Endothelial Glycocalyx Anomalies and Ocular Manifestations in Patients with Post-Acute COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Optical Coherence Tomography (B-Scan) and OCT-A
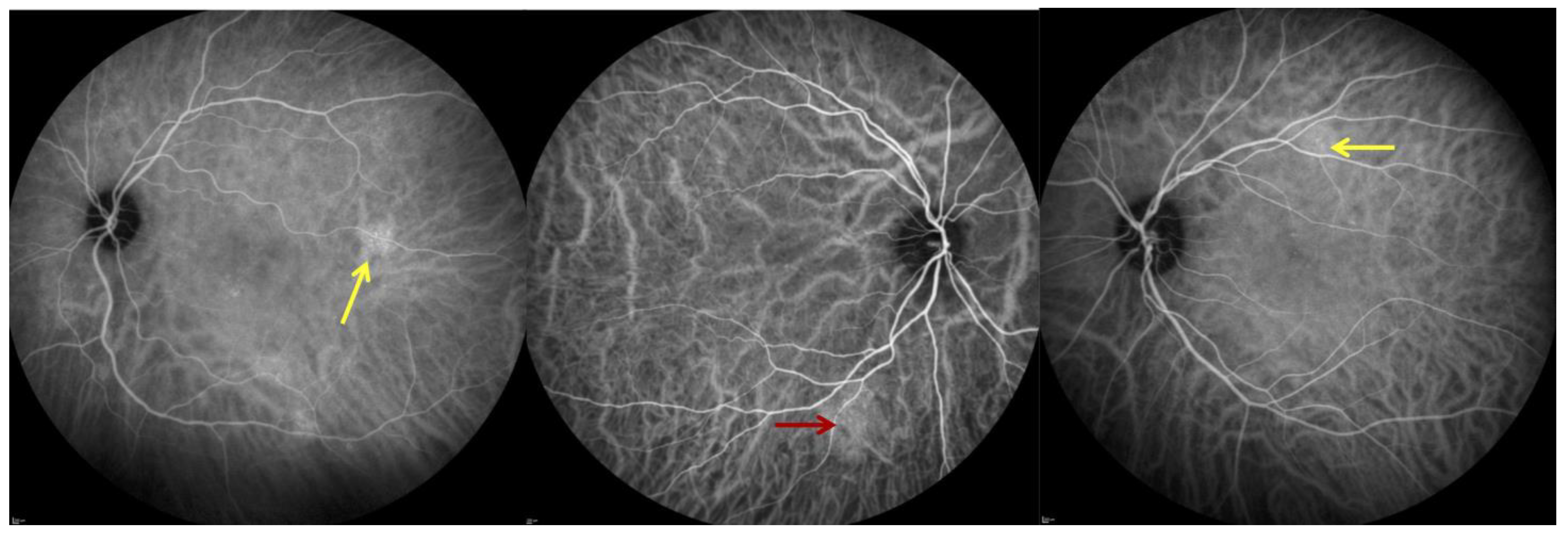
2.4. Indocyanine Green Angiography (ICGA)
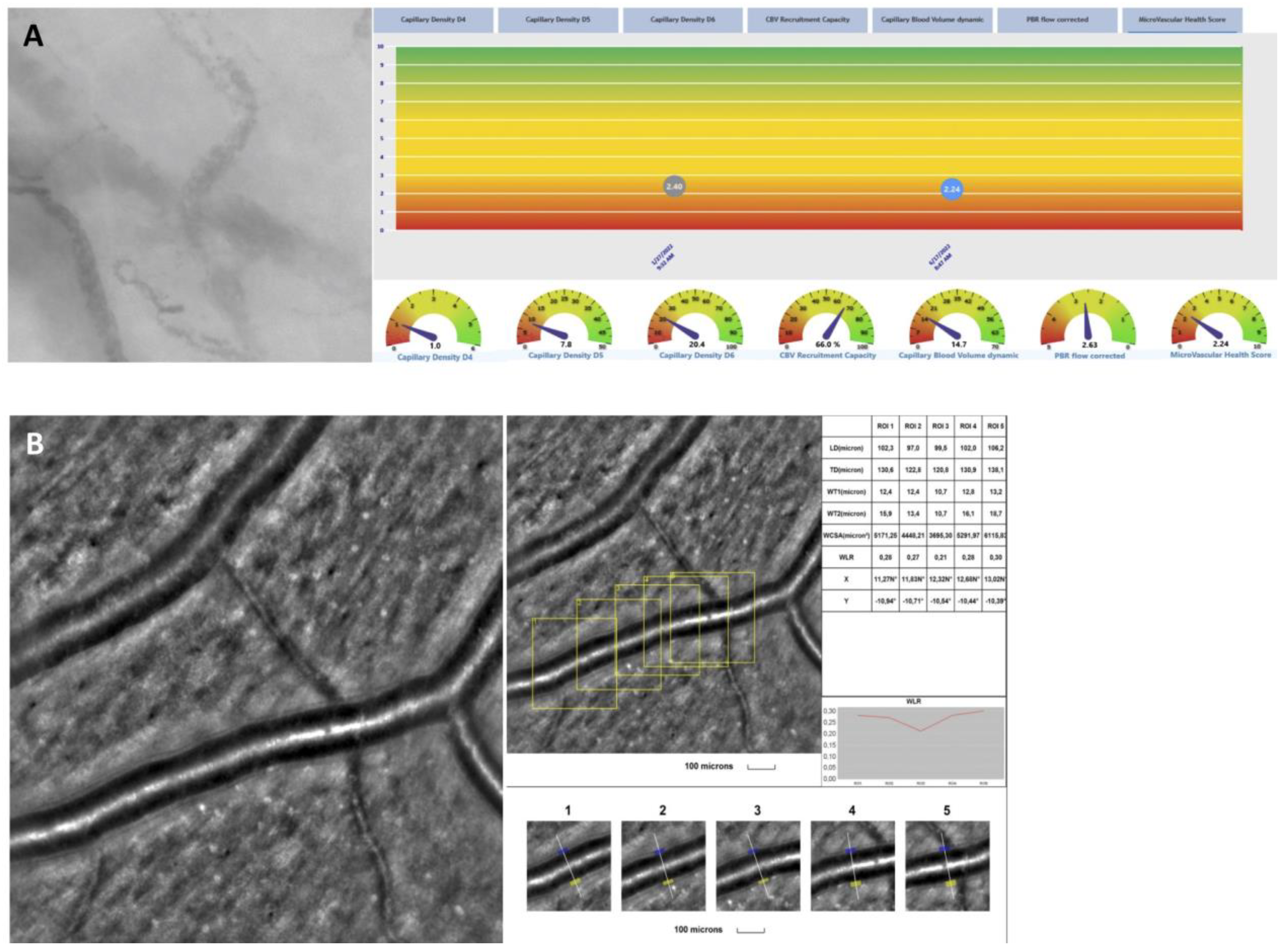
2.5. rtx1 Examination Protocol and Parameter Evaluation
2.6. In Vivo Assessment of the Microcirculation and Glycocalyx Dimensions
2.7. Statistical Analysis
3. Results
3.1. Study Population and Characteristics of Ocular Findings
3.2. Adaptive Optics
3.3. Sublingual Glycocalyx Parameters
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Callard, F.; Perego, E. How and why patients made Long COVID. Soc. Sci. Med. 2021, 268, 113426. [Google Scholar] [CrossRef] [PubMed]
- Parotto, M.; Gyöngyösi, M.; Howe, K.; Myatra, S.N.; Ranzani, O.; Shankar-Hari, M.; Herridge, M.S. Post-acute sequelae of COVID-19: Understanding and addressing the burden of multisystem manifestations. Lancet Respir. Med. 2023, 11, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Shah, W.; Hillman, T.; Playford, E.D.; Hishmeh, L. Managing the long term effects of COVID-19: Summary of NICE, SIGN, and RCGP rapid guideline. BMJ 2021, 372, n136. [Google Scholar] [CrossRef]
- Seeßle, J.; Waterboer, T.; Hippchen, T.; Simon, J.; Kirchner, M.; Lim, A.; Müller, B.; Merle, U. Persistent Symptoms in Adult Patients 1 Year After Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study. Clin. Infect. Dis. 2021, 74, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Morrone, M.C.; Patrono, C.; Santoro, M.G.; Schiaffino, S.; Remuzzi, G.; Bussolati, G.; Cappuccinelli, P.; Fitzgerald, G.; Bacci, M.L.; et al. Long COVID: Where we stand and challenges ahead. Cell Death Differ. 2022, 29, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Pacella, F.; Pacella, E.; Tiscione, G.; Oliva, A.; Violi, F. Conjunctivitis and COVID-19: A meta-analysis. J. Med. Virol. 2020, 92, 1413–1414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Stewart, J.M. Retinal and choroidal manifestations of COVID-19. Curr. Opin. Ophthalmol. 2021, 32, 536–540. [Google Scholar] [CrossRef]
- Marinho, P.M.; Marcos, A.A.A.; Romano, A.C.; Nascimento, H.; Belfort, R. Retinal findings in patients with COVID-19. Lancet 2020, 395, 1610. [Google Scholar] [CrossRef]
- Azar, G.; Bonnin, S.; Vasseur, V.; Faure, C.; Salviat, F.; Clermont, C.V.; Titah, C.; Farès, S.; Boulanger, E.; Derrien, S.; et al. Did the COVID-19 Pandemic Increase the Incidence of Acute Macular Neuroretinopathy? J. Clin. Med. 2021, 10, 5038. [Google Scholar] [CrossRef]
- Abdelmassih, Y.; Azar, G.; Bonnin, S.; Timsit, C.S.; Vasseur, V.; Spaide, R.F.; Behar-Cohen, F.; Mauget-Faysse, M. COVID-19 Associated Choroidopathy. J. Clin. Med. 2021, 10, 4686. [Google Scholar] [CrossRef]
- Tohamy, D.; Sharaf, M.; Abdelazeem, K.; Saleh, M.G.; Rateb, M.F.; Soliman, W.; Kedwany, S.M.; Abdelmalek, M.O.; Medhat, M.A.; Tohamy, A.M.; et al. Ocular Manifestations of Post-Acute COVID-19 Syndrome, Upper Egypt Early Report. J. Multidiscip. Health 2021, 14, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Goonewardena, S.N.; Grushko, O.G.; Wells, J.; Herty, L.; Rosenson, R.S.; Haus, J.M.; Hummel, S.L. Immune-Mediated Glycocalyx Remodeling in Hospitalized COVID-19 Patients. Cardiovasc. Drugs Ther. 2023, 37, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka-Tojo, M. Endothelial glycocalyx damage as a systemic inflammatory microvascular endotheliopathy in COVID-19. Biomed. J. 2020, 43, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, M.K.; Winther, S.A.; Hansen, T.W.; Diaz, L.J.; Persson, F.; Rossing, P.; Frimodt-Møller, M. Assessment of the sublingual microcirculation with the GlycoCheck system: Reproducibility and examination conditions. PLoS ONE 2020, 15, e0243737. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. The Ambiguity of Pachychoroid. Retina 2020, 41, 231–237. [Google Scholar] [CrossRef]
- Zaleska-Żmijewska, A.; Wawrzyniak, Z.M.; Dąbrowska, A.; Szaflik, J.P. Adaptive Optics (rtx1) High-Resolution Imaging of Photoreceptors and Retinal Arteries in Patients with Diabetic Retinopathy. J. Diabetes Res. 2019, 2019, 9548324. [Google Scholar] [CrossRef]
- Rovas, A.; Sackarnd, J.; Rossaint, J.; Kampmeier, S.; Pavenstädt, H.; Vink, H.; Kümpers, P. Identification of novel sublingual parameters to analyze and diagnose microvascular dysfunction in sepsis: The NOSTRADAMUS study. Crit. Care 2021, 25, 112. [Google Scholar] [CrossRef]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dysfunction in ‘long COVID’: Rationale, physiology and management strategies. Clin. Med. 2021, 21, e63–e67. [Google Scholar] [CrossRef]
- Aggarwal, K.; Agarwal, A.; Jaiswal, N.; Dahiya, N.; Ahuja, A.; Mahajan, S.; Tong, L.; Duggal, M.; Singh, M.; Agrawal, R.; et al. Ocular surface manifestations of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241661. [Google Scholar] [CrossRef]
- Wan, K.H.; Lui, G.C.Y.; Poon, K.C.F.; Ng, S.S.S.; Young, A.L.; Hui, D.S.C.; Tham, C.C.Y.; Chan, P.K.S.; Pang, C.P.; Chong, K.K.L. Ocular surface disturbance in patients after acute COVID-19. Clin. Exp. Ophthalmol. 2022, 50, 398–406. [Google Scholar] [CrossRef]
- Lambadiari, V.; Mitrakou, A.; Kountouri, A.; Thymis, J.; Katogiannis, K.; Korakas, E.; Varlamos, C.; Andreadou, I.; Tsoumani, M.; Triantafyllidi, H.; et al. Association of COVID-19 with impaired endothelial glycocalyx, vascular function and myocardial deformation 4 months after infection. Eur. J. Heart Fail. 2021, 23, 1916–1926. [Google Scholar] [CrossRef] [PubMed]
- Vollenberg, R.; Tepasse, P.-R.; Ochs, K.; Floer, M.; Strauss, M.; Rennebaum, F.; Kabar, I.; Rovas, A.; Nowacki, T. Indications of Persistent Glycocalyx Damage in Convalescent COVID-19 Patients: A Prospective Multicenter Study and Hypothesis. Viruses 2021, 13, 2324. [Google Scholar] [CrossRef]
- Osiaevi, I.; Schulze, A.; Evers, G.; Harmening, K.; Vink, H.; Kümpers, P.; Mohr, M.; Rovas, A. Persistent capillary rarefication in long COVID syndrome. Angiogenesis 2023, 26, 53–61. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Lambadiari, V.; Mitrakou, A.; Kountouri, A.; Katogiannis, K.; Thymis, J.; Korakas, E.; Pavlidis, G.; Kazakou, P.; Panagopoulos, G.; et al. Myocardial work and vascular dysfunction are partially improved at 12 months after COVID-19 infection. Eur. J. Heart Fail. 2022, 24, 727–729. [Google Scholar] [CrossRef]
- Smadja, D.M.; Mentzer, S.J.; Fontenay, M.; Laffan, M.A.; Ackermann, M.; Helms, J.; Jonigk, D.; Chocron, R.; Pier, G.B.; Gendron, N.; et al. COVID-19 is a systemic vascular hemopathy: Insight for mechanistic and clinical aspects. Angiogenesis 2021, 24, 755–788. [Google Scholar] [CrossRef] [PubMed]
- Warwick, A.; Khandhadia, S.; Ennis, S.; Lotery, A. Age-Related Macular Degeneration: A Disease of Systemic or Local Complement Dysregulation? J. Clin. Med. 2014, 3, 1234–1257. [Google Scholar] [CrossRef]
- Benmansour, N.C.; Carvelli, J.; Vivier, E. Complement cascade in severe forms of COVID-19: Recent advances in therapy. Eur. J. Immunol. 2021, 51, 1652–1659. [Google Scholar] [CrossRef]
- Mullins, R.F.; Johnson, M.N.; Faidley, E.A.; Skeie, J.M.; Huang, J. Choriocapillaris Vascular Dropout Related to Density of Drusen in Human Eyes with Early Age-Related Macular Degeneration. Investig. Opthalmology Vis. Sci. 2011, 52, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Dull, R.O.; Hahn, R.G. The glycocalyx as a permeability barrier: Basic science and clinical evidence. Crit. Care 2022, 26, 273. [Google Scholar] [CrossRef]
- Cífková, R.; Harazny, J.M.; Bruthans, J.; Wohlfahrt, P.; Krajčoviechová, A.H.; Lánská, V.; Gelžinský, J.; Mateřánková, M.; Mareš, Š.; Filipovský, J.; et al. Early vascular damage in retinal microcirculation in arterial hypertension: The Czech post-MONICA study. J. Hypertens. 2023, 42, 557–563. [Google Scholar] [CrossRef]
| Overall Population (n = 44) | |
|---|---|
| Age in years (Mean ± SD) | 47.5 ± 11.5 |
| Sex: | |
| 36 (81.8%) |
| 8 (18.2%) |
| Interval following initial infection in months (Mean ± SD) | >23.0 ± 3.8 |
| Diabetes mellitus (%) | 1 (2.3%) |
| Hypertension (%) | 6 (13.6%) |
| Cardiovascular disease (%) | 1 (2.3%) |
| Thromboembolic disease (%) | 0 (0%) |
| Allergy (%) | 18 (40.9%) |
| Auto-immune disease (%) | 11 (25.0%) |
| Treatment | |
| Corticosteroids (%) | 12 (27.3%) |
| Eyes (n = 88) | Female (n = 72) | Male (n = 16) | p-Value | |
|---|---|---|---|---|
| TBUT < 5 s (%) | 39 (44.3%) | 31 (43.1%) | 8 (50.0%) | 0.61 |
| Keratitis (%) | 30 (34.1%) | 27 (37.5%) | 3 (18.8%) | 0.15 |
| Blepharitis (%) | 17 (19.3%) | 15 (20.0%) | 2 (12.5%) | 0.44 |
| Choroidal thickness in μm (Mean ± SD) | 325.2 ± 110.9 | 324.8 ± 114.3 | 326.9 ± 97.8 | 0.94 |
| Presence of pachyvessels (%) | 52 (59.1%) | 42 (58.3%) | 10 (62.5%) | 0.76 |
| OCT-A abnormal superficial capillary plexus (%) | 42 (47.7%) | 33 (45.8%) | 9 (56.3%) | 0.45 |
| OCT-A abnormal deep capillary plexus (%) | 21 (23.9%) | 17 (23.6%) | 4 (25.0%) | 0.91 |
| Hyperreflective dots (%) | 32 (36.4%) | 26 (36.1%) | 6 (37.5%) | 0.92 |
| Hemangioma-like lesions (%) | 7 (8.0%) | 6 (8.3%) | 1 (6.3%) | 0.78 |
| Hyperpermeability (%) | 47 (53.4%) | 39 (54.2%) | 8 (50.0%) | 0.76 |
| Adaptive Optic (88 Eyes) | |
|---|---|
| WLR, μm (mean ± SD) | 0.3 ± 0.0 |
| WCSA, μm2 (mean ± SD) | 3931.4 ± 907.7 |
| WT, μm (mean ± SD) | 12.1 ± 1.9 |
| GlycoCheck (44 patients) | |
| Number of exams per patient (mean ± SD) | 14.6 ± 8.7 |
| PBR, μm (mean ± SD) | 2.2 ± 0.2 |
| Flow total density D4–D25, ×10−2 mm/mm2 (mean ± SD) | 166.8 ± 63.0 |
| Capillary density D4–D6, ×10−2 mm/mm2 (mean ± SD) | 23.9 ± 12.2 |
| CBVdynamic (mean ± SD) | 13.2 ± 8.4 |
| MVHS (mean ± SD) | 2.4 ± 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azar, G.; Abdelmassih, Y.; Bonnin, S.; Guindolet, D.; Vasseur, V.; Behar Cohen, F.; Salmon, D.; Mauget-Faÿsse, M. Endothelial Glycocalyx Anomalies and Ocular Manifestations in Patients with Post-Acute COVID-19. J. Clin. Med. 2024, 13, 7272. https://doi.org/10.3390/jcm13237272
Azar G, Abdelmassih Y, Bonnin S, Guindolet D, Vasseur V, Behar Cohen F, Salmon D, Mauget-Faÿsse M. Endothelial Glycocalyx Anomalies and Ocular Manifestations in Patients with Post-Acute COVID-19. Journal of Clinical Medicine. 2024; 13(23):7272. https://doi.org/10.3390/jcm13237272
Chicago/Turabian StyleAzar, Georges, Youssef Abdelmassih, Sophie Bonnin, Damien Guindolet, Vivien Vasseur, Francine Behar Cohen, Dominique Salmon, and Martine Mauget-Faÿsse. 2024. "Endothelial Glycocalyx Anomalies and Ocular Manifestations in Patients with Post-Acute COVID-19" Journal of Clinical Medicine 13, no. 23: 7272. https://doi.org/10.3390/jcm13237272
APA StyleAzar, G., Abdelmassih, Y., Bonnin, S., Guindolet, D., Vasseur, V., Behar Cohen, F., Salmon, D., & Mauget-Faÿsse, M. (2024). Endothelial Glycocalyx Anomalies and Ocular Manifestations in Patients with Post-Acute COVID-19. Journal of Clinical Medicine, 13(23), 7272. https://doi.org/10.3390/jcm13237272






