Coldness or Darkness? Which Places Greater Stress on the Thyroid? Seasonal Changes in Thyroid-Stimulating Hormone and Thyroid Hormones
Abstract
:1. Introduction
2. Material and Methods
3. Statistical Analysis
4. Examination of the Effects of Methodological Data on TSH, FT3, and FT4 Concentrations
5. Results
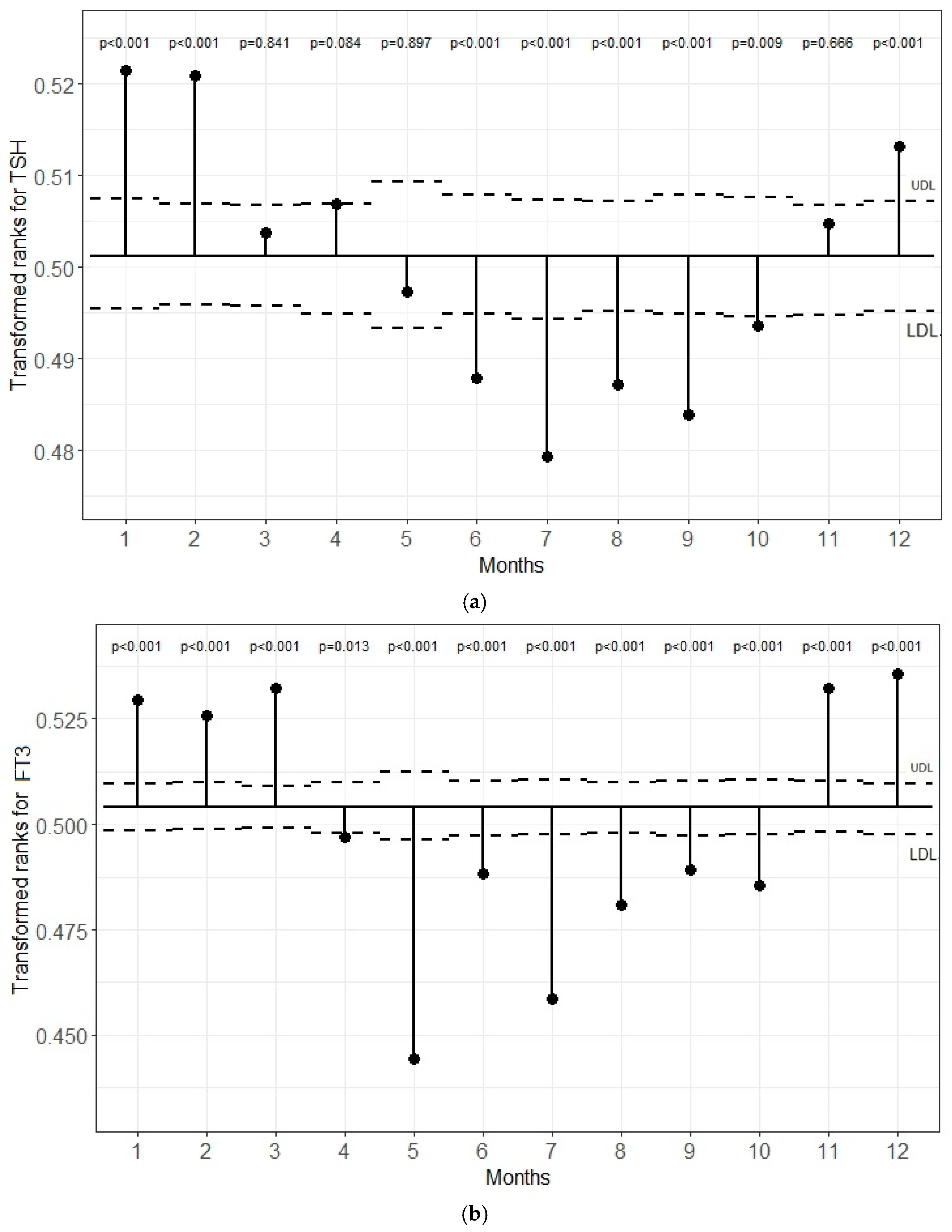
5.1. Transformed Rank Mean Comparison in Each Month for TSH, FT3, and FT4 Levels
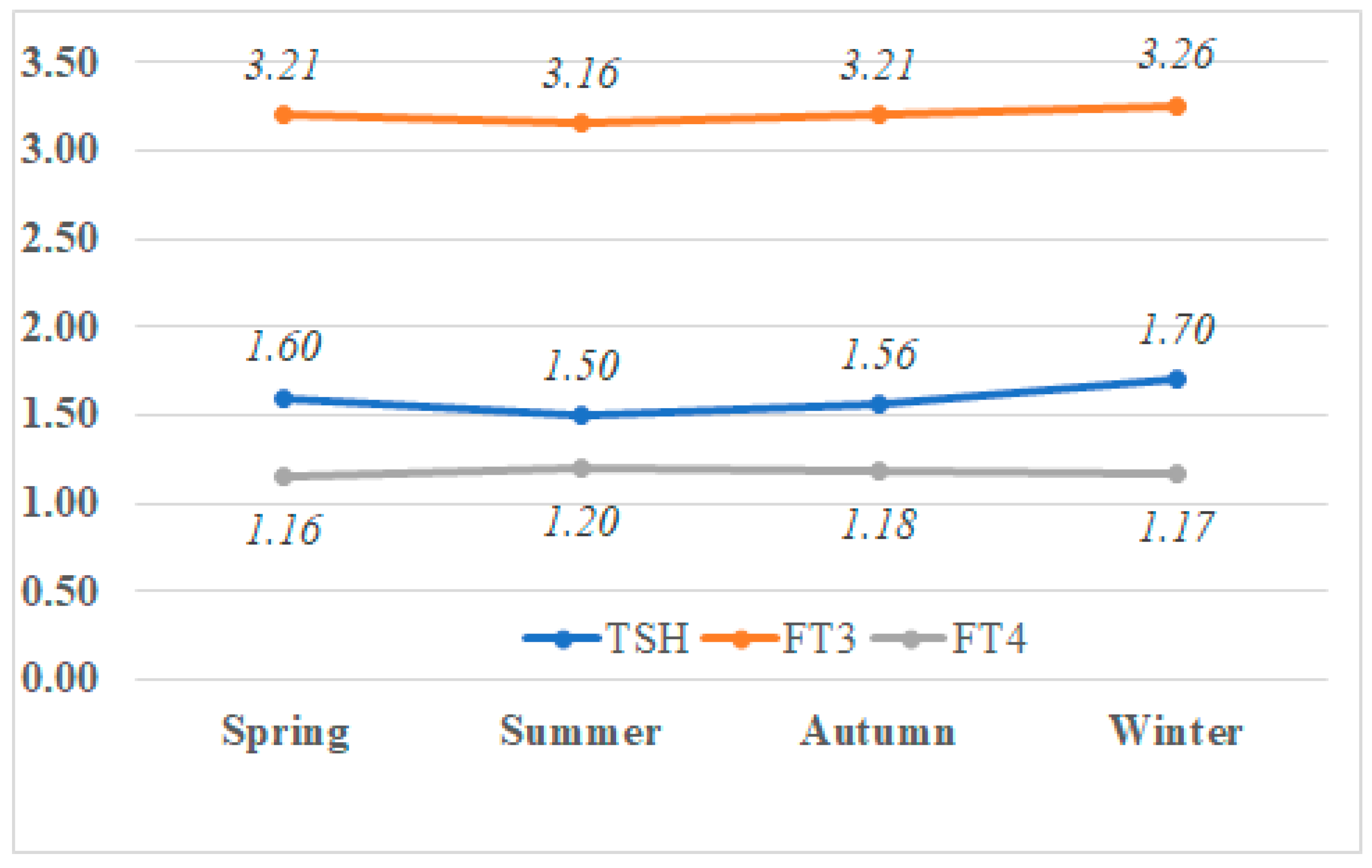
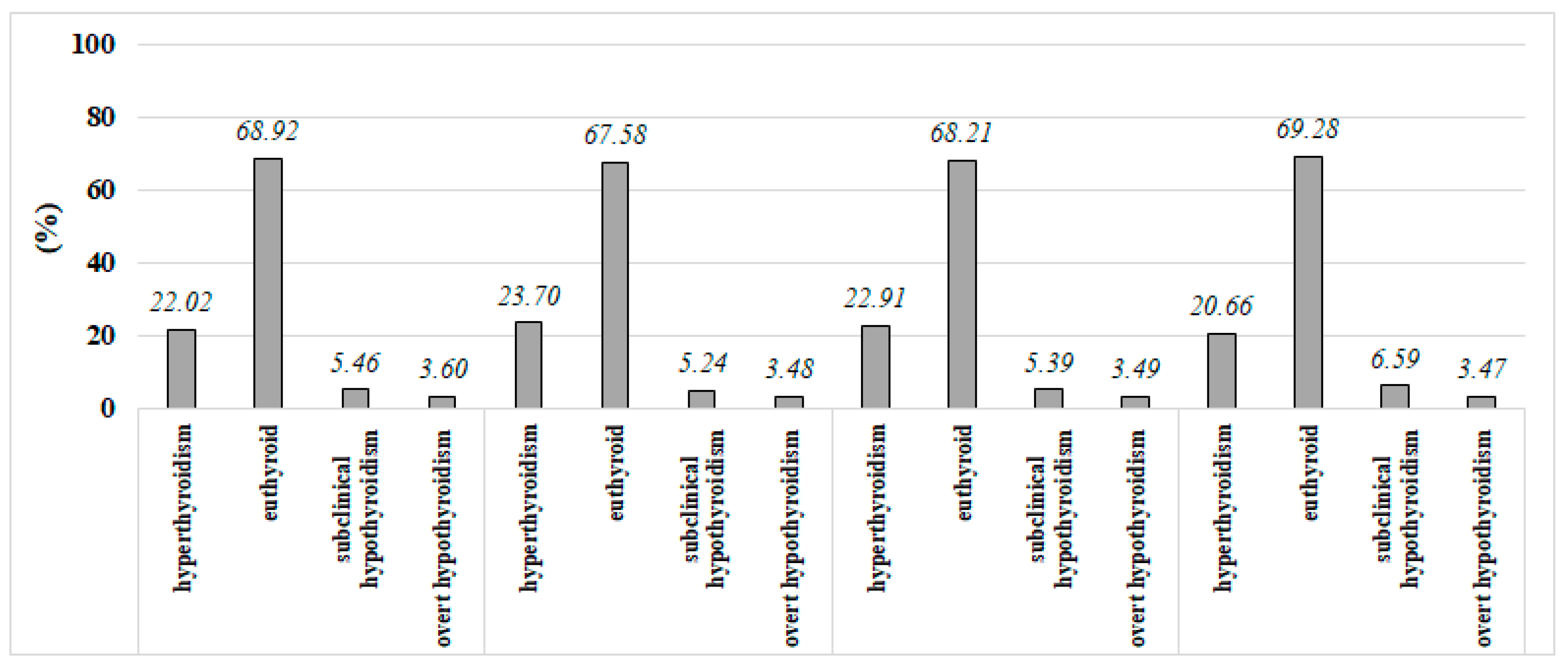
5.2. Seasonal Variations in Median TSH, T3, and T4 Levels
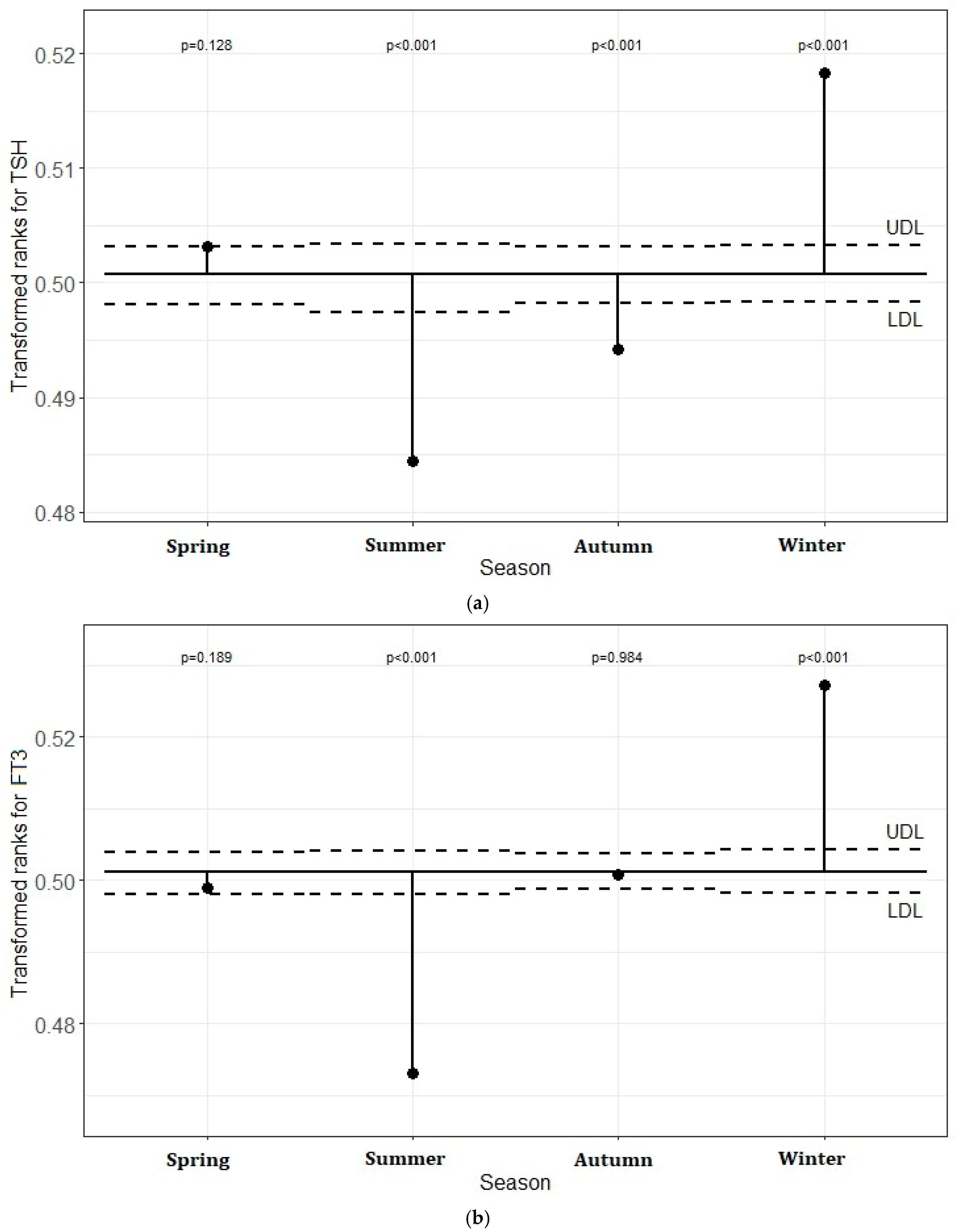
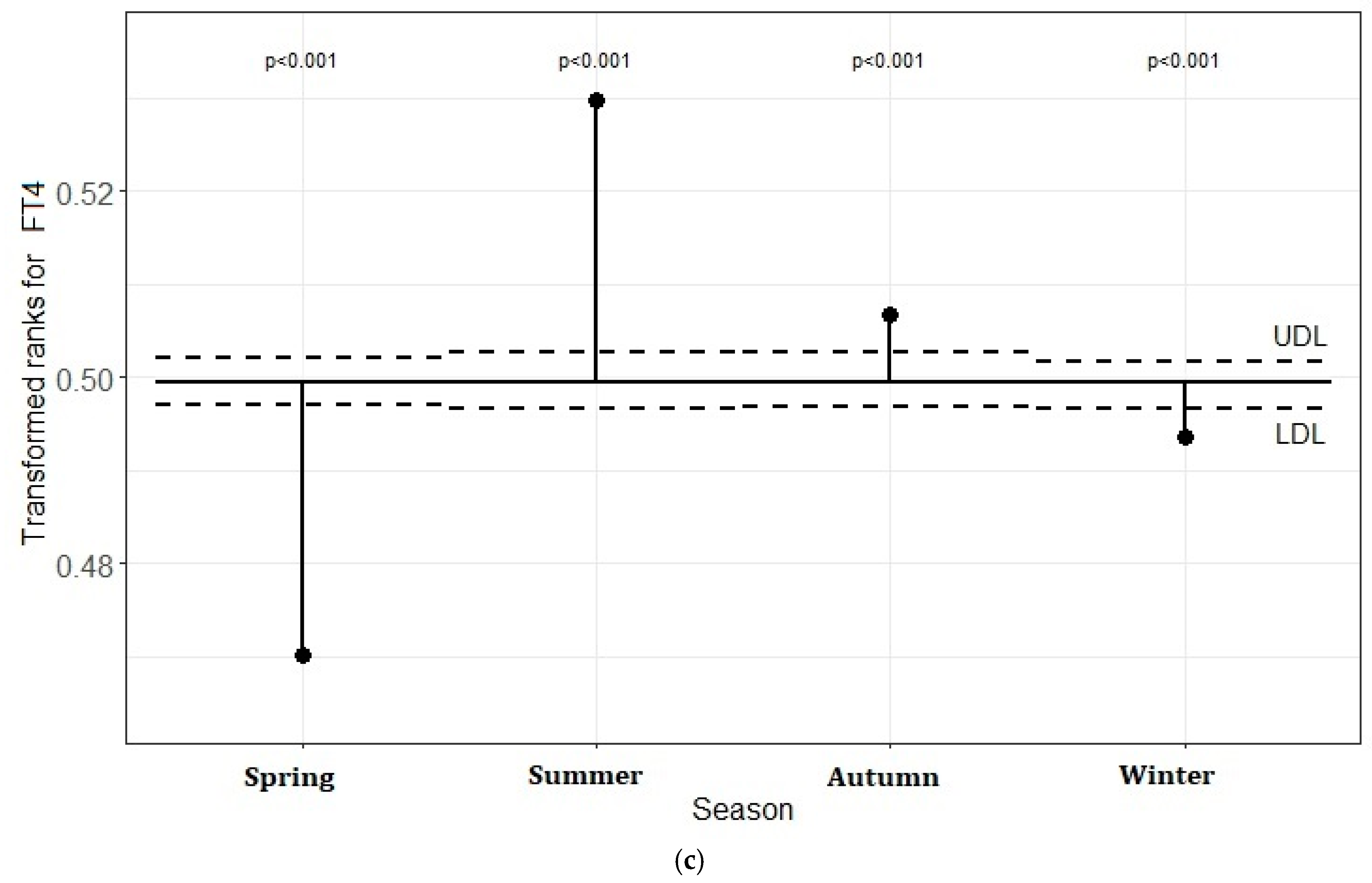
5.3. Transformed Rank Mean Comparison in Each Season for TSH, FT3, and FT4 Levels
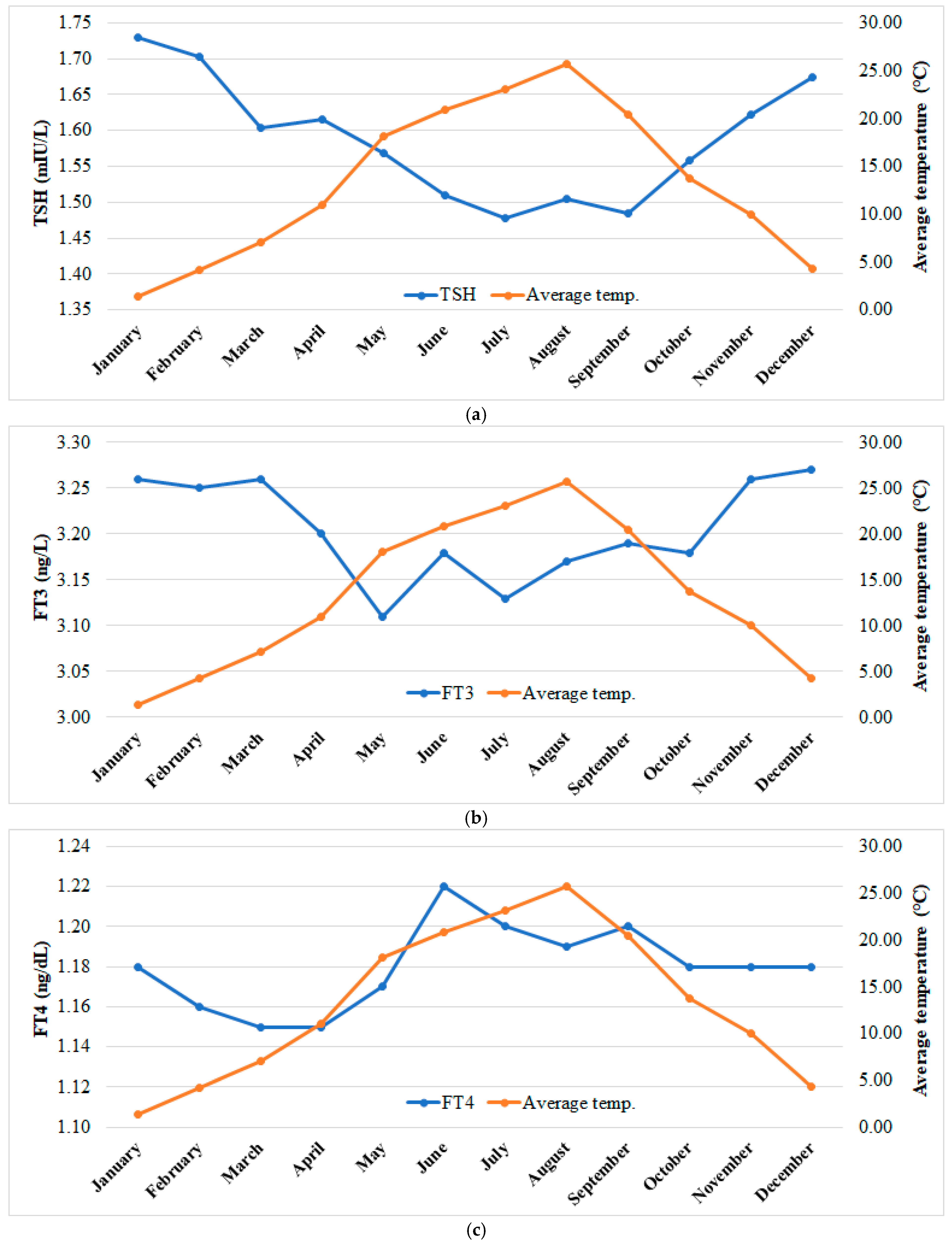
5.3.1. The Distribution of Monthly Average Air Temperature According to Season and the Values of TSH, FT3, and FT4
5.3.2. Effects of Meteorological Data on TSH, FT3, and FT4 Concentrations
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shinomiya, A.; Shimmura, T.; Nishiwaki-Ohkawa, T.; Yoshimura, T. Regulation of seasonal reproduction by hypothalamic activation of thyroid hormone. Front. Endocrinol. 2014, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.; Loudon, A. Clocks for all seasons: Unwinding the roles and mechanisms of circadian and interval timers in the hypothalamus and pituitary. J. Endocrinol. 2014, 222, R39–R59. [Google Scholar] [CrossRef]
- Nakayama, T.; Yoshimura, T. Seasonal rhythms: The role of thyrotropin and thyroid hormones. Thyroid 2018, 28, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Takashi, Y. Thyroid hormone and seasonal regulation of reproduction. Front. Neuroendocr. 2013, 34, 157–166. [Google Scholar]
- Ikegami, K.; Refetoff, S.; Van Cauter, E.; Yoshimura, T. Interconnection between circadian clocks and thyroid function. Nat. Rev. Endocrinol. 2019, 15, 590–600. [Google Scholar] [CrossRef]
- Philippe, J.; Dibner, C. Thyroid circadian timing: Roles in physiology and thyroid malignancies. J. Biol. Rhythms. 2015, 30, 76–83. [Google Scholar] [CrossRef]
- Yoshihara, A.; Noh, J.Y.; Watanabe, N.; Iwaku, K.; Kunji, Y.; Ohye, H.; Suzuki, M.; Matsumoto, M.; Suzuki, N.; Sugino, K. Seasonal changes in serum thyrotropin concentrations observed from big data obtained during six consecutive years from 2010 to 2015 at a single hospital in Japan. Thyroid 2018, 28, 429–436. [Google Scholar] [CrossRef]
- Kuzmenko, N.V.; Tsyrlin, V.A.; Pliss, M.G.; Galagudza, M.M. Seasonal variations in levels of human thyroid-stimulating hormone and thyroid hormones: A meta-analysis. Chronobiol. Int. 2021, 38, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Bellastella, G.; Maiorino, M.I.; Scappaticcio, L.; De Bellis, A.; Mercadante, S.; Esposito, K.; Bellastella, A. Chronothyroidology: Chronobiological aspects in thyroid function and diseases. Life 2021, 11, 426. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Brigante, G.; Setti, M.; Tagliavini, S.; Trenti, T.; Simoni, M. Semi-annual seasonal pattern of serum thyrotropin in adults. Sci. Rep. 2019, 9, 10786. [Google Scholar] [CrossRef] [PubMed]
- Van der Spoel, E.; Roelfsema, F.; van Heemst, D. Within-person variation in serum thyrotropin concentrations: Main sources, potential underlying biological mechanisms, and clinical implications. Front. Endocrinol. 2021, 12, 619568. [Google Scholar] [CrossRef]
- Yamada, S.; Horiguchi, K.; Akuzawa, M.; Sakamaki, K.; Shimomura, Y.; Kobayashi, I.; Andou, Y.; Yamada, M. Seasonal Variation in Thyroid Function in Over 7,000 Healthy Subjects in an Iodine-sufficient Area and Literature Review. J. Endocr. Soc. 2022, 6, bvac054. [Google Scholar] [CrossRef] [PubMed]
- Mahwi, T.O.; Abdulateef, D.S. Relation of different components of climate with human pituitary-thyroid axis and FT3/FT4 ratio: A study on euthyroid and SCH subjects in two different seasons. Int. J. Endocrinol. 2019, 2019, 2762978. [Google Scholar] [CrossRef]
- Zeng, Y.; He, H.; Wang, X.; Zhang, M.; An, Z. Climate and air pollution exposure are associated with thyroid function parameters: A retrospective cross-sectional study. J. Endocrinol. Investig. 2021, 44, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cheng, X.; Yu, S.; Qiu, L.; Lian, X.; Guo, X.; Hu, Y.; Lu, S.; Yang, G.; Liu, H. Data mining: Seasonal and temperature fluctuations in thyroid-stimulating hormone. Clin. Biochem. 2018, 60, 59–63. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, K.W.; Ahn, H.Y.; Won, H.; Choi, Y.; Cho, S.W.; Moon, J.H.; Yi, K.H.; Park, D.J.; Jang, H.C.; et al. Effect of seasonal changes on the transition between subclinical hypothyroid and euthyroid status. J. Clin. Endocrinol. Metab. 2013, 98, 3420–3429. [Google Scholar] [CrossRef]
- Konno, N.; Morikawa, K. Seasonal variation of serum thyrotropin concentration and thyrotropin response to thyrotropin-releasing hormone in patients with primary hypothyroidism on constant replacement dosage of thyroxine. J. Clin. Endocrinol. Metab. 1982, 54, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Simoni, M.; Velardo, A.; Montanini, V.; Faustini Fustini, M.; Seghedoni, S.; Marrama, P. Circannual rhythm of plasma thyrotropin in middle-aged and old euthyroid subjects. Horm. Res. 1990, 33, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Reed, H.L. Circannual changes in thyroid hormone physiology: The role of cold environmental temperatures. Arctic Med. Res. 1995, 54 (Suppl. 2), 9–15. [Google Scholar] [PubMed]
- Gullo, D.; Latina, A.; Frasca, F.; Squatrito, S.; Belfiore, A.; Vigneri, R. Seasonal variations in TSH serum levels in athyreotic patients under L-thyroxine replacement monotherapy. Clin. Endocrinol. 2017, 87, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Elgazzar, A. Influence of Chronic Exposure to Cold Environment on Thyroid Gland Function in Rabbits. Horm. Metab. Res. 2014, 46, 546–549. [Google Scholar] [CrossRef]
- O’Malley, B.P.; Cook, N.; Richardson, A.; Barnett, D.B.; Rosenthal, F.D. Circulating Catecholamine, Thyrotrophin, Thyroid Hormone and Prolactin Responses of Normal Subjects to Acute Cold Exposure. Clin. Endocrinol. 1984, 21, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Wahl, R. Pars Distalis and Pars Tuberalis Thyroid-Stimulating Hormones and Their Roles in Macro-Thyroid-Stimulating Hormone Formation. Int. J. Mol. Sci. 2023, 20, 11699. [Google Scholar] [CrossRef]
- Hassi, J.; Sikkila, K.; Ruokonen, A.; Leppaluoto, J. The pituitary-thyroid axis in healthy men living under subarctic climatological conditions. J. Endocrinol. 2001, 169, 195–203. [Google Scholar] [CrossRef]
- Tendler, A.; Bar, A.; Mendelsohn-Cohen, N.; Karin, O.; Korem Kohanim, Y.; Maimon, L.; Milo, T.; Raz, M.; Mayo, A.; Tanay, A.; et al. Hormone seasonality in medical records suggests circannual endocrine circuits. Proc. Natl. Acad. Sci. USA 2021, 118, e2003926118. [Google Scholar] [CrossRef]
- Belsare, P.; Bartolome, A.; Stanger, C.; Prioleau, T. Understanding Temporal Changes and Seasonal Variations in Glycemic Trends Using Wearable Data. Sci. Adv. 2023, 9, eadg2132. [Google Scholar] [CrossRef] [PubMed]
- Marti-Soler, H.; Gubelmann, C.; Aeschbacher, S.; Alves, L.; Bobak, M.; Bongard, V.; Clays, E.; de Gaetano, G.; di Castelnuovo, A.; Elosua, R.; et al. Seasonality of Cardiovascular Risk Factors: An Analysis Including Over 230,000 Participants in 15 Countries. Heart 2014, 100, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Olendzki, B.C.; Li, W.; Hafner, A.R.; Chiriboga, D.; Hebert, J.R.; Campbell, M.; Sarnie, M.; Ockene, I.S. Seasonal Variation in Food Intake, Physical Activity, and Body Weight in a Predominantly Overweight Population. Eur. J. Clin. Nutr. 2006, 60, 519–528. [Google Scholar] [CrossRef]

| Variables | TSH | FT3 | FT4 | ||||
|---|---|---|---|---|---|---|---|
| Coef | Importance | Coef | Importance | Coef | Importance | ||
| Monthly | Age | X | −0.024 | 100.00 | 0.001 | ||
| Gender | −0.006 | 37.53 | 0.019 | 77.63 | 0.004 | 3.34 | |
| Mat | X | 0.00003 | 0.11 | 0.009 | 10.44 | ||
| Dminta | X | −0.002 | 6.58 | 0.008 | 9.67 | ||
| Minrtm | X | 0.002 | 6.42 | −0.002 | 1.75 | ||
| Dmta | −0.017 | 100.00 | −0.006 | 26.31 | 0.001 | 0.29 | |
| Maxrtm | −0.0002 | 1.03 | 0.010 | 41.00 | −0.003 | 2.12 | |
| Maap | −0.002 | 14.22 | −0.004 | 16.01 | −0.004 | 3.76 | |
| Marh | X | 0.023 | 94.77 | −0.008 | 8.92 | ||
| Maws | −0.006 | 33.55 | −0.001 | 4.06 | 0.005 | 4.57 | |
| Dtsdam | X | 0.016 | 66.92 | −0.011 | 12.92 | ||
| Maxdd | X | 0.012 | 49.76 | 0.003 | 2.34 | ||
| Mindd | X | −0.020 | 80.48 | 0.064 | 82.98 | ||
| Cosine component | X | X | 0.077 | 100.0 | |||
| Sine component | 0.005 | 31.73 | 0.0002 | 0.76 | 0.010 | 12.18 | |
| Intercept | 0.011 | 0.511 | 0.075 | ||||
| Seasonal | Age | 0.0004 | 0.12 | −0.024 | 27.14 | 0.001 | 1.63 |
| Gender | −0.008 | 9.39 | 0.019 | 21.27 | 0.004 | 23.21 | |
| Mat | −0.024 | 28.88 | 0.0004 | 0.00 | −0.001 | 0.48 | |
| Dminta | 0.003 | 3.52 | −0.030 | 33.39 | −0.013 | 100.00 | |
| Minrtm | −0.0003 | 0.00 | −0.021 | 23.92 | 0.009 | 71.21 | |
| Dmta | −0.075 | 93.15 | −0.004 | 4.12 | −0.001 | 0.00 | |
| Maxrtm | −0.050 | 61.48 | −0.011 | 11.79 | −0.009 | 66.16 | |
| Maap | 0.014 | 16.44 | 0.020 | 21.90 | −0.001 | 5.37 | |
| Marh | −0.038 | 47.06 | 0.059 | 66.81 | −0.007 | 49.75 | |
| Maws | 0.008 | 9.88 | −0.005 | 5.03 | 0.007 | 48.00 | |
| Dtsdam | 0.005 | 5.71 | 0.040 | 45.63 | 0.004 | 24.73 | |
| Cosine component | −0.081 | 100.00 | −0.088 | 100.00 | −0.002 | 7.83 | |
| Sine component | −0.005 | 5.70 | −0.004 | 4.69 | −0.007 | 50.53 | |
| Intercept | 0.011 | 0.512 | 0.075 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tam, A.A.; Fakı, S.; Demir, P.; Ozdemir, D.; Topaloglu, O.; Ersoy, R.; Cakir, B. Coldness or Darkness? Which Places Greater Stress on the Thyroid? Seasonal Changes in Thyroid-Stimulating Hormone and Thyroid Hormones. J. Clin. Med. 2024, 13, 7293. https://doi.org/10.3390/jcm13237293
Tam AA, Fakı S, Demir P, Ozdemir D, Topaloglu O, Ersoy R, Cakir B. Coldness or Darkness? Which Places Greater Stress on the Thyroid? Seasonal Changes in Thyroid-Stimulating Hormone and Thyroid Hormones. Journal of Clinical Medicine. 2024; 13(23):7293. https://doi.org/10.3390/jcm13237293
Chicago/Turabian StyleTam, Abbas Ali, Sevgul Fakı, Pervin Demir, Didem Ozdemir, Oya Topaloglu, Reyhan Ersoy, and Bekir Cakir. 2024. "Coldness or Darkness? Which Places Greater Stress on the Thyroid? Seasonal Changes in Thyroid-Stimulating Hormone and Thyroid Hormones" Journal of Clinical Medicine 13, no. 23: 7293. https://doi.org/10.3390/jcm13237293
APA StyleTam, A. A., Fakı, S., Demir, P., Ozdemir, D., Topaloglu, O., Ersoy, R., & Cakir, B. (2024). Coldness or Darkness? Which Places Greater Stress on the Thyroid? Seasonal Changes in Thyroid-Stimulating Hormone and Thyroid Hormones. Journal of Clinical Medicine, 13(23), 7293. https://doi.org/10.3390/jcm13237293






