N. Hemoglobin Research and the Origins of Molecular Medicine. Blood 2008, 112, 3927–3938. [Google Scholar] [CrossRef]
- DeSouza, L.; Diehl, G.; Yang, E.C.C.; Guo, J.; Rodrigues, M.J.; Romaschin, A.D.; Colgan, T.J.; Siu, K.W.M. Proteomic Analysis of the Proliferative and Secretory Phases of the Human Endometrium: Protein Identification and Differential Protein Expression. Proteomics 2005, 5, 270–281. [Google Scholar] [CrossRef]
- Verma, P. Detection and Comparison of Normal and Menstrual Blood Samples Found at Crime Scene. J. Forensic Sci. Crim. Investig. 2018, 9, 2233–2236. [Google Scholar] [CrossRef]
- Weber, A.; Wójtowicz, A.; Lednev, I.K. Post Deposition Aging of Bloodstains Probed by Steady-State Fluorescence Spectroscopy. J. Photochem. Photobiol. B 2021, 221, 112251. [Google Scholar] [CrossRef]
- Yang, H.; Xu, L.; Hou, L.; Xu, T.C.; Ye, S.H. Stability of Vitamin A, E, C and Thiamine during Storage of Different Powdered Enteral Formulas. Heliyon 2022, 8, e11460. [Google Scholar] [CrossRef] [PubMed]
- Colak, A.; Toprak, B.; Dogan, N.; Ustuner, F. Effect of Sample Type, Centrifugation and Storage Conditions on Vitamin D Concentration. Biochem. Medica 2013, 23, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Reifen, R. Vitamin A as an Anti-Inflammatory Agent. Proc. Nutr. Soc. 2002, 61, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, L.; Xu, H.-J.; Li, Y.; Hu, C.-M.; Yang, J.-Y.; Sun, M.-Y. The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2736. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Feldman, D. Mechanisms of the Anti-Cancer and Anti-Inflammatory Actions of Vitamin, D. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 311–336. [Google Scholar] [CrossRef]
- Bonatz, G.; Hansmann, M.L.; Buchholz, F.; Mettler, L.; Radzun, H.J.; Semm, K. Macrophage- and Lymphocyte-Subtypes in the Endometrium during Different Phases of the Ovarian Cycle. Int. J. Gynecol. Obstet. 1992, 37, 29–36. [Google Scholar] [CrossRef]
- Evans, J.; Salamonsen, L.A. Inflammation, Leukocytes and Menstruation. Rev. Endocr. Metab. Disord. 2012, 13, 277–288. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Handa, Y.; Uematsu, Y.; Takeda, S.; Sekine, K.; Yoshihara, Y.; Kawakami, T.; Arioka, K.; Sato, H.; Uchiyama, Y.; et al. Mice Lacking the Vitamin D Receptor Exhibit Impaired Bone Formation, Uterine Hypoplasia and Growth Retardation after Weaning. Nat. Genet. 1997, 16, 391–396. [Google Scholar] [CrossRef]
- Cermisoni, G.C.; Alteri, A.; Corti, L.; Rabellotti, E.; Papaleo, E.; Viganò, P.; Sanchez, A.M. Vitamin D and Endometrium: A Systematic Review of a Neglected Area of Research. Int. J. Mol. Sci. 2018, 19, 2320. [Google Scholar] [CrossRef]
- Viganò, P.; Lattuada, D.; Mangioni, S.; Ermellino, L.; Vignali, M.; Caporizzo, E.; Panina-Bordignon, P.; Besozzi, M.; Di Blasio, A.M. Cycling and Early Pregnant Endometrium as a Site of Regulated Expression of the Vitamin D System. J. Mol. Endocrinol. 2006, 36, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Cordes, T.; Diesing, D.; Diedrich, K.; Friedrich, M. Expression of 25 Hydroxyvitamin D3-1α-Hydroxylase in Human Endometrial Tissue. J. Steroid Biochem. Mol. Biol. 2007, 103, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Chu, C.; Doebis, C.; von Baehr, V.; Hocher, B. Reference Values for Free 25-Hydroxy-Vitamin D Based on Established Total 25-Hydroxy-Vitamin D Reference Values. J. Steroid Biochem. Mol. Biol. 2021, 210, 105877. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Reddy, A.P.; Jacob, T.; Thomas, A.; Schneider, K.A.; Dasari, S.; Lapidus, J.A.; Lu, X.; Rodland, M.; Roberts, C.T.; et al. Identification of Novel Protein Biomarkers of Preterm Birth in Human Cervical−Vaginal Fluid. J. Proteome Res. 2007, 6, 1269–1276. [Google Scholar] [CrossRef]
- Fernando, M.; Ellery, S.J.; Marquina, C.; Lim, S.; Naderpoor, N.; Mousa, A. Vitamin D-Binding Protein in Pregnancy and Reproductive Health. Nutrients 2020, 12, 1489. [Google Scholar] [CrossRef]
- Bischof, M.G.; Heinze, G.; Vierhapper, H. Vitamin D Status and Its Relation to Age and Body Mass Index. Horm. Res. 2006, 66, 211–215. [Google Scholar] [CrossRef]
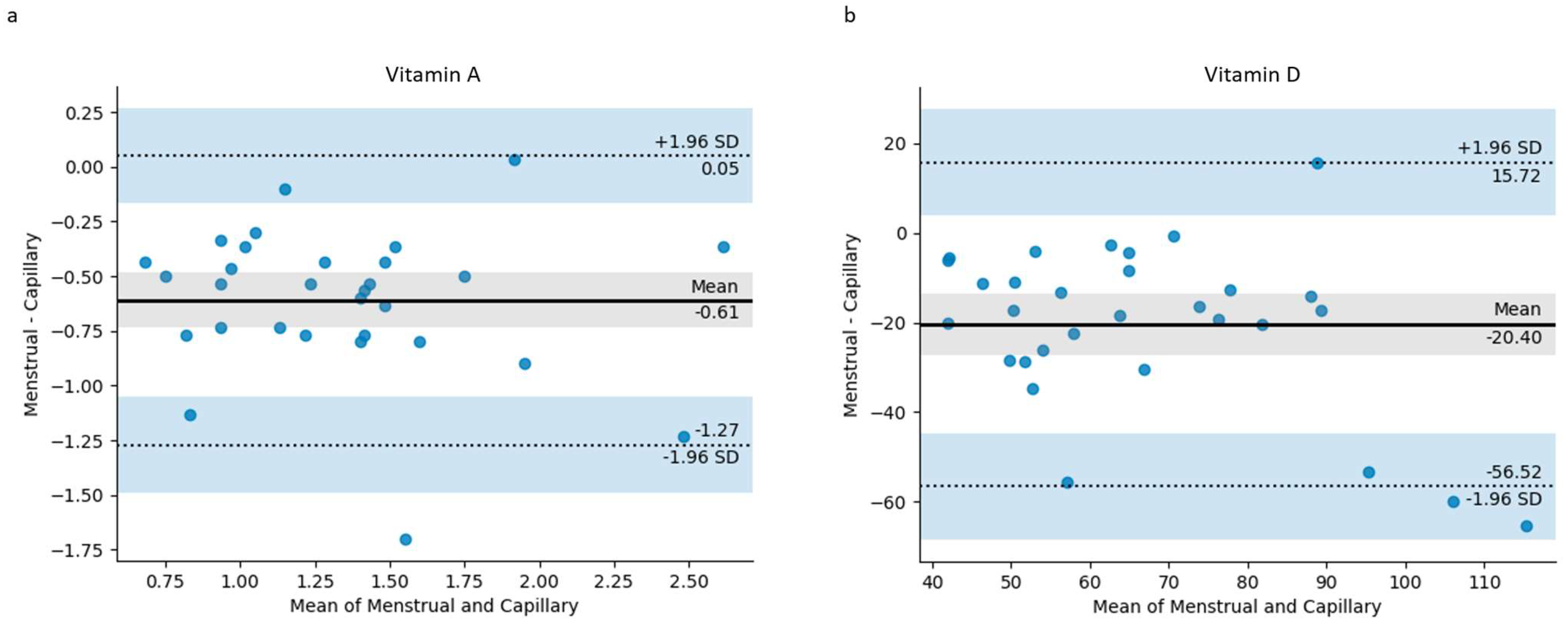
| Biomarker (Units) | Capillary Blood Mean ± SD | Menstrual Blood Mean ± SD | Paired t-Test (p < 0.05) | Mean Difference | 95% Confidence Interval of the Mean Difference |
|---|---|---|---|---|---|
| Vitamin A (µmol/L) | 1.65 ± 0.52 | 1.04 ± 0.47 | <0.001 | 0.61 | 0.36–0.86 |
| Vitamin D (nmol/L) | 76.60 ± 24.51 | 56.20 ± 17.76 | <0.001 | 20.40 | 9.57–31.23 |
| Hemoglobin (g/dL) | 12.12 ± 1.12 | 6.76 ± 2.93 | <0.001 | 5.36 | 4.23–6.49 |
| Biomarker | Pearson’s Correlation Coefficient (r) | p-Value | 95% Confidence Interval |
|---|---|---|---|
| Vitamin A | 0.77 | <0.001 | 0.57–0.89 |
| Vitamin D | 0.66 | <0.001 | 0.40–0.83 |
| Hemoglobin | 0.27 | 0.14 | −0.10–0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whitbread, A.L.; Mittelmeier, L.; Rao, R.P.; Mittelmeier, W.; Osmanski-Zenk, K. Menstrual Blood as a Non-Invasive Alternative for Monitoring Vitamin Levels. J. Clin. Med. 2024, 13, 7212. https://doi.org/10.3390/jcm13237212
Whitbread AL, Mittelmeier L, Rao RP, Mittelmeier W, Osmanski-Zenk K. Menstrual Blood as a Non-Invasive Alternative for Monitoring Vitamin Levels. Journal of Clinical Medicine. 2024; 13(23):7212. https://doi.org/10.3390/jcm13237212
Chicago/Turabian StyleWhitbread, Amy L., Lucas Mittelmeier, Rajnish P. Rao, Wolfram Mittelmeier, and Katrin Osmanski-Zenk. 2024. "Menstrual Blood as a Non-Invasive Alternative for Monitoring Vitamin Levels" Journal of Clinical Medicine 13, no. 23: 7212. https://doi.org/10.3390/jcm13237212
APA StyleWhitbread, A. L., Mittelmeier, L., Rao, R. P., Mittelmeier, W., & Osmanski-Zenk, K. (2024). Menstrual Blood as a Non-Invasive Alternative for Monitoring Vitamin Levels. Journal of Clinical Medicine, 13(23), 7212. https://doi.org/10.3390/jcm13237212






