CT-Guided Transthoracic Core-Needle Biopsy of Pulmonary Nodules: Current Practices, Efficacy, and Safety Considerations
Abstract
:1. Introduction
1.1. Background and Importance of CT-Guided Transthoracic Needle Biopsy
1.2. Objectives of the Review
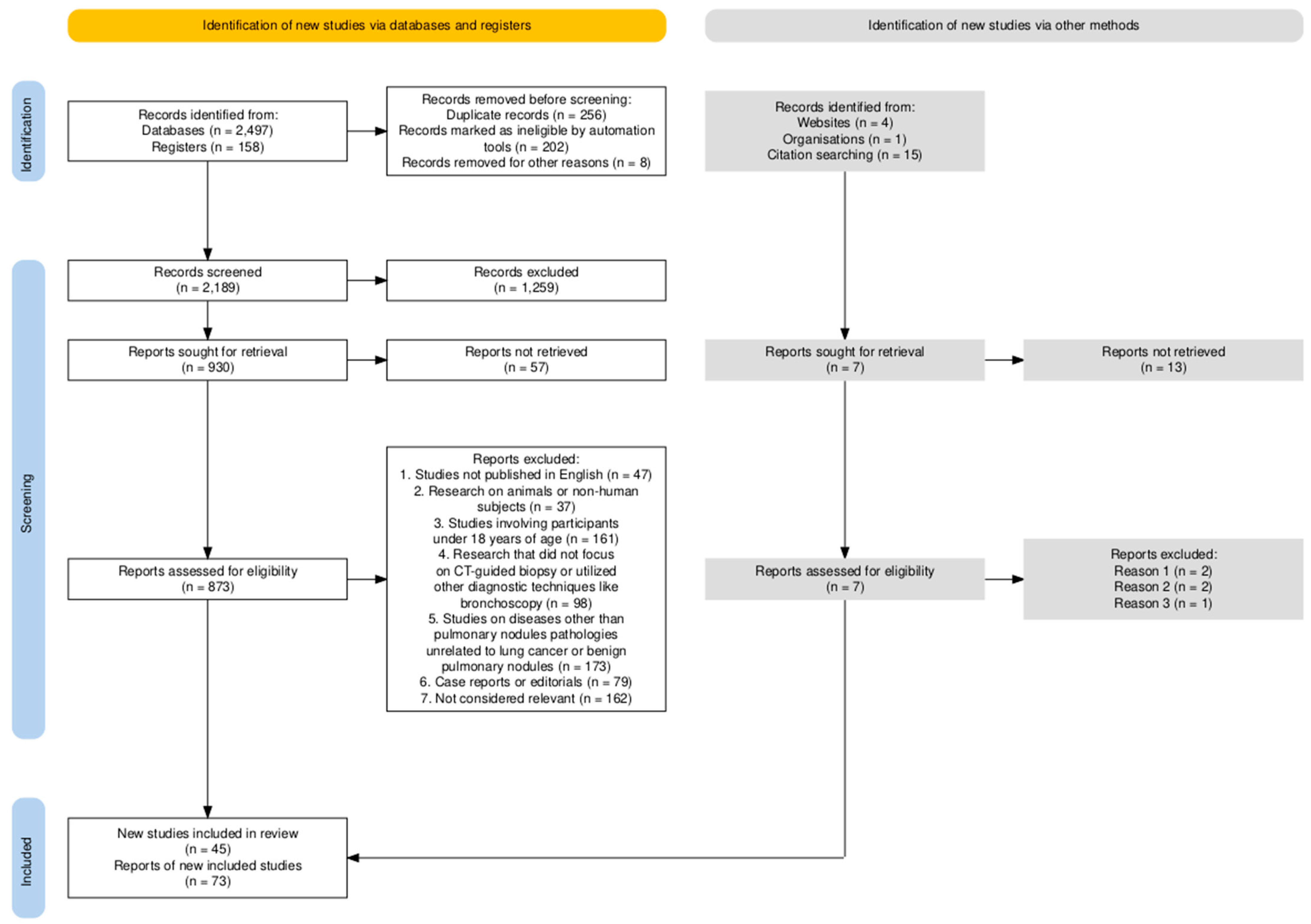
2. Materials and Methods
- Studies published in English;
- Research conducted on human subjects;
- Studies evaluating the diagnostic yield, efficacy, and safety of CT-guided core-needle biopsy for pulmonary nodules;
- Clinical trials, cohort studies, and comparative studies focusing on biopsy techniques.
- Studies not published in English;
- Research on animals or non-human subjects;
- Studies involving participants under 18 years of age;
- Research that did not focus on CT-guided biopsy or utilized other diagnostic techniques like bronchoscopy;
- Studies on diseases other than pulmonary nodules (e.g., pathologies unrelated to lung cancer or benign pulmonary nodules);
- Case reports or editorials.
3. Indications
3.1. Evaluation of Solitary Pulmonary Nodules
3.2. Metastatic Lung Disease
4. Contraindications
4.1. Absolute Contraindications
4.2. Relative Contraindications
5. Technique
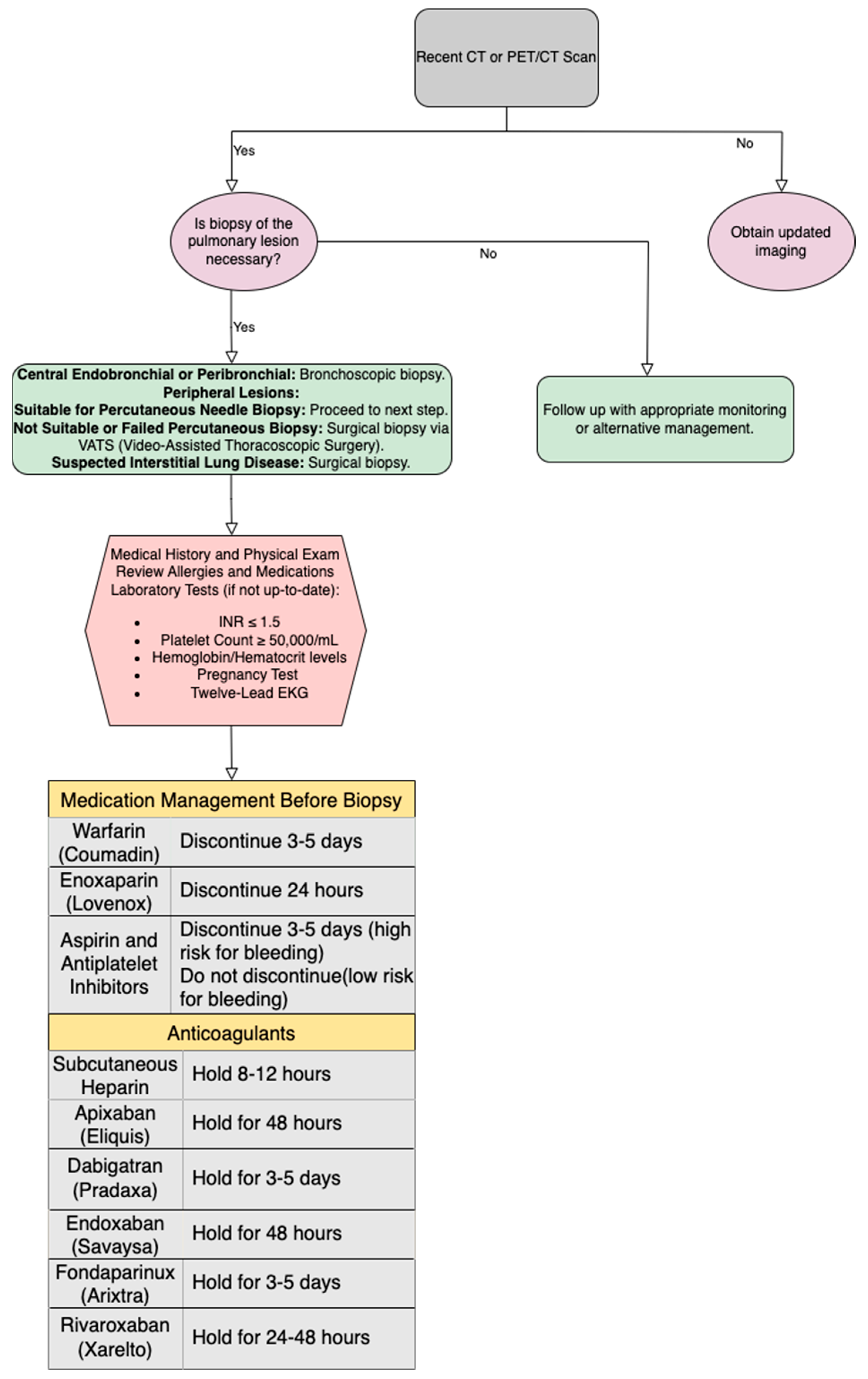
5.1. Patient Selection and Pre-Procedural Evaluation
5.2. Patient Positioning
5.3. Sedation and Anesthesia
5.4. Biopsy Needles and Equipment
5.4.1. Types of Needles (Aspiration, Cutting)
5.4.2. Selection Criteria for Needles
5.5. Procedural Steps
5.5.1. Planning the Access Route
5.5.2. Needle Insertion and Positioning
5.5.3. Sample Collection and Handling
5.5.4. Patient Monitoring
- Monitoring vital indicators like heart rate, blood pressure, respiratory rate, and oxygen saturation is essential. This helps detect PNX and bleeding early.
- Monitoring respiratory status is crucial due to the process. Dyspnea, hemoptysis, and diminished breath sounds may suggest a PNX or associated problems.
- Regular pain assessment using standard scales is necessary for effective pain management. Analgesics should be given to relieve procedure pain and guarantee patient comfort.
- Regularly check for problems such as PNX, pulmonary hemorrhage, and infection. Physical examination and patient-reported symptoms [32].
5.5.5. Immediate Post-Procedure Imaging
6. Efficacy
6.1. Diagnostic Yield and Accuracy
6.2. Sensitivity and Specificity for Malignancy
6.3. Factors Influencing Diagnostic Success
6.3.1. Lesion Size
6.3.2. Lesion Depth
6.3.3. Number of Biopsy Passes
6.3.4. Needle Path
6.3.5. Imaging Guidance and Techniques
7. Complications
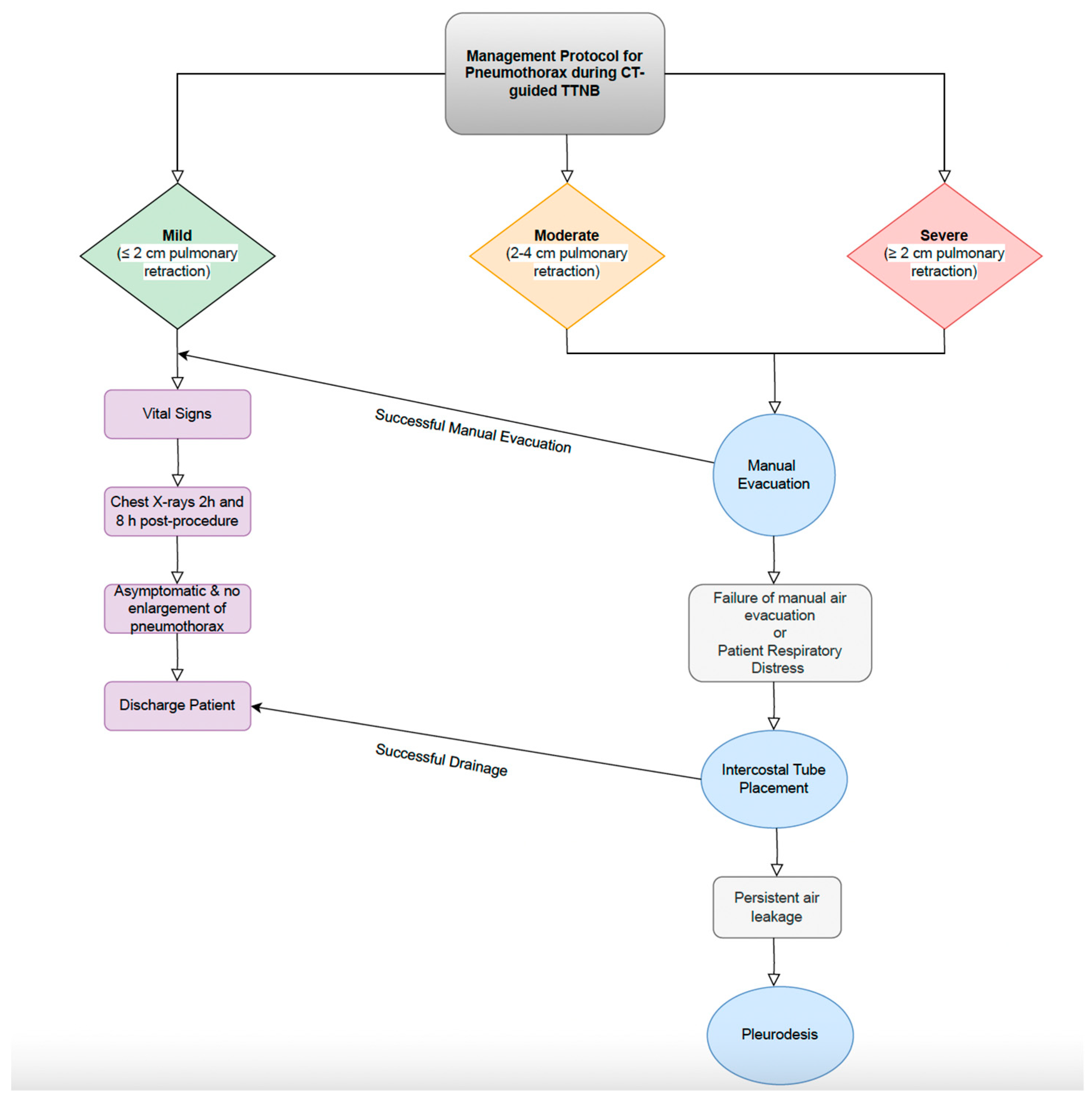
7.1. Pneumothorax
7.1.1. Incidence and Risk Factors
7.1.2. Management Strategies
7.2. Pulmonary Hemorrhage
7.2.1. Incidence and Risk Factors
7.2.2. Management Strategies
7.2.3. Air Embolism
7.2.4. Tumor Seeding
8. Discussion
8.1. Comparison with Other Biopsy Techniques
8.2. Future Directions and Innovations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heerink, W.J.; De Bock, G.H.; De Jonge, G.J.; Groen, H.J.M.; Vliegenthart, R.; Oudkerk, M. Complication Rates of CT-Guided Transthoracic Lung Biopsy: Meta-Analysis. Eur. Radiol. 2017, 27, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Chang, C.-C.; Chu, C.-Y.; Huang, L.-T.; Chung, T.-J.; Liu, Y.-S.; Yen, Y.-T. Computed Tomography-Guided Transthoracic Needle Biopsy: Predictors for Diagnostic Failure and Tissue Adequacy for Molecular Testing. Front. Med. 2021, 8, 650381. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, Y.; Lin, J.; Fu, L.; Sun, H.; Huang, Z.; Ooi, B.Y.; Xie, S. Improving CT-Guided Transthoracic Biopsy Diagnostic Yield of Lung Masses Using Intraprocedural CT and Prior PET/CT Fusion Imaging. BMC Pulm. Med. 2022, 22, 311. [Google Scholar] [CrossRef] [PubMed]
- Tahir, I.; Cahalane, A.M.; Saenger, J.A.; Leppelmann, K.S.; Abrishami Kashani, M.; Marquardt, J.P.; Silverman, S.G.; Shyn, P.B.; Mercaldo, N.D.; Fintelmann, F.J. Factors Associated with Hospital Length of Stay and Adverse Events Following Percutaneous Ablation of Lung Tumors. J. Vasc. Interv. Radiol. 2023, 34, 759–767.e2. [Google Scholar] [CrossRef]
- Callister, M.E.J.; Baldwin, D.R.; Akram, A.R.; Barnard, S.; Cane, P.; Draffan, J.; Franks, K.; Gleeson, F.; Graham, R.; Malhotra, P.; et al. British Thoracic Society Guidelines for the Investigation and Management of Pulmonary Nodules: Accredited by NICE. Thorax 2015, 70, ii1–ii54. [Google Scholar] [CrossRef] [PubMed]
- Snoeckx, A.; Reyntiens, P.; Desbuquoit, D.; Spinhoven, M.J.; Van Schil, P.E.; Van Meerbeeck, J.P.; Parizel, P.M. Evaluation of the Solitary Pulmonary Nodule: Size Matters, but Do Not Ignore the Power of Morphology. Insights Imaging 2018, 9, 73–86. [Google Scholar] [CrossRef]
- Jiang, B.; Takashima, S.; Miyake, C.; Hakucho, T.; Takahashi, Y.; Morimoto, D.; Numasaki, H.; Nakanishi, K.; Tomita, Y.; Higashiyama, M. Thin-Section CT Findings in Peripheral Lung Cancer of 3 Cm or Smaller: Are There Any Characteristic Features for Predicting Tumor Histology or Do They Depend Only on Tumor Size? Acta Radiol. 2014, 55, 302–308. [Google Scholar] [CrossRef]
- Chu, Z.; Zhang, Y.; Li, W.; Li, Q.; Zheng, Y.; Lv, F. Primary Solid Lung Cancerous Nodules with Different Sizes: Computed Tomography Features and Their Variations. BMC Cancer 2019, 19, 1060. [Google Scholar] [CrossRef]
- Horeweg, N.; Van Rosmalen, J.; Heuvelmans, M.A.; Van Der Aalst, C.M.; Vliegenthart, R.; Scholten, E.T.; Ten Haaf, K.; Nackaerts, K.; Lammers, J.-W.J.; Weenink, C.; et al. Lung Cancer Probability in Patients with CT-Detected Pulmonary Nodules: A Prespecified Analysis of Data from the NELSON Trial of Low-Dose CT Screening. Lancet Oncol. 2014, 15, 1332–1341. [Google Scholar] [CrossRef]
- Naidich, D.P.; Bankier, A.A.; MacMahon, H.; Schaefer-Prokop, C.M.; Pistolesi, M.; Goo, J.M.; Macchiarini, P.; Crapo, J.D.; Herold, C.J.; Austin, J.H.; et al. Recommendations for the Management of Subsolid Pulmonary Nodules Detected at CT: A Statement from the Fleischner Society. Radiology 2013, 266, 304–317. [Google Scholar] [CrossRef]
- Hu, H.; Wang, Q.; Tang, H.; Xiong, L.; Lin, Q. Multi-slice Computed Tomography Characteristics of Solitary Pulmonary Ground-glass Nodules: Differences between Malignant and Benign. Thorac. Cancer 2016, 7, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Lee, K.S. Ground-Glass Opacity Nodules: Histopathology, Imaging Evaluation, and Clinical Implications. J. Thorac. Imaging 2011, 26, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [PubMed]
- Herold, C.J. Management of Solid and Sub-Solid Lung Nodules. Cancer Imaging 2014, 14, O24. [Google Scholar] [CrossRef]
- Wu, F.; Tian, S.; Jin, X.; Jing, R.; Yang, Y.; Jin, M.; Zhao, S. CT and Histopathologic Characteristics of Lung Adenocarcinoma with Pure Ground-Glass Nodules 10 Mm or Less in Diameter. Eur. Radiol. 2017, 27, 4037–4043. [Google Scholar] [CrossRef]
- Chung, K.; Jacobs, C.; Scholten, E.T.; Goo, J.M.; Prosch, H.; Sverzellati, N.; Ciompi, F.; Mets, O.M.; Gerke, P.K.; Prokop, M.; et al. Lung-RADS Category 4X: Does It Improve Prediction of Malignancy in Subsolid Nodules? Radiology 2017, 284, 264–271. [Google Scholar] [CrossRef]
- Sim, Y.T.; Poon, F.W. Imaging of Solitary Pulmonary Nodule—A Clinical Review. Quant. Imaging Med. Surg. 2013, 3, 316. [Google Scholar]
- Shinohara, S.; Hanagiri, T.; Takenaka, M.; Chikaishi, Y.; Oka, S.; Shimokawa, H.; Nakagawa, M.; Uramoto, H.; So, T.; Aoki, T.; et al. Evaluation of Undiagnosed Solitary Lung Nodules According to the Probability of Malignancy in the American College of Chest Physicians (ACCP) Evidence-Based Clinical Practice Guidelines. Radiol. Oncol. 2014, 48, 50–55. [Google Scholar] [CrossRef]
- Henschke, C.I.; Yankelevitz, D.F.; Reeves, A.P.; Cham, M.D. Image Analysis of Small Pulmonary Nodules Identified by Computed Tomography. Mt. Sinai J. Med. J. Transl. Pers. Med. 2011, 78, 882–893. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, X.; Qu, Y.; Wen, Z. CT-Guided Core Needle Biopsy of the Lung in Patients with Primary Malignancy Suspected of Lung Metastasis: 5-Year Experience from a Single Institution. Diagn. Interv. Radiol. 2021, 27, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Cerci, J.J.; Tabacchi, E.; Bogoni, M.; Delbeke, D.; Pereira, C.C.; Cerci, R.J.; Krauzer, C.; Sakamoto, D.G.; Fanti, S.; Vitola, J.V. Comparison of CT and PET/CT for Biopsy Guidance in Oncological Patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Saggiante, L.; Biondetti, P.; Lanza, C.; Carriero, S.; Ascenti, V.; Piacentino, F.; Shehab, A.; Ierardi, A.M.; Venturini, M.; Carrafiello, G. Computed-Tomography-Guided Lung Biopsy: A Practice-Oriented Document on Techniques and Principles and a Review of the Literature. Diagnostics 2024, 14, 1089. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lee, S.M.; Park, C.H.; Lee, J.H.; Kim, H.; Chae, K.J.; Jin, K.N.; Lee, K.H.; Kim, J.I.; Hong, J.H.; et al. 2020 Clinical Practice Guideline for Percutaneous Transthoracic Needle Biopsy of Pulmonary Lesions: A Consensus Statement and Recommendations of the Korean Society of Thoracic Radiology. Korean J. Radiol. 2021, 22, 263. [Google Scholar] [CrossRef]
- Veltri, A.; Bargellini, I.; Giorgi, L.; Almeida, P.A.M.S.; Akhan, O. CIRSE Guidelines on Percutaneous Needle Biopsy (PNB). Cardiovasc. Intervent. Radiol. 2017, 40, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.C.; Tahir, I.; Saenger, J.A.; Abrishami Kashani, M.; Muniappan, A.; Levesque, V.M.; Shyn, P.B.; Silverman, S.G.; Fintelmann, F.J. Safety and Effectiveness of Percutaneous Image-Guided Thermal Ablation of Juxtacardiac Lung Tumors. J. Vasc. Interv. Radiol. 2023, 34, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Yoon, S.H.; Goo, J.M.; Park, C.M. Cone-Beam CT-Guided Percutaneous Transthoracic Needle Lung Biopsy of Juxtaphrenic Lesions: Diagnostic Accuracy and Complications. Korean J. Radiol. 2021, 22, 1203. [Google Scholar] [CrossRef] [PubMed]
- Graur, A.; Mercaldo, N.D.; Simon, J.; Alici, C.; Saenger, J.A.; Cahalane, A.M.; Vazquez, R.; Fintelmann, F.J. High-Frequency Jet Ventilation Versus Spontaneous Respiration for Percutaneous Cryoablation of Lung Tumors: Comparison of Adverse Events and Procedural Efficiency. Am. J. Roentgenol. 2024, 222, e2330557. [Google Scholar] [CrossRef]
- Cronin, C.G.; Sharma, A.; Digumarthy, S.R.; Gilman, M.D.; McLoud, T.; Muse, V.V.; Shepard, J.O. Percutaneous Lung Biopsy After Pneumonectomy: Factors for Improving Success in the Care of Patients at High Risk. Am. J. Roentgenol. 2011, 196, 929–934. [Google Scholar] [CrossRef]
- Wu, C.C.; Maher, M.M.; Shepard, J.-A.O. CT-Guided Percutaneous Needle Biopsy of the Chest: Preprocedural Evaluation and Technique. Am. J. Roentgenol. 2011, 196, W511–W514. [Google Scholar] [CrossRef]
- Cham, M.; Lane, M.; Henschke, C.; Yankelevitz, D. Lung Biopsy: Special Techniques. Semin. Respir. Crit. Care Med. 2008, 29, 335–349. [Google Scholar] [CrossRef]
- Manhire, A. Guidelines for Radiologically Guided Lung Biopsy. Thorax 2003, 58, 920–936. [Google Scholar] [CrossRef] [PubMed]
- Borelli, C.; Vergara, D.; Simeone, A.; Pazienza, L.; Castorani, G.; Graziano, P.; Di Micco, C.; Quarato, C.M.I.; Sperandeo, M. CT-Guided Transthoracic Biopsy of Pulmonary Lesions: Diagnostic versus Nondiagnostic Results. Diagnostics 2022, 12, 359. [Google Scholar] [CrossRef] [PubMed]
- Coley, S.M.; Crapanzano, J.P.; Saqi, A. FNA, Core Biopsy, or Both for the Diagnosis of Lung Carcinoma: Obtaining Sufficient Tissue for a Specific Diagnosis and Molecular Testing. Cancer Cytopathol. 2015, 123, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Frank, N. An Overview of Percutaneous CT-Guided Lung Biopsies. J. Radiol. Nurs. 2018, 37, 2–8. [Google Scholar] [CrossRef]
- Lal, H.; Neyaz, Z.; Nath, A.; Borah, S. CT-Guided Percutaneous Biopsy of Intrathoracic Lesions. Korean J. Radiol. 2012, 13, 210. [Google Scholar] [CrossRef]
- Schneider, F.; Smith, M.A.; Lane, M.C.; Pantanowitz, L.; Dacic, S.; Ohori, N.P. Adequacy of Core Needle Biopsy Specimens and Fine-Needle Aspirates for Molecular Testing of Lung Adenocarcinomas. Am. J. Clin. Pathol. 2015, 143, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Anzidei, M.; Porfiri, A.; Andrani, F.; Di Martino, M.; Saba, L.; Catalano, C.; Bezzi, M. Imaging-Guided Chest Biopsies: Techniques and Clinical Results. Insights Imaging 2017, 8, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.-C.; Tsai, W.-L.; Chen, M.-C.; Chang, G.-C.; Tzeng, W.-S.; Chan, S.-W.; Chen, C.C.-C. CT-Guided Core Biopsy of Lung Lesions: A Primer. Am. J. Roentgenol. 2009, 193, 1228–1235. [Google Scholar] [CrossRef]
- Najafi, A.; Al Ahmar, M.; Bonnet, B.; Delpla, A.; Kobe, A.; Madani, K.; Roux, C.; Deschamps, F.; De Baère, T.; Tselikas, L. The PEARL Approach for CT-Guided Lung Biopsy: Assessment of Complication Rate. Radiology 2022, 302, 473–480. [Google Scholar] [CrossRef]
- Brown, R.W.; Speranza, V.D.; Alvarez, J.O.; Eisen, R.N.; Frishberg, D.P.; Rosai, J.; Santiago, J.; Tunnicliffe, J.; Colasacco, C.; Lacchetti, C.; et al. Uniform Labeling of Blocks and Slides in Surgical Pathology: Guideline From the College of American Pathologists Pathology and Laboratory Quality Center and the National Society for Histotechnology. Arch. Pathol. Lab. Med. 2015, 139, 1515–1524. [Google Scholar] [CrossRef]
- Lott, R.; Tunnicliffe, J.; Sheppard, E.; Santiago, J.; Hladik, C.; Nasim, M.; Zeitner, K.; Haas, T.; Kohl, S.; Movahedi-Lankarani, S. Practical Guide to Specimen Handling in Surgical Pathology; College of American Pathologists: Northfield, MN, USA, 2023. [Google Scholar]
- Roy-Chowdhuri, S.; Dacic, S.; Ghofrani, M.; Illei, P.B.; Layfield, L.J.; Lee, C.; Michael, C.W.; Miller, R.A.; Mitchell, J.W.; Nikolic, B.; et al. Guideline From the College of American Pathologists in Collaboration With the American College of Chest Physicians, Association for Molecular Pathology, American Society of Cytopathology, American Thoracic Society, Pulmonary Pathology Society, Papanicolaou Society of Cytopathology, Society of Interventional Radiology, and Society of Thoracic Radiology. Arch. Pathol. Lab. Med. 2020, 144. [Google Scholar]
- Graur, A.; Saenger, J.A.; Mercaldo, N.D.; Simon, J.; Abston, E.D.; Price, M.C.; Lanciotti, K.; Swisher, L.A.; Colson, Y.L.; Willers, H.; et al. Multimodality Management of Thoracic Tumors: Initial Experience With a Multidisciplinary Thoracic Ablation Conference. Ann. Surg. Oncol. 2024, 31, 3426–3436. [Google Scholar] [CrossRef] [PubMed]
- Weinand, J.T.; Du Pisanie, L.; Ngeve, S.; Commander, C.; Yu, H. Pneumothorax after Computed Tomography-Guided Lung Biopsy: Utility of Immediate Post-Procedure Computed Tomography and One-Hour Delayed Chest Radiography. PLoS ONE 2023, 18, e0284145. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yoon, S.H.; Hong, H.; Rho, J.Y.; Goo, J.M. Incidence, Risk Factors, and Prognostic Indicators of Symptomatic Air Embolism after Percutaneous Transthoracic Lung Biopsy: A Systematic Review and Pooled Analysis. Eur. Radiol. 2021, 31, 2022–2033. [Google Scholar] [CrossRef]
- Brzezinski, R.Y.; Vigiser, I.; Fomin, I.; Israeli, L.; Shenhar-Tsarfaty, S.; Bar-Shai, A. The Yield of Immediate Post Lung Biopsy CT in Predicting Iatrogenic Pneumothorax. BMC Pulm. Med. 2020, 20, 91. [Google Scholar] [CrossRef]
- Tomiyama, N.; Yasuhara, Y.; Nakajima, Y.; Adachi, S.; Arai, Y.; Kusumoto, M.; Eguchi, K.; Kuriyama, K.; Sakai, F.; Noguchi, M.; et al. CT-Guided Needle Biopsy of Lung Lesions: A Survey of Severe Complication Based on 9783 Biopsies in Japan. Eur. J. Radiol. 2006, 59, 60–64. [Google Scholar] [CrossRef]
- Choi, S.H.; Chae, E.J.; Kim, J.-E.; Kim, E.Y.; Oh, S.Y.; Hwang, H.J.; Lee, H.J. Percutaneous CT-Guided Aspiration and Core Biopsy of Pulmonary Nodules Smaller Than 1 Cm: Analysis of Outcomes of 305 Procedures From a Tertiary Referral Center. Am. J. Roentgenol. 2013, 201, 964–970. [Google Scholar] [CrossRef]
- Baratella, E.; Cernic, S.; Minelli, P.; Furlan, G.; Crimì, F.; Rocco, S.; Ruaro, B.; Cova, M.A. Accuracy of CT-Guided Core-Needle Biopsy in Diagnosis of Thoracic Lesions Suspicious for Primitive Malignancy of the Lung: A Five-Year Retrospective Analysis. Tomography 2022, 8, 2828–2838. [Google Scholar] [CrossRef]
- Minot, D.M.; Gilman, E.A.; Aubry, M.; Voss, J.S.; Van Epps, S.G.; Tuve, D.J.; Sciallis, A.P.; Henry, M.R.; Salomao, D.R.; Lee, P.; et al. An Investigation into False-negative Transthoracic Fine Needle Aspiration and Core Biopsy Specimens. Diagn. Cytopathol. 2014, 42, 1063–1068. [Google Scholar] [CrossRef]
- Tsukada, H.; Satou, T.; Iwashima, A.; Souma, T. Diagnostic Accuracy of CT-Guided Automated Needle Biopsy of Lung Nodules. Am. J. Roentgenol. 2000, 175, 239–243. [Google Scholar] [CrossRef]
- Huang, M.-D.; Weng, H.-H.; Hsu, S.-L.; Hsu, L.-S.; Lin, W.-M.; Chen, C.-W.; Tsai, Y.-H. Accuracy and Complications of CT-Guided Pulmonary Core Biopsy in Small Nodules: A Single-Center Experience. Cancer Imaging 2019, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Boiselle, P.M.; Shepard, J.O.; Trotman-Dickenson, B.; McLoud, T.C. Diagnostic Accuracy and Safety of CT-Guided Percutaneous Needle Aspiration Biopsy of the Lung: Comparison of Small and Large Pulmonary Nodules. Am. J. Roentgenol. 1996, 167, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sun, W.; Li, Q.; Yao, Y.; Lv, T.; Zeng, J.; Liang, W.; Zhou, X.; Song, Y. Diagnostic Accuracy of CT-Guided Transthoracic Needle Biopsy for Solitary Pulmonary Nodules. PLoS ONE 2015, 10, e0131373. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Gomes, M.M.; Tsao, M.S.; Allen, C.J.; Geddie, W.; Sekhon, H. Fine-Needle Sspiration Biopsy versus Core-Needle Biopsy in Diagnosing Lung Cancer: A Systematic Review. Curr. Oncol. 2012, 19, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Marhana, I.A.; Widianiti, K.; Kusumastuti, E.H. Conformity of Fine Needle Aspiration Biopsy (FNAB) and Core Needle Biopsy (CNB) in Peripheral Lung Tumor Patients: A Cross-Sectional Study. Ann. Med. Surg. 2022, 75, 103423. [Google Scholar] [CrossRef]
- Chen, L.; Jing, H.; Gong, Y.; Tam, A.L.; Stewart, J.; Staerkel, G.; Guo, M. Diagnostic Efficacy and Molecular Testing by Combined Fine-needle Aspiration and Core Needle Biopsy in Patients with a Lung Nodule. Cancer Cytopathol. 2020, 128, 201–206. [Google Scholar] [CrossRef]
- Paulson, E.K.; Sheafor, D.H.; Enterline, D.S.; McAdams, H.P.; Yoshizumi, T.T. CT Fluoroscopy-Guided Interventional Procedures: Techniques and Radiation Dose to Radiologists. Radiology 2001, 220, 161–167. [Google Scholar] [CrossRef]
- Yamamoto, N.; Watanabe, T.; Yamada, K.; Nakai, T.; Suzumura, T.; Sakagami, K.; Yoshimoto, N.; Sato, K.; Tanaka, H.; Mitsuoka, S.; et al. Efficacy and Safety of Ultrasound (US) Guided Percutaneous Needle Biopsy for Peripheral Lung or Pleural Lesion: Comparison with Computed Tomography (CT) Guided Needle Biopsy. J. Thorac. Dis. 2019, 11, 936–943. [Google Scholar] [CrossRef]
- Beslic, S.; Zukic, F.; Milisic, S. Percutaneous Transthoracic CT Guided Biopsies of Lung Lesions; Fine Needle Aspiration Biopsy versus Core Biopsy. Radiol. Oncol. 2012, 46, 19–22. [Google Scholar] [CrossRef]
- Larici, A.R.; Farchione, A.; Franchi, P.; Ciliberto, M.; Cicchetti, G.; Calandriello, L.; Del Ciello, A.; Bonomo, L. Lung Nodules: Size Still Matters. Eur. Respir. Rev. 2017, 26, 170025. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.F.; Peng, J.; Li, B.; Zhang, C.; Yu, J.H. Small (≤20 Mm) Ground-Glass Opacity Pulmonary Lesions: Which Factors Influence the Diagnostic Accuracy of CT-Guided Percutaneous Core Needle Biopsy? BMC Pulm. Med. 2022, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Loverdos, K.; Fotiadis, A.; Kontogianni, C.; Iliopoulou, M.; Gaga, M. Lung Nodules: A Comprehensive Review on Current Approach and Management. Ann. Thorac. Med. 2019, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Krishnamurthy, S.; Broemeling, L.D.; Morello, F.A.; Wallace, M.J.; Ahrar, K.; Madoff, D.C.; Murthy, R.; Hicks, M.E. Small (≤2-Cm) Subpleural Pulmonary Lesions: Short- versus Long-Needle-Path CT-Guided Biopsy—Comparison of Diagnostic Yields and Complications. Radiology 2005, 234, 631–637. [Google Scholar] [CrossRef]
- Laurent, F.; Michel, P.; Latrabe, V.; Tunon De Lara, M.; Marthan, R. Pneumothoraces and Chest Tube Placement after CT-Guided Transthoracic Lung Biopsy Using a Coaxial Technique: Incidence and Risk Factors. Am. J. Roentgenol. 1999, 172, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.E.; Heuvelmans, M.A.; Bock, G.H.D.; Yousaf-Khan, U.; Groen, H.J.M.; Aalst, C.M.V.D.; Nackaerts, K.; Ooijen, P.M.A.V.; Koning, H.J.D.; Vliegenthart, R.; et al. Characteristics of New Solid Nodules Detected in Incidence Screening Rounds of Low-Dose CT Lung Cancer Screening: The NELSON Study. Thorax 2018, 73, 741–747. [Google Scholar] [CrossRef]
- Yeow, K.-M.; Su, I.-H.; Pan, K.-T.; Tsay, P.-K.; Lui, K.-W.; Cheung, Y.-C.; Chou, A.S.-B. Risk Factors of Pneumothorax and Bleeding. Chest 2004, 126, 748–754. [Google Scholar] [CrossRef]
- Khan, M.F.; Straub, R.; Moghaddam, S.R.; Maataoui, A.; Gurung, J.; Wagner, T.O.F.; Ackermann, H.; Thalhammer, A.; Vogl, T.J.; Jacobi, V. Variables Affecting the Risk of Pneumothorax and Intrapulmonal Hemorrhage in CT-Guided Transthoracic Biopsy. Eur. Radiol. 2008, 18, 1356–1363. [Google Scholar] [CrossRef]
- Moulton, J.S.; Moore, P.T. Coaxial percutaneous biopsy technique with automated biopsy devices: Value in improving accuracy and negative predictive value. Radiology 1993, 186, 515–522. [Google Scholar] [CrossRef]
- Görgülü, F.F.; Öksüzler, F.Y.; Arslan, S.A.; Arslan, M.; Özsoy, İ.E.; Görgülü, O. Computed Tomography-Guided Transthoracic Biopsy: Factors Influencing Diagnostic and Complication Rates. J. Int. Med. Res. 2017, 45, 808–815. [Google Scholar] [CrossRef]
- Lim, C.; Lee, K.Y.; Kim, Y.K.; Ko, J.M.; Han, D.H. CT -guided Core Biopsy of Malignant Lung Lesions: How Many Needle Passes Are Needed? J. Med. Imaging Radiat. Oncol. 2013, 57, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Park, C.M.; Lee, K.H.; Lim, K.Y.; Suh, Y.J.; Im, D.J.; Hur, J.; Han, D.H.; Kang, M.-J.; Choo, J.Y.; et al. Analysis of Complications of Percutaneous Transthoracic Needle Biopsy Using CT-Guidance Modalities In a Multicenter Cohort of 10568 Biopsies. Korean J. Radiol. 2019, 20, 323. [Google Scholar] [CrossRef]
- Cerci, J.J.; Bogoni, M.; Cerci, R.J.; Masukawa, M.; Neto, C.C.P.; Krauzer, C.; Fanti, S.; Sakamoto, D.G.; Barreiros, R.B.; Nanni, C.; et al. PET/CT-Guided Biopsy of Suspected Lung Lesions Requires Less Rebiopsy Than CT-Guided Biopsy Due to Inconclusive Results. J. Nucl. Med. 2021, 62, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.-C.; Li, T.-F.; Han, X.-W.; Wu, G.; Ma, J.; Fu, M.-T.; Sun, Q.; Beilner, J. Clinical Applications of the C-Arm Cone-Beam CT-Based 3D Needle Guidance System in Performing Percutaneous Transthoracic Needle Biopsy of Pulmonary Lesions. Diagn. Interv. Radiol. 2014, 20, 470–474. [Google Scholar] [CrossRef]
- Hwang, E.J.; Kim, H.; Park, C.M.; Yoon, S.H.; Lim, H.; Goo, J.M. Cone Beam Computed Tomography Virtual Navigation-Guided Transthoracic Biopsy of Small (≤1 cm) Pulmonary Nodules: Impact of Nodule Visibility during Real-Time Fluoroscopy. Br. J. Radiol. 2018, 91, 20170805. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Cui, N.; Ma, Q.; Lin, Z.; Zhang, J.; Li, X. Conventional versus Cone-Beam Computed Tomography in Lung Biopsy: Diagnostic Performance, Risks, and the Advantages of Tract Embolization with Gelfoam Particle Suspension. Quant. Imaging Med. Surg. 2024, 14, 6479–6492. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Li, L.; Liu, D.; Wu, X.; Zhang, W.; Pan, F.; Liang, H.; Zheng, C. C-Arm Cone-Beam CT Virtual Navigation versus Conventional CT Guidance in the Transthoracic Lung Biopsy: A Case-Control Study. Diagnostics 2022, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Wallace, M.J.; Cardella, J.F.; Kundu, S.; Miller, D.L.; Rose, S.C. Quality Improvement Guidelines for Percutaneous Needle Biopsy. J. Vasc. Interv. Radiol. 2010, 21, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.R.D.; Rocha, R.D.; Falsarella, P.M.; Rahal Junior, A.; Santos, R.S.D.; Franceschini, J.P.; Fernando, H.C.; Garcia, R.G. CT-Guided Percutaneous Core Needle Biopsy of Pulmonary Nodules Smaller than 2 Cm: Technical Aspects and Factors Influencing Accuracy. J. Bras. Pneumol. 2018, 44, 307–314. [Google Scholar] [CrossRef]
- Tanaka, J.; Sonomura, T.; Shioyama, Y.; Kutsukake, Y.; Tomita, K.; Ushimi, T.; Yokoyama, Y.; Abe, K.; Suzuki, K. ‘“Oblique Path”’—The Optimal Needle Path for Computed Tomography-Guided Biopsy of Small Subpleural Lesions. Cardiovasc. Interv. Radiol. 1996, 19, 332–334. [Google Scholar]
- Covey, A.M.; Gandhi, R.; Brody, L.A.; Getrajdman, G.; Thaler, H.T.; Brown, K.T. Factors Associated With Pneumothorax and Pneumothorax Requiring Treatment after Percutaneous Lung Biopsy in 443 Consecutive Patients. J. Vasc. Interv. Radiol. 2004, 15, 479–483. [Google Scholar] [CrossRef]
- He, C.; Zhao, L.; Yu, H.-L.; Zhao, W.; Li, D.; Li, G.-D.; Wang, H.; Huo, B.; Huang, Q.-M.; Liang, B.-W.; et al. Pneumothorax after Percutaneous CT-Guided Lung Nodule Biopsy: A Prospective, Multicenter Study. Quant. Imaging Med. Surg. 2024, 14, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Kuban, J.D.; Tam, A.L.; Huang, S.Y.; Ensor, J.E.; Philip, A.S.; Chen, G.J.; Ahrar, J.; Murthy, R.; Avritscher, R.; Madoff, D.C.; et al. The Effect of Needle Gauge on the Risk of Pneumothorax and Chest Tube Placement After Percutaneous Computed Tomographic (CT)-Guided Lung Biopsy. Cardiovasc. Intervent. Radiol. 2015, 38, 1595–1602. [Google Scholar] [CrossRef]
- Beck, K.S.; Kim, T.-J.; Lee, K.Y.; Kim, Y.K.; Kang, J.H.; Han, D.H. CT-Guided Coaxial Biopsy of Malignant Lung Lesions: Are Cores from 20-Gauge Needle Adequate for Histologic Diagnosis and Molecular Analysis? J. Thorac. Dis. 2019, 11, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Nour-Eldin, N.-E.A.; Alsubhi, M.; Emam, A.; Lehnert, T.; Beeres, M.; Jacobi, V.; Gruber-Rouh, T.; Scholtz, J.-E.; Vogl, T.J.; Naguib, N.N. Pneumothorax Complicating Coaxial and Non-Coaxial CT-Guided Lung Biopsy: Comparative Analysis of Determining Risk Factors and Management of Pneumothorax in a Retrospective Review of 650 Patients. Cardiovasc. Intervent. Radiol. 2016, 39, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Ruud, E.A.; Stavem, K.; Geitung, J.T.; Borthne, A.; Søyseth, V.; Ashraf, H. Predictors of Pneumothorax and Chest Drainage after Percutaneous CT-Guided Lung Biopsy: A Prospective Study. Eur. Radiol. 2021, 31, 4243–4252. [Google Scholar] [CrossRef] [PubMed]
- Nour-Eldin, N.-E.; Naguib, N.N.N.; Tawfik, A.M.; Koitka, K.; Saeed, A.S.; Vogl, T.J. Outcomes of an Algorithmic Approach to Management of Pneumothorax Complicating Thermal Ablation of Pulmonary Neoplasms. J. Vasc. Interv. Radiol. 2011, 22, 1279–1286. [Google Scholar] [CrossRef]
- Winokur, R.; Pua, B.; Sullivan, B.; Madoff, D. Percutaneous Lung Biopsy: Technique, Efficacy, and Complications. Semin. Interv. Radiol. 2013, 30, 121–127. [Google Scholar] [CrossRef]
- Wells, B.J.; Roberts, D.J.; Grondin, S.; Navsaria, P.H.; Kirkpatrick, A.W.; Dunham, M.B.; Ball, C.G. To Drain or Not to Drain? Predictors of Tube Thoracostomy Insertion and Outcomes Associated with Drainage of Traumatic Hemothoraces. Injury 2015, 46, 1743–1748. [Google Scholar] [CrossRef]
- Tai, R.; Dunne, R.M.; Trotman-Dickenson, B.; Jacobson, F.L.; Madan, R.; Kumamaru, K.K.; Hunsaker, A.R. Frequency and Severity of Pulmonary Hemorrhage in Patients Undergoing Percutaneous CT-Guided Transthoracic Lung Biopsy: Single-Institution Experience of 1175 Cases. Radiology 2016, 279, 287–296. [Google Scholar] [CrossRef]
- Zhu, J.; Qu, Y.; Wang, X.; Jiang, C.; Mo, J.; Xi, J.; Wen, Z. Risk Factors Associated with Pulmonary Hemorrhage and Hemoptysis Following Percutaneous CT-Guided Transthoracic Lung Core Needle Biopsy: A Retrospective Study of 1090 Cases. Quant. Imaging Med. Surg. 2020, 10, 1008–1020. [Google Scholar] [CrossRef]
- Filippo, M.D.; Saba, L.; Silva, M.; Zagaria, R.; Concari, G.; Nizzoli, R.; Bozzetti, C.; Tiseo, M.; Ardizzoni, A.; Lipia, S.; et al. CT-Guided Biopsy of Pulmonary Nodules: Is Pulmonary Hemorrhage a Complication or an Advantage? Diagn. Interv. Radiol. 2014, 20, 421–425. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhao, L.; Yu, H.; Zhao, W.; Li, D.; Li, G.; Wang, H.; Huo, B.; Huang, Q.; Liang, B.; et al. Incidence and Risk Factors for Pulmonary Hemorrhage after Percutaneous CT-Guided Pulmonary Nodule Biopsy: An Observational Study. Sci. Rep. 2024, 14, 7348. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, R.; Narsinh, K.; Tuan, A.; Kinney, T. Systemic Air Embolism Following Percutaneous Lung Biopsy. Semin. Interv. Radiol. 2014, 31, 375–377. [Google Scholar] [CrossRef]
- Kuo, H.-L.; Cheng, L.; Chung, T.-J. Systemic Air Embolism Detected during Percutaneous Transthoracic Needle Biopsy: Report of Two Cases and a Proposal for a Routine Postprocedure Computed Tomography Scan of the Aorto-Cardiac Region. Clin. Imaging 2010, 34, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Fiore, L.; Frenk, N.E.; Martins, G.L.P.; Viana, P.C.C.; Menezes, M.R.D. Systemic Air Embolism after Percutaneous Lung Biopsy: A Manageable Complication. J. Radiol. Case Rep. 2017, 11, 6–14. [Google Scholar] [CrossRef]
- Fintelmann, F.J.; Sharma, A.; Shepard, J.O. Prevention of Air Embolism During Transthoracic Biopsy of the Lung. Am. J. Roentgenol. 2017, 209, W404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, S.; Zhong, F.; Liao, M. Risk Factors for Air Embolism Following Computed Tomography-Guided Percutaneous Transthoracic Needle Biopsy: A Systematic Review and Meta-Analysis. Diagn. Interv. Radiol. 2023, 29, 478–491. [Google Scholar] [CrossRef]
- Malik, N.; Claus, P.L.; Illman, J.E.; Kligerman, S.J.; Moynagh, M.R.; Levin, D.L.; Woodrum, D.A.; Arani, A.; Arunachalam, S.P.; Araoz, P.A. Air Embolism: Diagnosis and Management. Future Cardiol. 2017, 13, 365–378. [Google Scholar] [CrossRef]
- Wang, T.; Luo, L.; Zhou, Q. Risk of Pleural Recurrence in Early Stage Lung Cancer Patients after Percutaneous Transthoracic Needle Biopsy: A Meta-Analysis. Sci. Rep. 2017, 7, 42762. [Google Scholar] [CrossRef]
- Li, H.; Chen, R.; Zhao, J. Correlation between Percutaneous Transthoracic Needle Biopsy and Recurrence in Stage I Lung Cancer: A Systematic Review and Meta-Analysis. BMC Pulm. Med. 2020, 20, 198. [Google Scholar] [CrossRef]
- Toyoda, T.; Suzuki, H.; Hata, A.; Yamamoto, T.; Morimoto, J.; Sakairi, Y.; Wada, H.; Nakajima, T.; Yoshino, I. Is Needle Biopsy a Risk Factor of Pleural Recurrence after Surgery for Non-Small Cell Lung Cancer? J. Thorac. Dis. 2020, 12, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Date, H.; Toyooka, S.; Oto, T.; Yamane, M.; Hiraki, T.; Gobara, H.; Mimura, H.; Kanazawa, S. Percutaneous Computed Tomography-guided Lung Biopsy and Pleural Dissemination: An Assessment by Intraoperative Pleural Lavage Cytology. Cancer 2009, 115, 5526–5533. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, J.; Masago, K.; Kato, R.; Hata, A.; Kaji, R.; Fujita, S.; Katakami, N. CT-Guided Fine-Needle Aspiration and Core Needle Biopsies of Pulmonary Lesions: A Single-Center Experience With 750 Biopsies in Japan. Am. J. Roentgenol. 2015, 204, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, F.; Huang, Y.-Y.; Cao, W. Comparison between Computed Tomography-Guided Core and Fine Needle Lung Biopsy: A Meta-Analysis. Medicine 2022, 101, e29016. [Google Scholar] [CrossRef]
- Zhang, H.; Guang, Y.; He, W.; Cheng, L.; Yu, T.; Tang, Y.; Song, H.; Liu, X.; Zhang, Y. Ultrasound-Guided Percutaneous Needle Biopsy Skill for Peripheral Lung Lesions and Complications Prevention. J. Thorac. Dis. 2020, 12, 3697–3705. [Google Scholar] [CrossRef]
- Yang, R.J.; Yang, Y.L.; Ruan, L.T.; Yuan, L.J.; Chao, L.J.; Chen, S.; Duan, Y.Y. Clinical Value of Ultrasound-Guided Percutaneous Biopsy of Pulmonary Lesions. Genet. Mol. Res. 2015, 14, 3784–3790. [Google Scholar] [CrossRef]
- Wang Memoli, J.S.; Nietert, P.J.; Silvestri, G.A. Meta-Analysis of Guided Bronchoscopy for the Evaluation of the Pulmonary Nodule. Chest 2012, 142, 385–393. [Google Scholar] [CrossRef]
- Chen, C.; Mu, C.-Y.; Su, M.-Q.; Mao, J.-Y.; Zhu, Y.-H.; Huang, J.-A. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Increases the Yield of Transbronchial Lung Biopsy for the Evaluation of Peribronchial Lesions. Chin. Med. J. 2017, 130, 11–14. [Google Scholar] [CrossRef]
- Cheng, F.; Su, L.; Qian, C. Circulating Tumor DNA: A Promising Biomarker in the Liquid Biopsy of Cancer. Oncotarget 2016, 7, 48832–48841. [Google Scholar] [CrossRef]
- Rivera, M.P.; Mehta, A.C.; Wahidi, M.M. Establishing the Diagnosis of Lung Cancer. Chest 2013, 143, e142S–e165S. [Google Scholar] [CrossRef]
- Bogoni, M.; Cerci, J.J.; Cornelis, F.H.; Nanni, C.; Tabacchi, E.; Schöder, H.; Shyn, P.B.; Sofocleous, C.T.; Solomon, S.B.; Kirov, A.S. Practice and Prospects for PET/CT Guided Interventions. Q. J. Nucl. Med. Mol. Imaging 2021, 65, 20. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, M.; Li, W.; Li, X.; Hou, H.; Shan, H. Computed Tomography Image: Guided Needle Biopsy in the Diagnosis of Lung Malignant Tumors under Artificial Intelligence Algorithm. Sci. Program. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Lanza, C.; Carriero, S.; Buijs, E.F.M.; Mortellaro, S.; Pizzi, C.; Sciacqua, L.V.; Biondetti, P.; Angileri, S.A.; Ianniello, A.A.; Ierardi, A.M.; et al. Robotics in Interventional Radiology: Review of Current and Future Applications. Technol. Cancer Res. Treat. 2023, 22, 153303382311520. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Cone-Beam CT (CBCT) | Conventional CT (CCT) |
|---|---|---|
| Sensitivity | 92% to 97% | 93% to 97% |
| Specificity | 96% to 100% | 85% to 95% |
| Advantages |
|
|
| Limitations |
|
|
| Complication | Cone-Beam CT (CBCT) | Conventional CT (CCT) |
|---|---|---|
| Pneumothorax | 15% to 20% | 18% to 25% |
| Pulmonary Hemorrhage | 10% to 24% | 15% to 25% |
| Chest Tube Insertion | 1% to 4% | 3% to 6% |
| Hemoptysis | 1% to 3% | 2% to 5% |
| Radiation Exposure | 10% to 30% lower compared to CCT | Higher compared to CBCT |
| Needle Repositioning | Reduced by 84% (real-time guidance) | Requires more repositioning, static images |
| Procedure Time | Typically longer (due to real-time adjustments) | Shorter, more straightforward for simpler cases |
| Complication | Causes | Notes |
|---|---|---|
| Pneumothorax |
| Occurs in 12–45% of cases, with a higher risk in patients with emphysema or multiple needle passes. Chest tube insertion is required in 2–15% of cases. |
| Pulmonary Hemorrhage |
| Hemorrhage may self-resolve in minor cases; serious cases require interventions like bronchoscopy or embolization. |
| Air Embolism |
| Rare but potentially fatal. Requires immediate identification and treatment. |
| Tumor Seeding |
| Very rare (<0.01%) but can occur with aggressive tumors. Larger needles and multiple passes increase the risk. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantinescu, A.; Stoicescu, E.R.; Iacob, R.; Chira, C.A.; Cocolea, D.M.; Nicola, A.C.; Mladin, R.; Oancea, C.; Manolescu, D. CT-Guided Transthoracic Core-Needle Biopsy of Pulmonary Nodules: Current Practices, Efficacy, and Safety Considerations. J. Clin. Med. 2024, 13, 7330. https://doi.org/10.3390/jcm13237330
Constantinescu A, Stoicescu ER, Iacob R, Chira CA, Cocolea DM, Nicola AC, Mladin R, Oancea C, Manolescu D. CT-Guided Transthoracic Core-Needle Biopsy of Pulmonary Nodules: Current Practices, Efficacy, and Safety Considerations. Journal of Clinical Medicine. 2024; 13(23):7330. https://doi.org/10.3390/jcm13237330
Chicago/Turabian StyleConstantinescu, Amalia, Emil Robert Stoicescu, Roxana Iacob, Cosmin Alexandru Chira, Daiana Marina Cocolea, Alin Ciprian Nicola, Roxana Mladin, Cristian Oancea, and Diana Manolescu. 2024. "CT-Guided Transthoracic Core-Needle Biopsy of Pulmonary Nodules: Current Practices, Efficacy, and Safety Considerations" Journal of Clinical Medicine 13, no. 23: 7330. https://doi.org/10.3390/jcm13237330
APA StyleConstantinescu, A., Stoicescu, E. R., Iacob, R., Chira, C. A., Cocolea, D. M., Nicola, A. C., Mladin, R., Oancea, C., & Manolescu, D. (2024). CT-Guided Transthoracic Core-Needle Biopsy of Pulmonary Nodules: Current Practices, Efficacy, and Safety Considerations. Journal of Clinical Medicine, 13(23), 7330. https://doi.org/10.3390/jcm13237330








