Multidisciplinary Digital Therapeutics for Chronic Low Back Pain Versus In-Person Therapeutic Exercise with Education: A Randomized Controlled Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
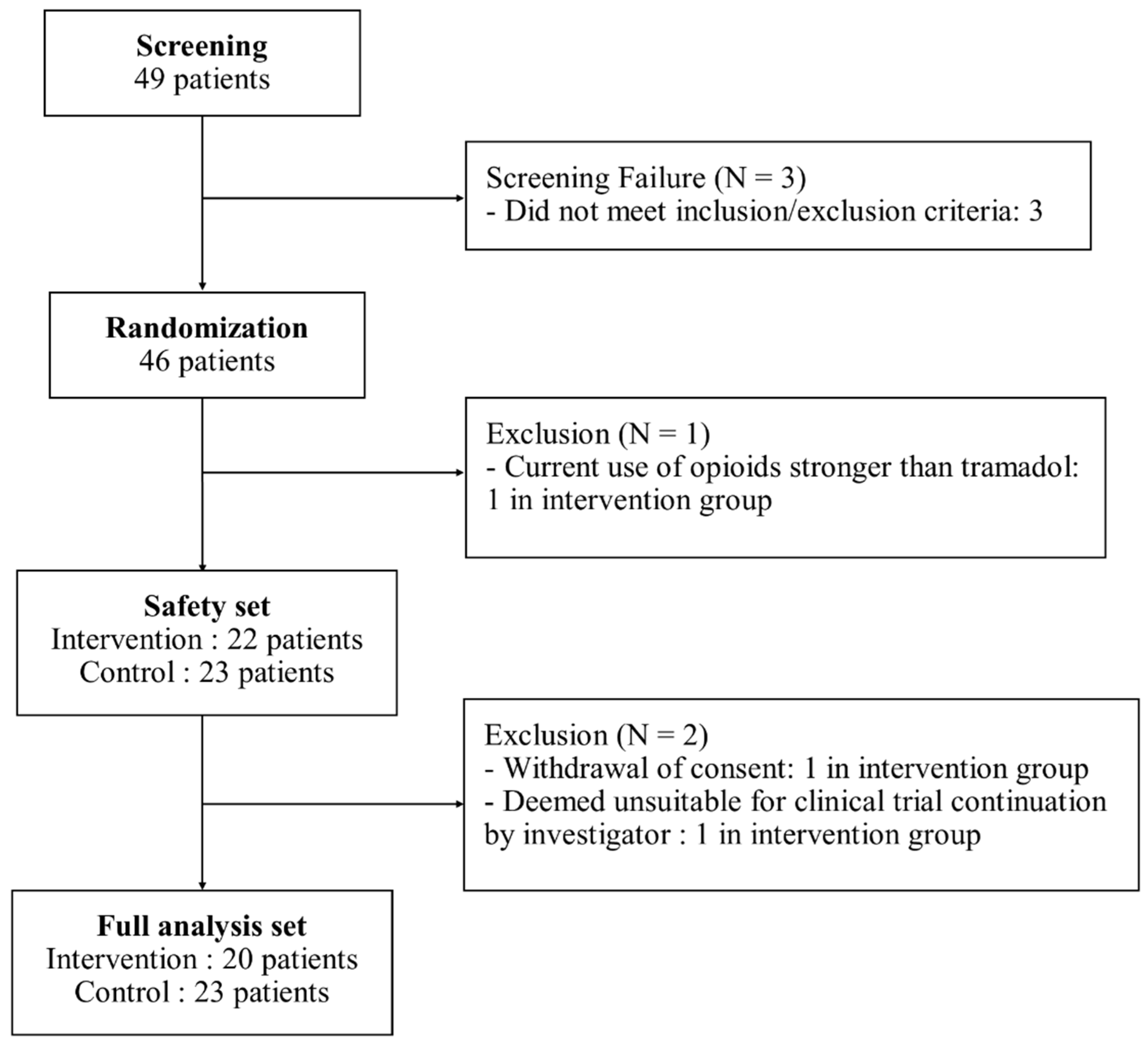
2.2. Participants
2.3. Randomization and Blinding
2.4. Interventions
2.5. Outcome Measures and Data Collection
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
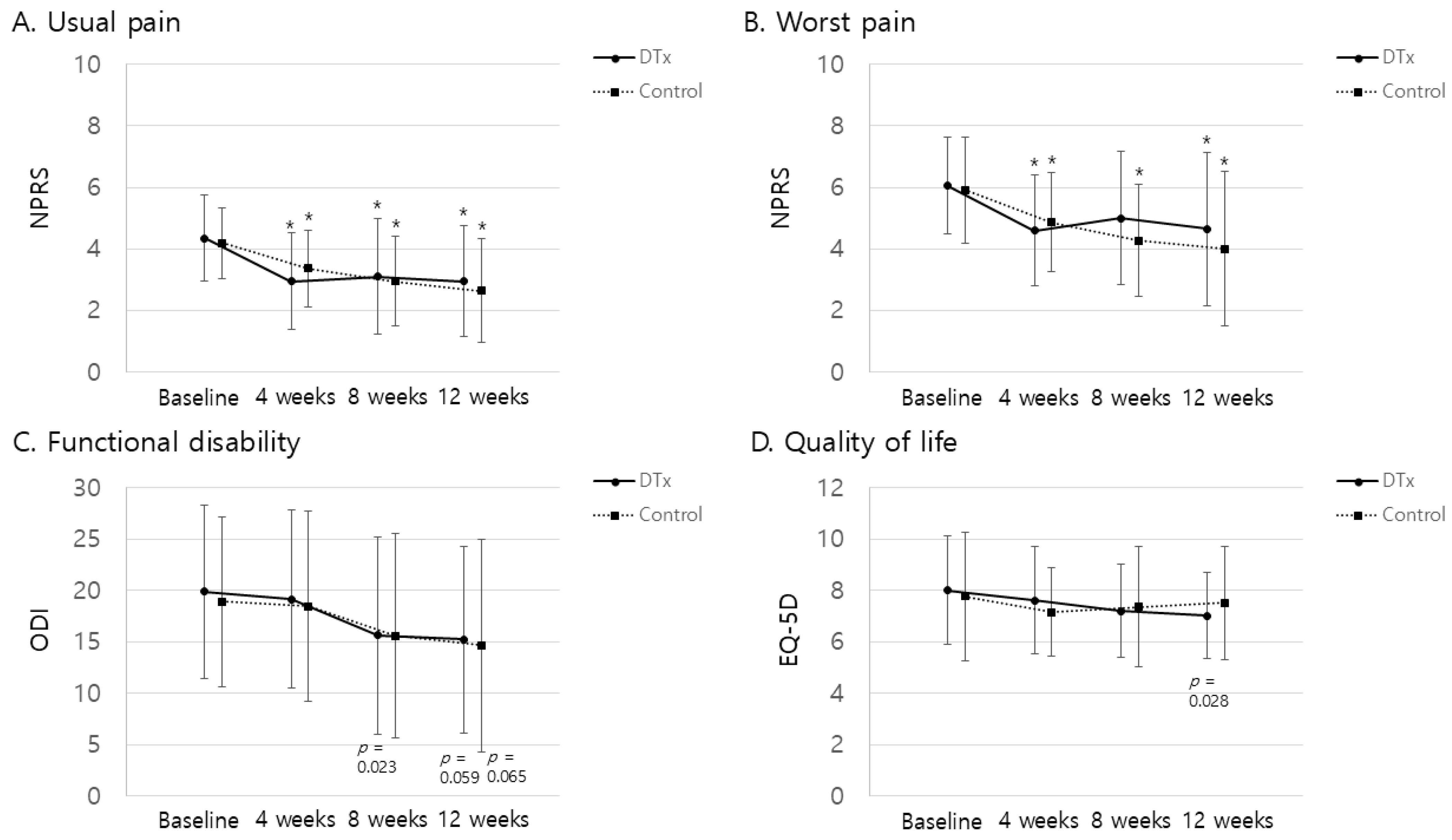
3.2. Primary Outcomes
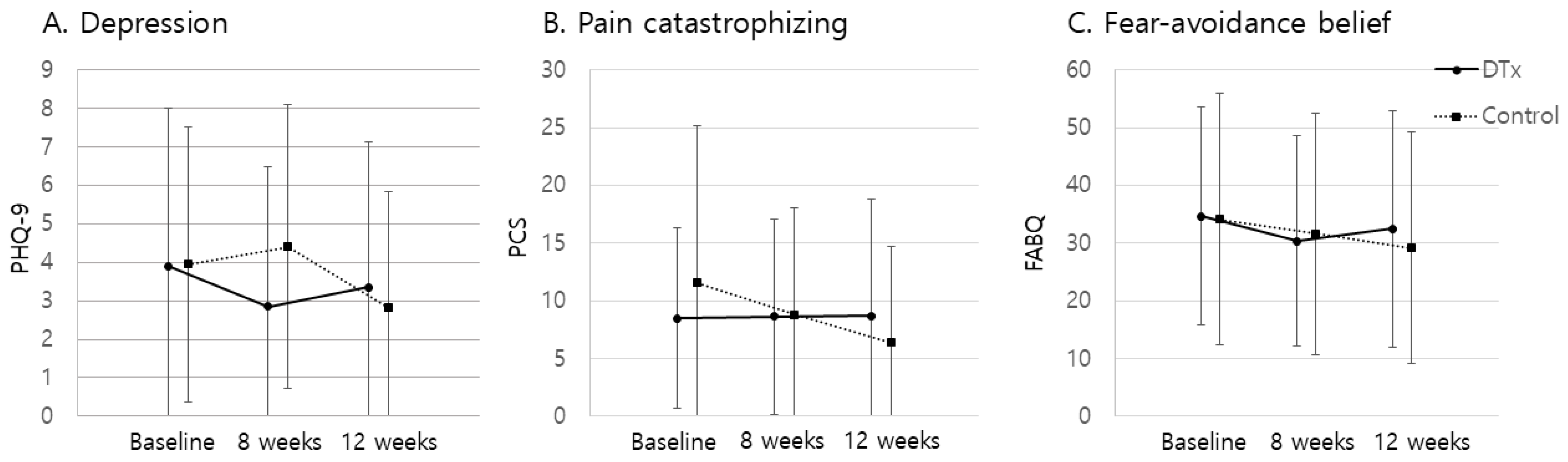
3.3. Secondary Outcomes
3.4. Safety Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Tulder, M.; Becker, A.; Bekkering, T.; Breen, A.; del Real, M.T.G.; Hutchinson, A.; Koes, B.; Laerum, E.; Malmivaara, A.; COST B13 Working Group on Guidelines for the Management of Acute Low Back Pain in Primary Care. European guidelines for the management of acute nonspecific low back pain in primary care. Eur. Spine J. 2006, 15 (Suppl. 2), S169–S191. [Google Scholar] [CrossRef]
- van Dieën, J.H.; Kuijer, P.P.; Burdorf, A.; Marras, W.S.; Adams, M.A. Non-specific low back pain. Lancet 2012, 379, 1874–1875. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.E.; Anema, J.R.; Cherkin, D.; Chou, R.; Cohen, S.P.; Gross, D.P.; Ferreira, P.H.; Fritz, J.M.; Koes, B.W.; Peul, W.; et al. Prevention and treatment of low back pain: Evidence, challenges, and promising directions. Lancet 2018, 391, 2368–2383. [Google Scholar] [CrossRef] [PubMed]
- Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef]
- Costa, L.D.M.; Henschke, N.; Maher, C.G.; Refshauge, K.M.; Herbert, D.R.; McAuley, J.H.; Das, A.; Costa, L.O.P. Prognosis of chronic low back pain: Design of an inception cohort study. BMC Musculoskelet. Disord. 2007, 8, 4. [Google Scholar] [CrossRef][Green Version]
- Hayden, J.A.; Chou, R.; Hogg-Johnson, S.; Bombardier, C. Systematic reviews of low back pain prognosis had variable methods and results: Guidance for future prognosis reviews. J. Clin. Epidemiol. 2009, 62, 781–796.e1. [Google Scholar] [CrossRef]
- Hong, J.Y.; Song, K.S.; Cho, J.H.; Lee, J.H.; Kim, N.H. An updated overview of low back pain management. Asian Spine J. 2022, 16, 968–982. [Google Scholar] [CrossRef]
- Perrot, S.; Doane, M.J.; Jaffe, D.H.; Dragon, E.; Abraham, L.; Viktrup, L.; Bushmakin, A.G.; Cappelleri, J.C.; Conaghan, P.G. Burden of chronic low back pain: Association with pain severity and prescription medication use in five large European countries. Pain Pract. 2022, 22, 359–371. [Google Scholar] [CrossRef]
- Baroncini, A.; Maffulli, N.; Schäfer, L.; Manocchio, N.; Bossa, M.; Foti, C.; Klimuch, A.; Migliorini, F. Physiotherapeutic and non-conventional approaches in patients with chronic low-back pain: A level I Bayesian network meta-analysis. Sci. Rep. 2024, 14, 11546. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, T.; van Middelkoop, M.; Rubinstein, S.M.; Ostelo, R.; Verhagen, A.; Koes, B.W.; van Tulder, M.W. A systematic review on the effectiveness of pharmacological interventions for chronic non-specific low-back pain. Eur. Spine J. 2011, 20, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, D.S.; Matz, P.; Bono, C.M.; Cho, C.H.; Easa, J.E.; Ghiselli, G.; Ghogawala, Z.; Reitman, C.A.; Resnick, D.K.; Watters, W.C.; et al. Guideline summary review: An evidence -based clinical guideline for the diagnosis and treatment of low back pain. Spine J. 2020, 20, 998–1024. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, K.; O’Keeffe, M.; O’Sullivan, P. NICE low back pain guidelines: Opportunities and obstacles to change practice. Br. J. Sports Med. 2017, 51, 1632–1633. [Google Scholar] [CrossRef] [PubMed]
- Nalamachu, S.; Rauck, R.L.; Hale, M.E.; Florete, O.G.; Robinson, C.Y.; Farr, S.J. A long-term, open-label safety study of single-entity hydrocodone bitartrate extended release for the treatment of moderate to severe chronic pain. J. Pain Res. 2014, 7, 669–678. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.; Negi, M.P.S.; Sharma, V.P.; Shukla, R.; Dev, R.; Mishra, U.K. Efficacy of two multimodal treatments on physical strength of occupationally subgrouped male with low back pain. J. Back Musculoskelet. Rehabil. 2009, 22, 179–188. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Ferreira, P.H.; Latimer, J.; Herbert, R.D.; Hodges, P.W.; Jennings, M.D.; Maher, C.G.; Refshauge, K.M. Comparison of general exercise, motor control exercise and spinal manipulative therapy for chronic low back pain: A randomized trial. Pain 2007, 131, 31–37. [Google Scholar] [CrossRef]
- Koldaş Doğan, S.; Sonel Tur, B.; Kurtaiş, Y.; Atay, M.B. Comparison of three different approaches in the treatment of chronic low back pain. Clin. Rheumatol. 2008, 27, 873–881. [Google Scholar] [CrossRef]
- Clarke, J.; van Tulder, M.; Blomberg, S.; de Vet, H.; van der Heijden, G.; Bronfort, G. Traction for low back pain with or without sciatica: An updated systematic review within the framework of the Cochrane Collaboration. Spine 2006, 31, 1591–1599. [Google Scholar] [CrossRef]
- Ho, E.; Ferreira, M.; Chen, L.X.; Simic, M.; Ashton-James, C.; Comachio, J.; Hayden, J.; Ferreira, P.; Wang, D.X.M.; Ferreira, P.H. Psychological interventions for chronic, non-specific low back pain: Systematic review with network meta-analysis. Br. Med. J. 2022, 376, 24. [Google Scholar] [CrossRef]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry disability index. Spine 2000, 25, 2940–2952, discussion 2952. [Google Scholar] [CrossRef] [PubMed]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Darnall, B.D.; Sturgeon, J.A.; Cook, K.F.; Taub, C.J.; Roy, A.; Burns, J.W.; Sullivan, M.; Mackey, S.C. Development and validation of a daily pain catastrophizing scale. J. Pain 2017, 18, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Waddell, G.; Newton, M.; Henderson, I.; Somerville, D.; Main, C.J. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain 1993, 52, 157–168. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Steffl, M.; Glenney, S.S.; Green, M.; Cashwell, L.; Prajerova, K.; Bunn, J. The prone bridge test: Performance, validity, and reliability among older and younger adults. J. Bodyw. Mov. Ther. 2018, 22, 385–389. [Google Scholar] [CrossRef]
- Balogun, J.A.; Ajayi, L.O.; Alawale, F. Determinants of single limb stance balance performance. Afr. J. Med. Med. Sci. 1997, 26, 153–157. [Google Scholar]
- Toelle, T.R.; Utpadel-Fischler, D.A.; Haas, K.K.; Priebe, J.A. App-based multidisciplinary back pain treatment versus combined physiotherapy plus online education: A randomized controlled trial. npj Digit. Med. 2019, 2, 34. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Y.; Bian, Y.; Chen, L.; Yuan, W.; Zhang, H.; Feng, Q.; Zhang, H.; Liu, D.; Lin, Y. The physical and psychological effects of telerehabilitation-based exercise for patients with nonspecific low back pain: Prospective randomized controlled trial. JMIR mHealth uHealth 2024, 12, e56580. [Google Scholar] [CrossRef]
- Shebib, R.; Bailey, J.F.; Smittenaar, P.; Perez, D.A.; Mecklenburg, G.; Hunter, S. Randomized controlled trial of a 12-week digital care program in improving low back pain. npj Digit. Med. 2019, 2, 1. [Google Scholar] [CrossRef]
- Rughani, G.; Nilsen, T.I.L.; Wood, K.; Mair, F.S.; Hartvigsen, J.; Mork, P.J.; Nicholl, B.I. The selfBACK artificial intelligence-based smartphone app can improve low back pain outcome even in patients with high levels of depression or stress. Eur. J. Pain 2023, 27, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, H.S.; Sharma, S.; Verma, S. Smartphone app in self-management of chronic low back pain: A randomized controlled trial. Eur. Spine J. 2018, 27, 2862–2874. [Google Scholar] [CrossRef] [PubMed]
- Almhdawi, K.A.; Obeidat, D.S.; Kanaan, S.F.; Oteir, A.O.; Mansour, Z.M.; Alrabbaei, H. Efficacy of an innovative smartphone application for office workers with chronic non-specific low back pain: A pilot randomized controlled trial. Clin. Rehabil. 2020, 34, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Chiauzzi, E.; Pujol, L.A.; Wood, M.; Bond, K.; Black, R.; Yiu, E.; Zacharoff, K. painACTION-back pain: A self-management Website for people with chronic back pain. Pain Med. 2010, 11, 1044–1058. [Google Scholar] [CrossRef]
- Yang, J.Y.; Wei, Q.; Ge, Y.L.; Meng, L.J.; Zhao, M.D. Smartphone-based remote self-management of chronic low back pain: A preliminary study. J. Healthc. Eng. 2019, 2019, 4632946. [Google Scholar] [CrossRef]
- Nordstoga, A.L.; Bach, K.; Sani, S.; Wiratunga, N.; Mork, P.J.; Villumsen, M.; Cooper, K. Usability and acceptability of an app (SELFBACK) to support self-management of low back pain: Mixed methods study. JMIR Rehabil. Assist. Technol. 2020, 7, e18729. [Google Scholar] [CrossRef]
- Sitges, C.; Terrasa, J.L.; García-Dopico, N.; Segur-Ferrer, J.; Velasco-Roldán, O.; Crespí-Palmer, J.; González-Roldán, A.M.; Montoya, P. An educational and exercise mobile phone-based intervention to elicit electrophysiological changes and to improve psychological functioning in adults with nonspecific chronic low back pain (BackFit app): Nonrandomized clinical trial. JMIR mHealth uHealth 2022, 10, e29171. [Google Scholar] [CrossRef]
- Vad, V.B.; Madrazo-Ibarra, A.; Estrin, D.; Pollak, J.P.; Carroll, K.M.; Vojta, D.; Vad, A.; Trapness, C. Back Rx, a personalized mobile phone application for discogenic chronic low back pain: A prospective pilot study. BMC Musculoskelet. Disord. 2022, 23, 923. [Google Scholar] [CrossRef]
- Biebl, J.T.; Rykala, M.; Strobel, M.; Kaur Bollinger, P.K.; Ulm, B.; Kraft, E.; Huber, S.; Lorenz, A. App-based feedback for rehabilitation exercise correction in patients with knee or hip osteoarthritis: Prospective cohort study. J. Med. Internet Res. 2021, 23, e26658. [Google Scholar] [CrossRef]
- Marcuzzi, A.; Nordstoga, A.L.; Bach, K.; Aasdahl, L.; Nilsen, T.I.L.; Bardal, E.M.; Boldermo, N.Ø.; Falkener Bertheussen, G.; Marchand, G.H.; Gismervik, S.; et al. Effect of an Artificial Intelligence-Based Self-Management App on musculoskeletal Health in Patients with Neck and/or Low Back Pain Referred to Specialist Care: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2320400. [Google Scholar] [CrossRef]
- Hartmann, R.; Avermann, F.; Zalpour, C.; Griefahn, A. Impact of an AI app-based exercise program for people with low back pain compared to standard care: A longitudinal cohort-study. Health Sci. Rep. 2023, 6, e1060. [Google Scholar] [CrossRef] [PubMed]
- Dillingham, T.; Kenia, J.; Popescu, A.; Plastaras, C.; Becker, S.; Shofer, F. Pain outcomes with an elliptical regimen (POWER) study: Identifying the proper dosage of exercise for therapeutic effect in persons with chronic back pain. J. Phys. Med. Rehabil. 2020, 2, 23–28. [Google Scholar] [PubMed]
- Li, Y.; Gong, Y.; Zheng, B.; Fan, F.; Yi, T.; Zheng, Y.; He, P.; Fang, J.; Jia, J.; Zhu, Q.; et al. Effects on adherence to a mobile app-based self-management digital therapeutics among patients with coronary heart disease: Pilot randomized controlled trial. JMIR mHealth uHealth 2022, 10, e32251. [Google Scholar] [CrossRef] [PubMed]
- Areias, A.C.; Costa, F.; Janela, D.; Molinos, M.; Moulder, R.G.; Lains, J.; Scheer, J.K.; Bento, V.; Yanamadala, V.; Correia, F.D. Long-term clinical outcomes of a remote digital musculoskeletal program: An ad hoc analysis from a longitudinal study with a non-participant comparison group. Healthcare 2022, 10, 2349. [Google Scholar] [CrossRef] [PubMed]

| Intervention (n = 20) | Control (n = 23) | p Value | |
|---|---|---|---|
| Age (years) | 38.1 ± 10.0 | 38.5 ± 7.4 | 0.930 |
| Female (n [%]) | 16 [80.0] | 18 [81.8] | 0.594 |
| Body mass index (kg/m2) | 22.6 ± 4.4 | 23.4 ± 3.3 | 0.399 |
| Prior pain medication use (n [%]) | 5 [25.0] | 4 [17.4] | 0.711 |
| Onset of backpain (months) | 88.9 ± 55.5 | 58.7 ± 52.6 | 0.078 |
| Usual pain (NPRS) | 4.4 ± 1.4 | 4.2 ± 1.1 | 0.792 |
| Worst pain (NPRS) | 6.1 ± 1.6 | 5.9 ± 1.7 | 0.679 |
| Functional disability (ODI) | 19.9 ± 8.4 | 18.9 ± 8.3 | 0.724 |
| QoL (EQ-5D) | 8.0 ± 2.2 | 7.8 ± 2.5 | 0.600 |
| Depression (PHQ-9) | 3.9 ± 4.1 | 3.8 ± 3.6 | 0.961 |
| Pain catastrophizing (PCS) | 8.5 ± 7.8 | 11.9 ± 13.4 | 0.654 |
| Fear-avoidance beliefs (FABQ) | 32.5 ± 20.4 | 29.4 ± 20.2 | 0.689 |
| Muscle endurance (Prone Bridge, s) | 63.2 ± 39.2 | 46.9 ± 32.6 | 0.161 |
| Balance ability (Single Limb Stance) | 4.3 ± 2.0 | 4.3 ± 2.4 | 0.897 |
| Pelvic incidence (°) | 48.3 ± 9.5 | 49.5 ± 7.1 | 0.649 |
| Lumbar lordosis (°) | 46.3 ± 12.3 | 49.8 ± 10.5 | 0.335 |
| Sacral slope (°) | 34.0 ± 8.0 | 38.6 ± 6.1 | 0.035 |
| Pelvic tilt (°) | 14.2 ± 8.5 | 10.9 ± 6.8 | 0.150 |
| Sagittal vertical axis (mm) | 5.3 ± 29.6 | −3.7 ± 23.6 | 0.265 |
| Cobb’s angle (°) | 3.3 ± 2.3 | 2.8 ± 2.6 | 0.350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.-H.; Park, J.H.; Yoon, C.; Choi, C.-H.; Lee, S.; Park, T.H.; Chang, S.Y.; Jang, S.-H. Multidisciplinary Digital Therapeutics for Chronic Low Back Pain Versus In-Person Therapeutic Exercise with Education: A Randomized Controlled Pilot Study. J. Clin. Med. 2024, 13, 7377. https://doi.org/10.3390/jcm13237377
Kang D-H, Park JH, Yoon C, Choi C-H, Lee S, Park TH, Chang SY, Jang S-H. Multidisciplinary Digital Therapeutics for Chronic Low Back Pain Versus In-Person Therapeutic Exercise with Education: A Randomized Controlled Pilot Study. Journal of Clinical Medicine. 2024; 13(23):7377. https://doi.org/10.3390/jcm13237377
Chicago/Turabian StyleKang, Dong-Ho, Jae Hyeon Park, Chan Yoon, Chi-Hyun Choi, Sanghee Lee, Tae Hyun Park, Sam Yeol Chang, and Seong-Ho Jang. 2024. "Multidisciplinary Digital Therapeutics for Chronic Low Back Pain Versus In-Person Therapeutic Exercise with Education: A Randomized Controlled Pilot Study" Journal of Clinical Medicine 13, no. 23: 7377. https://doi.org/10.3390/jcm13237377
APA StyleKang, D.-H., Park, J. H., Yoon, C., Choi, C.-H., Lee, S., Park, T. H., Chang, S. Y., & Jang, S.-H. (2024). Multidisciplinary Digital Therapeutics for Chronic Low Back Pain Versus In-Person Therapeutic Exercise with Education: A Randomized Controlled Pilot Study. Journal of Clinical Medicine, 13(23), 7377. https://doi.org/10.3390/jcm13237377






