Challenges in AAV-Based Retinal Gene Therapies and the Role of Magnetic Nanoparticle Platforms
Abstract
:1. Introduction
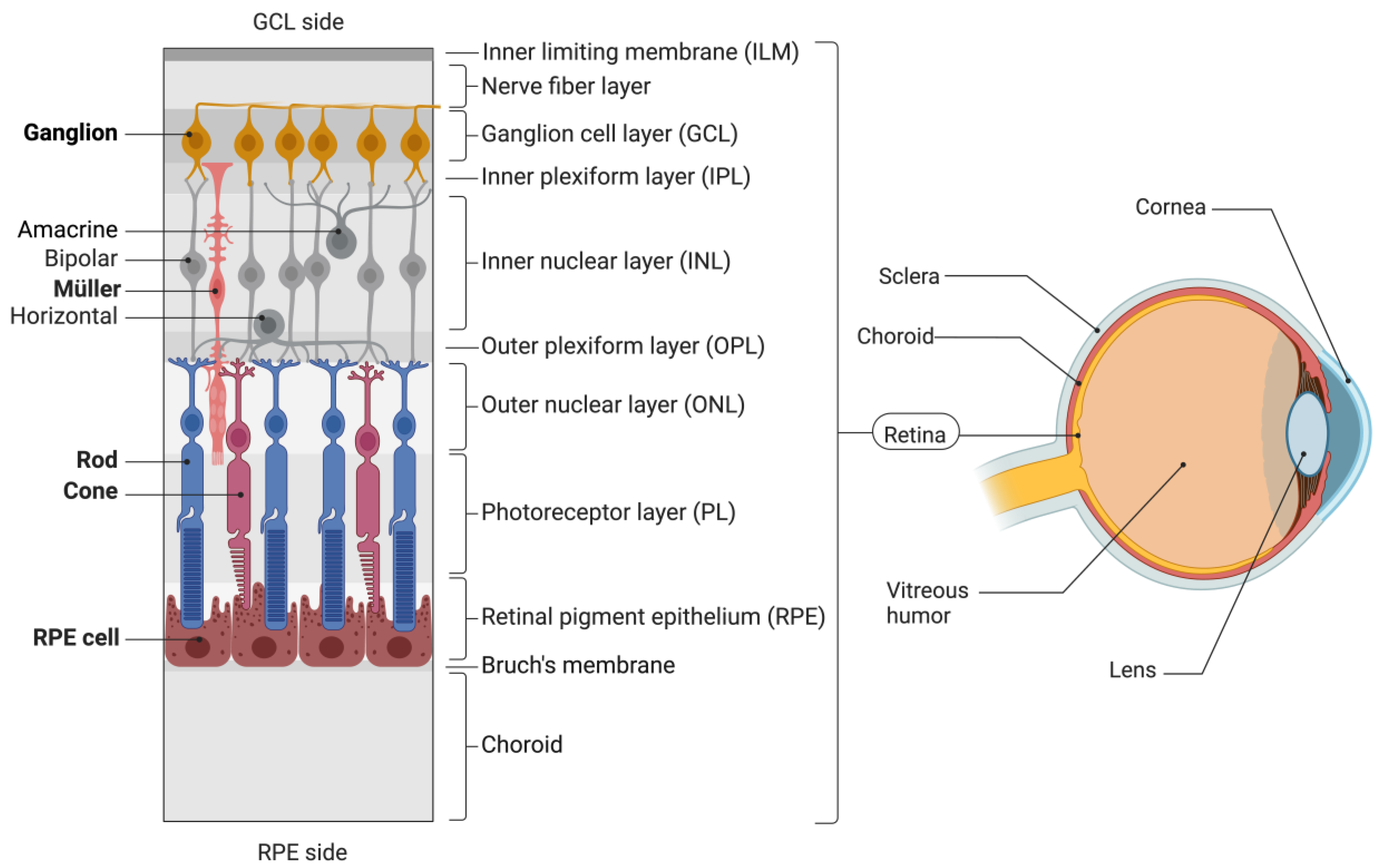
2. Viral Vectors in Retinal Gene Therapy
| AAV | Ganglion | Müller | Rod/Cone | RPE | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IVI | SRI | SCI | IVI | SRI | SCI | IVI | SRI | SCI | IVI | SRI | SCI | ||
| 2/2 | x | x | x | x | [33,34,35,58,63,64,65,66] | ||||||||
| 2/6 | x | x | x | x | [31,66,67] | ||||||||
| 2/8 | x | x | x | x | x | x | [31,34,57,65,68,69,70,71] | ||||||
| 2/9 | x | x | x | x | [31,65,68] | ||||||||
| 9/PHP.eB | x | x | x | [34] | |||||||||
| 2/ShH10 | x | [72] | |||||||||||
| 2/7m8 | x | x | x | x | x | x | x | x | [73,74,75,76] | ||||
| 2/Y-F mutants | x | x | x | x | x | x | x | x | [73,77,78,79] | ||||
| 2/NHP mutants | x | x | x | x | [61] | ||||||||
| 2/K9 mutants | x | x | x | x | [60] | ||||||||
| 2/GL | x | x | x | x | x | x | x | x | [74] | ||||
| 2/NN | x | x | x | x | x | x | x | x | [74] | ||||
| DJ | x | x | x | [80] | |||||||||
| 2/1 | x | x | x | x | [31,64,81] | ||||||||
| 2/4 | x | x | [19,81,82] | ||||||||||
| 2/5 | x | [19,63,64,65,68,69,83] | |||||||||||
| 2/7 | x | [65,68] | |||||||||||
| 8 | x | x | x | x | [35,76,84,85] | ||||||||
| v128 | x | x | x | x | x | x | [76] | ||||||
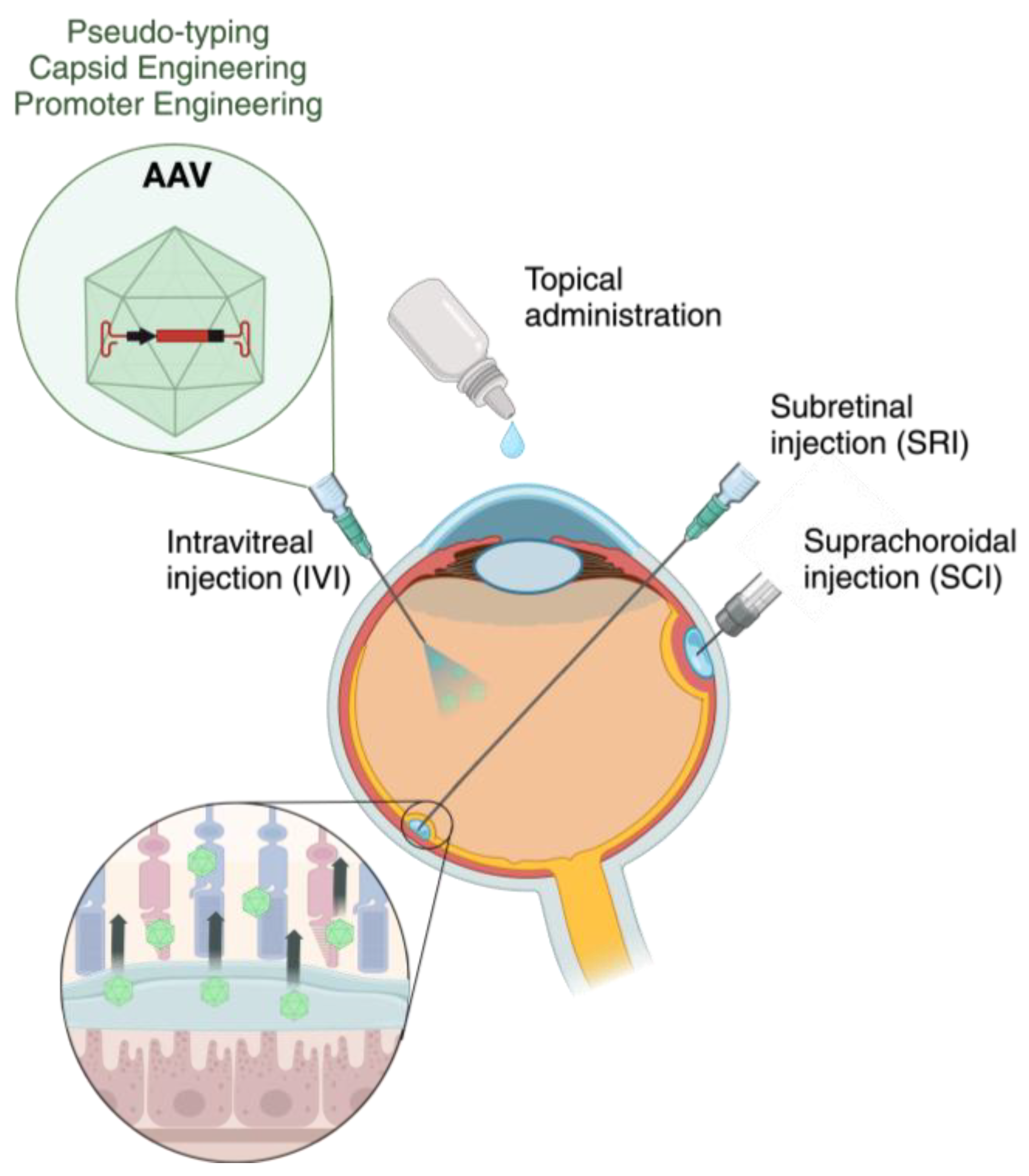
3. Obstacles in AAV-Based Retinal Gene Therapy
| Mouse | Monkey | Pig | Human | |
|---|---|---|---|---|
| Size | 3 mm | 20 mm | 24 mm | 24 mm |
| Retinal thickness | 204 μm | 292 μm | 300 μm | 310 μm |
| Ganglion cell density | 4000 cells/mm2 | 3100 cells/mm2 | 6000 cells/mm2 | 5700 cells/mm2 |
| Peak cone density | 1.8 × 104 cells/mm2 | 14 × 104 cells/mm2 | 3.9 × 104 cells/mm2 | 15 × 104 cells/mm2 |
| RPE thickness | 18 μm | 16.5 μm | N/A | 1–2 μm |
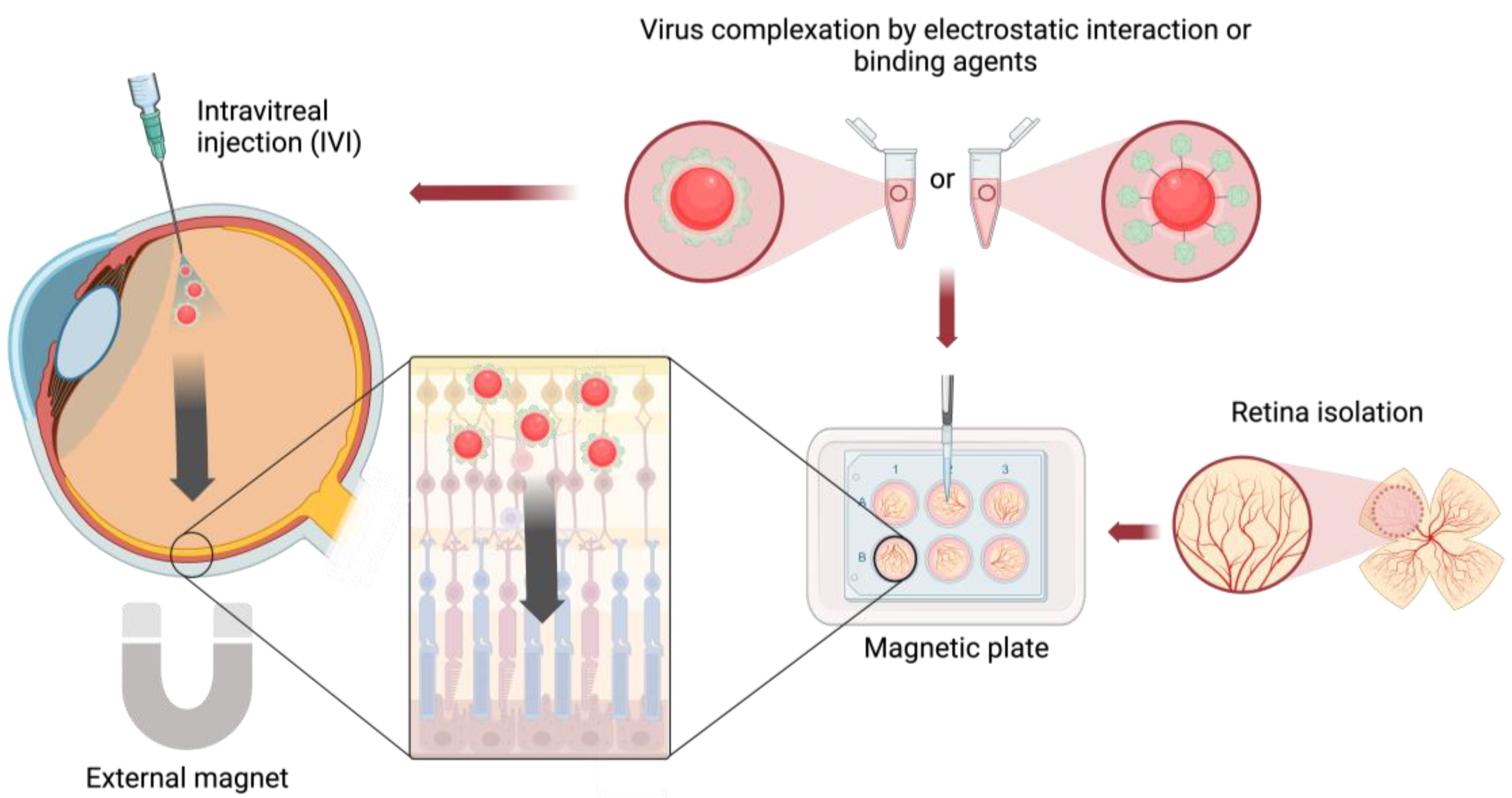
4. Magnetic Nanoparticles for AAV Delivery
| Species | Tissue or Organ | MNPs | Modifying Agent | Virus Type | Reference |
|---|---|---|---|---|---|
| Mouse | Brain (in vivo) | SPION | PEI, Streptavidin | Biotinylated adenovirus | [130] |
| Mouse | Tumor xenografts (in vivo) | IOP | PEI | Adenovirus | [138] |
| Mouse | Blood vessels (in vivo, ex vivo) | CombiMag, TransMag (Chemicell, Berlin, Germany) | - | Lentivirus | [139] |
| Mouse | Intestine (in vivo) | IOP | N-hexanoyl chitosan | Adenovirus | [140] |
| Rat | Intestine (in vivo), rlood vessels (in vivo) | SPION | PEI | Retrovirus, Adenovirus | [133] |
| Rat | Brain (in vivo) | AdenoMag (OZ Biosciences, Marseille, France) | - | Adenovirus | [129] |
| Mouse | Brain (in vivo), retina (ex vivo) | SuperMag Silica beads (Alpha Biobeads, San Diego, CA, USA) | AEEA | Rabiesvirus, Lentivirus, AAV | [125] |
| ViroMag (OZ Biosciences, Marseille, France) | - | ||||
| Mouse | Lung (in vivo) | Magnetite particle | HSPG | AAV | [131] |
| Pig | Retina (ex vivo), eye (ex vivo) | FluoMag-V (OZ Biosciences, Marseille, France) | - | AAV | [126] |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thapa, R.; Khanal, S.; Tan, H.; Thapa, S.; Rens, G. Prevalence, Pattern and Risk Factors of Retinal Diseases Among an Elderly Population in Nepal: The Bhaktapur Retina Study. Clin. Ophthalmol. 2020, 14, 2109–2118. [Google Scholar] [PubMed]
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global Data on Visual Impairment in the Year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Cheloni, R.; Gandolfi, S.A.; Signorelli, C.; Odone, A. Global Prevalence of Diabetic Retinopathy: Protocol for a Systematic Review and Meta-Analysis. BMJ Open 2019, 9, e022188. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wang, J.-H.; Chen, J.; Li, F.; Edwards, T.L.; Hewitt, A.W.; Liu, G.-S. Gene Therapy for Visual Loss: Opportunities and Concerns. Prog. Retin. Eye Res. 2019, 68, 31–53. [Google Scholar] [CrossRef]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and Safety of Voretigene Neparvovec (AAV2-hRPE65v2) in Patients with RPE65 -Mediated Inherited Retinal Dystrophy: A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Maguire, A.M.; Russell, S.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Drack, A.V.; Simonelli, F.; Leroy, B.P.; Reape, K.Z.; High, K.A.; et al. Durability of Voretigene Neparvovec for Biallelic RPE65-Mediated Inherited Retinal Disease. Ophthalmology 2021, 128, 1460–1468. [Google Scholar] [CrossRef]
- Bucher, K.; Rodríguez-Bocanegra, E.; Dauletbekov, D.; Fischer, M.D. Immune Responses to Retinal Gene Therapy Using Adeno-Associated Viral Vectors—Implications for Treatment Success and Safety. Prog. Retin. Eye Res. 2021, 83, 100915. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-Viral Vectors for Gene-Based Therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Moraru, A.; Costin, D.; Iorga, R.; Munteanu, M.; Moraru, R.; Branisteanu, D. Current Trends in Gene Therapy for Retinal Diseases (Review). Exp. Ther. Med. 2021, 23, 26. [Google Scholar] [CrossRef]
- Zinn, E.; Vandenberghe, L.H. Adeno-Associated Virus: Fit to Serve. Curr. Opin. Virol. 2014, 8, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Samulski, R.J. Engineering Adeno-Associated Virus Vectors for Gene Therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Hudry, E.; Vandenberghe, L.H. Therapeutic AAV Gene Transfer to the Nervous System: A Clinical Reality. Neuron 2019, 101, 839–862. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Lai, M.; Chiuppesi, F.; Ceccherini-Nelli, L.; Pistello, M. Viral Vectors: A Look Back and Ahead on Gene Transfer Technology. New Microbiol. 2013, 36, 1–22. [Google Scholar] [PubMed]
- Lu, P.; Lai, J.; Tabata, Y.; Hsiue, G.A. Methodology Based on the “Anterior Chamber of Rabbit Eyes” Model for Noninvasively Determining the Biocompatibility of Biomaterials in an Immune Privileged Site. J. Biomed. Mater. Res. 2008, 86, 108–116. [Google Scholar] [CrossRef]
- Fuller-Carter, P.I.; Basiri, H.; Harvey, A.R.; Carvalho, L.S. Focused Update on AAV-Based Gene Therapy Clinical Trials for Inherited Retinal Degeneration. BioDrugs 2020, 34, 763–781. [Google Scholar] [CrossRef]
- Trapani, I.; Auricchio, A. Seeing the Light after 25 Years of Retinal Gene Therapy. Trends Mol. Med. 2018, 24, 669–681. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral Vector Platforms within the Gene Therapy Landscape. Sig Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Wiley, L.A.; Burnight, E.R.; Kaalberg, E.E.; Jiao, C.; Riker, M.J.; Halder, J.A.; Luse, M.A.; Han, I.C.; Russell, S.R.; Sohn, E.H.; et al. Assessment of Adeno-Associated Virus Serotype Tropism in Human Retinal Explants. Hum. Gene Ther. 2018, 29, 424–436. [Google Scholar] [CrossRef]
- Milone, M.C.; O’Doherty, U. Clinical Use of Lentiviral Vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef]
- Sweigard, J.H.; Cashman, S.M.; Kumar-Singh, R. Adenovirus Vectors Targeting Distinct Cell Types in the Retina. Invest. Ophthalmol. Vis. Sci. 2010, 51, 2219. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.; Sun, J.W.D.; Forbes, B.; Maguire, A. Adenovirus Vector-Mediated In Vivo Gene Transfer Into Adult Murine Retina. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2535–2542. [Google Scholar]
- Puppo, A.; Cesi, G.; Marrocco, E.; Piccolo, P.; Jacca, S.; Shayakhmetov, D.M.; Parks, R.J.; Davidson, B.L.; Colloca, S.; Brunetti-Pierri, N.; et al. Retinal Transduction Profiles by High-Capacity Viral Vectors. Gene Ther. 2014, 21, 855–865. [Google Scholar] [CrossRef]
- Li, T.; Adamian, M.; Roof, D.J.; Berson, E.L.; Dryja, T.P.; Roessler, B.J.; Davidson, B.L. In Vivo Transfer of a Reporter Gene to the Retina Mediated by an Adenoviral Vector. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2543–2549. [Google Scholar]
- Han, I.C.; Burnight, E.R.; Ulferts, M.J.; Worthington, K.S.; Russell, S.R.; Sohn, E.H.; Mullins, R.F.; Stone, E.M.; Tucker, B.A.; Wiley, L.A. Helper-Dependent Adenovirus Transduces the Human and Rat Retina but Elicits an Inflammatory Reaction When Delivered Subretinally in Rats. Hum. Gene Ther. 2019, 30, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.J.; Chen, L.; Anton, M.; Sankar, U.; Rudnicki, M.A.; Graham, F.L. A Helper-Dependent Adenovirus Vector System: Removal of Helper Virus by Cre-Mediated Excision of the Viral Packaging Signal. Proc. Natl. Acad. Sci. USA 1996, 93, 13565–13570. [Google Scholar] [CrossRef]
- DiCarlo, J.E.; Mahajan, V.B.; Tsang, S.H. Gene Therapy and Genome Surgery in the Retina. J. Clin. Investig. 2018, 128, 2177–2188. [Google Scholar] [CrossRef]
- Trapani, I.; Puppo, A.; Auricchio, A. Vector Platforms for Gene Therapy of Inherited Retinopathies. Prog. Retin. Eye Res. 2014, 43, 108–128. [Google Scholar] [CrossRef]
- Kontogiannis, T.; Braybrook, J.; McElroy, C.; Foy, C.; Whale, A.S.; Quaglia, M.; Smales, C.M. Characterization of AAV Vectors: A Review of Analytical Techniques and Critical Quality Attributes. Mol. Ther. Methods Clin. Dev. 2024, 32, 101309. [Google Scholar] [CrossRef]
- Bennett, J.; Ashtari, M.; Wellman, J.; Marshall, K.A.; Cyckowski, L.L.; Chung, D.C.; McCague, S.; Pierce, E.A.; Chen, Y.; Bennicelli, J.L.; et al. AAV2 Gene Therapy Readministration in Three Adults with Congenital Blindness. Sci. Transl. Med. 2012, 4, 120ra15. [Google Scholar] [CrossRef]
- Han, I.C.; Cheng, J.L.; Burnight, E.R.; Ralston, C.L.; Fick, J.L.; Thomsen, G.J.; Tovar, E.F.; Russell, S.R.; Sohn, E.H.; Mullins, R.F.; et al. Retinal Tropism and Transduction of Adeno-Associated Virus Varies by Serotype and Route of Delivery (Intravitreal, Subretinal, or Suprachoroidal) in Rats. Hum. Gene Ther. 2020, 31, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Vandenberghe, L.H.; Alvira, M.R.; Lu, Y.; Calcedo, R.; Zhou, X.; Wilson, J.M. Clades of Adeno-Associated Viruses Are Widely Disseminated in Human Tissues. J. Virol. 2004, 78, 6381–6388. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yung, J.; Mak, H.; Leung, C.K.S. Factors Governing the Transduction Efficiency of Adeno-Associated Virus in the Retinal Ganglion Cells Following Intravitreal Injection. Gene Ther. 2019, 26, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Palfi, A.; Chadderton, N.; Millington-Ward, S.; Post, I.; Humphries, P.; Kenna, P.F.; Farrar, G.J. AAV-PHP.eB Transduces Both the Inner and Outer Retina with High Efficacy in Mice. Mol. Ther. Methods Clin. Dev. 2022, 25, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, L.H.; Bell, P.; Maguire, A.M.; Cearley, C.N.; Xiao, R.; Calcedo, R.; Wang, L.; Castle, M.J.; Maguire, A.C.; Grant, R.; et al. Dosage Thresholds for AAV2 and AAV8 Photoreceptor Gene Therapy in Monkey. Sci. Transl. Med. 2011, 3, 88ra54. [Google Scholar] [CrossRef]
- Kotterman, M.A.; Schaffer, D.V. Engineering Adeno-Associated Viruses for Clinical Gene Therapy. Nat. Rev. Genet. 2014, 15, 445–451. [Google Scholar] [CrossRef]
- Gao, G.; Vandenberghe, L.H.; Wilson, J.M. New Recombinant Serotypes of AAV Vectors. Curr. Gene Ther. 2005, 5, 285–297. [Google Scholar] [CrossRef]
- Yokoi, K.; Kachi, S.; Zhang, H.S.; Gregory, P.D.; Spratt, S.K.; Samulski, R.J.; Campochiaro, P.A. Ocular Gene Transfer with Self-Complementary AAV Vectors. Invest. Ophthalmol. Vis. Sci. 2007, 48, 3324. [Google Scholar] [CrossRef]
- Wu, L.; Lam, S.; Cao, H.; Guan, R.; Duan, R.; Van Der Kooy, D.; Bremner, R.; Molday, R.S.; Hu, J. Subretinal Gene Delivery Using Helper-Dependent Adenoviral Vectors. Cell Biosci. 2011, 1, 15. [Google Scholar] [CrossRef]
- Léveillard, T.; Sahel, J.-A. Rod-Derived Cone Viability Factor for Treating Blinding Diseases: From Clinic to Redox Signaling. Sci. Transl. Med. 2010, 2, ps16–ps26. [Google Scholar] [CrossRef]
- Dalkara, D.; Kolstad, K.D.; Caporale, N.; Visel, M.; Klimczak, R.R.; Schaffer, D.V.; Flannery, J.G. Inner Limiting Membrane Barriers to AAV-Mediated Retinal Transduction From the Vitreous. Mol. Ther. 2009, 17, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Schön, C.; Biel, M.; Michalakis, S. Retinal Gene Delivery by Adeno-Associated Virus (AAV) Vectors: Strategies and Applications. Eur. J. Pharm. Biopharm. 2015, 95, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Öztürk, B.E.; Johnson, M.E.; Turunç, S.; Stauffer, W.R.; Byrne, L.C. Quantitative Single-Cell Transcriptome-Based Ranking of Engineered AAVs in Human Retinal Explants. Mol. Ther. Methods Clin. Dev. 2022, 25, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.S.; Xu, J.; Pearson, R.A.; Smith, A.J.; Bainbridge, J.W.; Morris, L.M.; Fliesler, S.J.; Ding, X.-Q.; Ali, R.R. Long-Term and Age-Dependent Restoration of Visual Function in a Mouse Model of CNGB3-Associated Achromatopsia Following Gene Therapy. Hum. Mol. Genet. 2011, 20, 3161–3175. [Google Scholar] [CrossRef]
- Michalakis, S.; Mühlfriedel, R.; Tanimoto, N.; Krishnamoorthy, V.; Koch, S.; Fischer, M.D.; Becirovic, E.; Bai, L.; Huber, G.; Beck, S.C.; et al. Restoration of Cone Vision in the CNGA3−/− Mouse Model of Congenital Complete Lack of Cone Photoreceptor Function. Mol. Ther. 2010, 18, 2057–2063. [Google Scholar] [CrossRef]
- Alexander, J.J.; Umino, Y.; Everhart, D.; Chang, B.; Min, S.H.; Li, Q.; Timmers, A.M.; Hawes, N.L.; Pang, J.; Barlow, R.B.; et al. Restoration of Cone Vision in a Mouse Model of Achromatopsia. Nat. Med. 2007, 13, 685–687. [Google Scholar] [CrossRef]
- Komaromy, A.; Alexander, J.; Cooper, A.; Chiodo, V.; Glushakova, L.; Acland, G.; Hauswirth, W.; Aguirre, G. Targeting Gene Expression to Cones with Human Cone Opsin Promoters in Recombinant AAV. Gene Ther. 2008, 15, 1073. [Google Scholar] [CrossRef]
- Flannery, J.G.; Zolotukhin, S.; Vaquero, M.; LaVail, M.; Muzyczka, N.; Hauswirth, W. Efficient Photoreceptor-Targeted Gene Expression in Vivo by Recombinant Adeno-Associated Virus. Proc. Natl. Acad. Sci. USA 1997, 94, 6916–6921. [Google Scholar] [CrossRef]
- Allocca, M.; Mussolino, C.; Garcia-Hoyos, M.; Sanges, D.; Io, C.; Petrillo, M.; Vandenberghe, L.; Wilson, J.; Marigo, V.; Surace, E.; et al. Novel Adeno-Associated Virus Serotypes Efficiently Transduce Murine Photoreceptors. J. Virol. 2007, 81, 11372–11380. [Google Scholar] [CrossRef]
- Young, J. A Short, Highly Active Photoreceptor-Specific Enhancer/Promoter Region Upstream of the Human Rhodopsin Kinase Gene. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4076–4085. [Google Scholar] [CrossRef]
- Beltran, W.A.; Cideciyan, A.V.; Lewin, A.S.; Iwabe, S.; Khanna, H.; Sumaroka, A.; Chiodo, V.A.; Fajardo, D.S.; Román, A.J.; Deng, W.-T.; et al. Gene Therapy Rescues Photoreceptor Blindness in Dogs and Paves the Way for Treating Human X-Linked Retinitis Pigmentosa. Proc. Natl. Acad. Sci. USA 2012, 109, 2132–2137. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, A.; Kawase, K.; Thompson, D.A. Promoter Analysis of RPE65, the Gene Encoding a 61-kDa Retinal Pigment Epithelium-Specific Protein. Investig. Ophthalmol. Vis. Sci. 1998, 39, 637–644. [Google Scholar]
- Esumi, N.; Oshima, Y.; Li, Y.; Campochiaro, P.A.; Zack, D.J. Analysis of the VMD2 Promoter and Implication of E-Box Binding Factors in Its Regulation *. J. Biol. Chem. 2004, 279, 19064–19073. [Google Scholar] [CrossRef] [PubMed]
- Doroudchi, M.; Greenberg, K.; Liu, J.; Silka, K.; Boyden, E.; Lockridge, J.; Arman, A.; Janani, R.; Boye, S.; Boye, S.; et al. Virally Delivered Channelrhodopsin-2 Safely and Effectively Restores Visual Function in Multiple Mouse Models of Blindness. Mol. Ther. J. Am. Soc. Gene Ther. 2011, 19, 1220–1229. [Google Scholar] [CrossRef]
- Macé, E.; Caplette, R.; Marre, O.; Sengupta, A.; Chaffiol, A.; Barbe, P.; Desrosiers, M.; Bamberg, E.; Sahel, J.-A.; Picaud, S.; et al. Targeting Channelrhodopsin-2 to ON-Bipolar Cells With Vitreally Administered AAV Restores ON and OFF Visual Responses in Blind Mice. Mol. Ther. 2015, 23, 7–16. [Google Scholar] [CrossRef]
- Ali, R.R.; Reichel, M.B.; Thrasher, A.J.; Levinsky, R.J.; Kinnon, C.; Kanuga, N.; Hunt, D.M.; Bhattacharya, S.S. Gene Transfer into the Mouse Retina Mediated by an Adeno-Associated Viral Vector. Hum. Mol. Genet. 1996, 5, 591–594. [Google Scholar] [CrossRef]
- Manfredi, A.; Marrocco, E.; Puppo, A.; Cesi, G.; Sommella, A.; Della Corte, M.; Rossi, S.; Giunti, M.; Craft, C.M.; Bacci, M.L.; et al. Combined Rod and Cone Transduction by Adeno-Associated Virus 2/8. Hum. Gene Ther. 2013, 24, 982–992. [Google Scholar] [CrossRef]
- Yin, L.; Greenberg, K.; Hunter, J.J.; Dalkara, D.; Kolstad, K.D.; Masella, B.D.; Wolfe, R.; Visel, M.; Stone, D.; Libby, R.T.; et al. Intravitreal Injection of AAV2 Transduces Macaque Inner Retina. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2775–2783. [Google Scholar] [CrossRef]
- Stieger, K.; Le Meur, G.; Lasne, F.; Weber, M.; Deschamps, J.-Y.; Nivard, D.; Mendes-Madeira, A.; Provost, N.; Martin, L.; Moullier, P.; et al. Long-Term Doxycycline-Regulated Transgene Expression in the Retina of Nonhuman Primates Following Subretinal Injection of Recombinant AAV Vectors. Mol. Ther. 2006, 13, 967–975. [Google Scholar] [CrossRef]
- Öztürk, B.E.; Johnson, M.E.; Kleyman, M.; Turunç, S.; He, J.; Jabalameli, S.; Xi, Z.; Visel, M.; Dufour, V.L.; Iwabe, S.; et al. scAAVengr, a Transcriptome-Based Pipeline for Quantitative Ranking of Engineered AAVs with Single-Cell Resolution. eLife 2021, 10, e64175. [Google Scholar] [CrossRef]
- Byrne, L.C.; Day, T.P.; Visel, M.; Strazzeri, J.A.; Fortuny, C.; Dalkara, D.; Merigan, W.H.; Schaffer, D.V.; Flannery, J.G. In Vivo–Directed Evolution of Adeno-Associated Virus in the Primate Retina. JCI Insight 2020, 5, e135112. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, B.; Laperrousaz, E.; Tribble, J.; Verhaagen, J.; Fawcett, J.; Martin, K.; Williams, P.; Osborne, A. Improving Adeno-Associated Viral (AAV) Vector-Mediated Transgene Expression in Retinal Ganglion Cells: Comparison of Five Promoters. Gene Ther. 2023, 30, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Charbel Issa, P.; Silva, S.; Lipinski, D.; Singh, M.; Mouravlev, A.; You, Q.S.; Barnard, A.; Hankins, M.; During, M.; Maclaren, R. Assessment of Tropism and Effectiveness of New Primate-Derived Hybrid Recombinant AAV Serotypes in the Mouse and Primate Retina. PLoS ONE 2013, 8, e60361. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, A. Exchange of Surface Proteins Impacts on Viral Vector Cellular Specificity and Transduction Characteristics: The Retina as a Model. Hum. Mol. Genet. 2001, 10, 3075–3081. [Google Scholar] [CrossRef] [PubMed]
- Lebherz, C.; Maguire, A.; Tang, W.; Bennett, J.; Wilson, J.M. Novel AAV Serotypes for Improved Ocular Gene Transfer. J. Gene Med. 2008, 10, 375–382. [Google Scholar] [CrossRef]
- Hellström, M.; Ruitenberg, M.J.; Pollett, M.A.; Ehlert, E.M.E.; Twisk, J.; Verhaagen, J.; Harvey, A.R. Cellular Tropism and Transduction Properties of Seven Adeno-Associated Viral Vector Serotypes in Adult Retina after Intravitreal Injection. Gene Ther. 2009, 16, 521–532. [Google Scholar] [CrossRef]
- Yang, G.S.; Schmidt, M.; Yan, Z.; Lindbloom, J.D.; Harding, T.C.; Donahue, B.A.; Engelhardt, J.F.; Kotin, R.; Davidson, B.L. Virus-Mediated Transduction of Murine Retina with Adeno-Associated Virus: Effects of Viral Capsid and Genome Size. J. Virol. 2002, 76, 7651–7660. [Google Scholar] [CrossRef]
- Allocca, M.; Doria, M.; Petrillo, M.; Colella, P.; Garcia-Hoyos, M.; Gibbs, D.; Kim, S.R.; Maguire, A.; Rex, T.S.; Di Vicino, U.; et al. Serotype-Dependent Packaging of Large Genes in Adeno-Associated Viral Vectors Results in Effective Gene Delivery in Mice. J. Clin. Invest. 2008, 118, 1955–1964. [Google Scholar] [CrossRef]
- Mussolino, C.; Della Corte, M.; Rossi, S.; Viola, F.; Di Vicino, U.; Marrocco, E.; Neglia, S.; Doria, M.; Testa, F.; Giovannoni, R.; et al. AAV-Mediated Photoreceptor Transduction of the Pig Cone-Enriched Retina. Gene Ther. 2011, 18, 637–645. [Google Scholar] [CrossRef]
- Stieger, K.; Colle, M.-A.; Dubreil, L.; Mendes-Madeira, A.; Weber, M.; le Meur, G.; Deschamps, J.-Y.; Nathalie, P.; Nivard, D.; Cherel, Y.; et al. Subretinal Delivery of Recombinant AAV Serotype 8 Vector in Dogs Results in Gene Transfer to Neurons in the Brain. Mol. Ther. J. Am. Soc. Gene Ther. 2008, 16, 916–923. [Google Scholar] [CrossRef]
- Igarashi, T.; Miyake, K.; Asakawa, N.; Miyake, N.; Shimada, T.; Takahashi, H. Direct Comparison of Administration Routes for AAV8-Mediated Ocular Gene Therapy. Curr. Eye Res. 2013, 38, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, R.R.; Koerber, J.T.; Dalkara, D.; Flannery, J.G.; Schaffer, D.V. A Novel Adeno-Associated Viral Variant for Efficient and Selective Intravitreal Transduction of Rat Müller Cells. PLoS ONE 2009, 4, e7467. [Google Scholar] [CrossRef] [PubMed]
- Hickey, D.G.; Edwards, T.L.; Barnard, A.R.; Singh, M.S.; De Silva, S.R.; McClements, M.E.; Flannery, J.G.; Hankins, M.W.; MacLaren, R.E. Tropism of Engineered and Evolved Recombinant AAV Serotypes in the Rd1 Mouse and Ex Vivo Primate Retina. Gene Ther. 2017, 24, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, M.; Schön, C.; Occelli, L.M.; Rossi, A.; Meumann, N.; Boyd, R.F.; Bartoe, J.T.; Siedlecki, J.; Gerhardt, M.J.; Babutzka, S.; et al. Novel AAV Capsids for Intravitreal Gene Therapy of Photoreceptor Disorders. EMBO Mol. Med. 2021, 13, e13392. [Google Scholar] [CrossRef]
- Dalkara, D.; Byrne, L.C.; Klimczak, R.R.; Visel, M.; Yin, L.; Merigan, W.H.; Flannery, J.G.; Schaffer, D.V. In Vivo–Directed Evolution of a New Adeno-Associated Virus for Therapeutic Outer Retinal Gene Delivery from the Vitreous. Sci. Transl. Med. 2013, 5, 189ra76. [Google Scholar] [CrossRef]
- Luo, S.; Jiang, H.; Li, Q.; Qin, Y.; Yang, S.; Li, J.; Xu, L.; Gou, Y.; Zhang, Y.; Liu, F.; et al. An Adeno-associated Virus Variant Enabling Efficient Ocular-directed Gene Delivery Across Species. Nat. Commun. 2024, 15, 3780. [Google Scholar] [CrossRef]
- Petrs-Silva, H.; Dinculescu, A.; Li, Q.; Min, S.-H.; Chiodo, V.; Pang, J.-J.; Zhong, L.; Zolotukhin, S.; Srivastava, A.; Lewin, A.S.; et al. High-Efficiency Transduction of the Mouse Retina by Tyrosine-Mutant AAV Serotype Vectors. Mol. Ther. 2009, 17, 463–471. [Google Scholar] [CrossRef]
- Petrs-Silva, H.; Dinculescu, A.; Li, Q.; Deng, W.-T.; Pang, J.; Min, S.-H.; Chiodo, V.; Neeley, A.W.; Govindasamy, L.; Bennett, A.; et al. Novel Properties of Tyrosine-Mutant AAV2 Vectors in the Mouse Retina. Mol. Ther. 2011, 19, 293–301. [Google Scholar] [CrossRef]
- Mowat, F.M.; Gornik, K.R.; Dinculescu, A.; Boye, S.L.; Hauswirth, W.W.; Petersen-Jones, S.M.; Bartoe, J.T. Tyrosine Capsid-Mutant AAV Vectors for Gene Delivery to the Canine Retina from a Subretinal or Intravitreal Approach. Gene Ther. 2014, 21, 96–105. [Google Scholar] [CrossRef]
- Katada, Y.; Kobayashi, K.; Tsubota, K.; Kurihara, T. Evaluation of AAV-DJ Vector for Retinal Gene Therapy. PeerJ 2019, 7, e6317. [Google Scholar] [CrossRef]
- Rabinowitz, E.; Rolling, F.; Li, C.; Conrath, H.; Xiao, W.; Xiao, X.; Samulski, R. Cross-Packaging of a Single Adeno-Associated Virus (AAV) Type 2 Vector Genome into Multiple AAV Serotypes Enables Transduction with Broad Specificity. J. Virol. 2002, 76, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Rabinowitz, J.; Provost, N.; Conrath, H.; Folliot, S.; Briot, D.; Chérel, Y.; Chenuaud, P.; Samulski, J.; Moullier, P.; et al. Recombinant Adeno-Associated Virus Serotype 4 Mediates Unique and Exclusive Long-Term Transduction of Retinal Pigmented Epithelium in Rat, Dog, and Nonhuman Primate after Subretinal Delivery. Mol. Ther. 2003, 7, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Lotery, A.J.; Yang, G.S.; Mullins, R.F.; Russell, S.R.; Schmidt, M.; Stone, E.M.; Lindbloom, J.D.; Chiorini, J.A.; Kotin, R.M.; Davidson, B.L. Adeno-Associated Virus Type 5: Transduction Efficiency and Cell-Type Specificity in the Primate Retina. Hum. Gene Ther. 2003, 14, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Yiu, G.; Chung, S.H.; Mollhoff, I.N.; Nguyen, U.T.; Thomasy, S.M.; Yoo, J.; Taraborelli, D.; Noronha, G. Suprachoroidal and Subretinal Injections of AAV Using Transscleral Microneedles for Retinal Gene Delivery in Nonhuman Primates. Mol. Ther. Methods Clin. Dev. 2020, 16, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Shen, J.; Hafiz, Z.; Hackett, S.F.; Silva, R.L.E.; Khan, M.; Lorenc, V.E.; Chen, D.; Chadha, R.; Zhang, M.; et al. AAV8-Vectored Suprachoroidal Gene Transfer Produces Widespread Ocular Transgene Expression. J. Clin. Investig. 2019, 129, 4901–4911. [Google Scholar] [CrossRef]
- Büning, H. AAV Entry: Filling in the Blanks. Mol. Ther. 2020, 28, 346–347. [Google Scholar] [CrossRef]
- Dudek, A.M.; Zabaleta, N.; Zinn, E.; Pillay, S.; Zengel, J.; Porter, C.; Franceschini, J.S.; Estelien, R.; Carette, J.E.; Zhou, G.L.; et al. GPR108 Is a Highly Conserved AAV Entry Factor. Mol. Ther. 2020, 28, 367–381. [Google Scholar] [CrossRef]
- Wagner, H.J.; Weber, W.; Fussenegger, M. Synthetic Biology: Emerging Concepts to Design and Advance Adeno-Associated Viral Vectors for Gene Therapy. Adv. Sci. 2021, 8, 2004018. [Google Scholar] [CrossRef]
- Smith, T.J.; Fusco, R.M.; Elmore, Z.C.; Asokan, A. Interplay between Furin and Sialoglycans in Modulating Adeno-Associated Viral Cell Entry. J. Virol. 2023, 97, e00093-23. [Google Scholar] [CrossRef]
- Candace, S.; Jude, S.R. Membrane-Associated Heparan Sulfate Proteoglycan Is a Receptor for Adeno-Associated Virus Type 2 Virions. J. Virol. 1998, 72, 1438–1445. [Google Scholar] [CrossRef]
- Kaludov, N.; Brown, K.E.; Walters, R.W.; Zabner, J.; Chiorini, J.A. Adeno-Associated Virus Serotype 4 (AAV4) and AAV5 Both Require Sialic Acid Binding for Hemagglutination and Efficient Transduction but Differ in Sialic Acid Linkage Specificity. J. Virol. 2001, 75, 6884–6893. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Bryant, K.D.; Brown, S.M.; Randell, S.H.; Asokan, A. Terminal N-Linked Galactose Is the Primary Receptor for Adeno-Associated Virus 9 *. J. Biol. Chem. 2011, 286, 13532–13540. [Google Scholar] [CrossRef] [PubMed]
- Halbert Christine, L.; Allen James, M.; Miller, A. Dusty Adeno-Associated Virus Type 6 (AAV6) Vectors Mediate Efficient Transduction of Airway Epithelial Cells in Mouse Lungs Compared to That of AAV2 Vectors. J. Virol. 2001, 75, 6615–6624. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.; Yi, S.; Keshavjee, S.; Brown, K.; Chiorini, J.; Zabner, J. Binding of Adeno-Associated Virus Type 5 to 2,3-Linked Sialic Acid Is Required for Gene Transfer. J. Biol. Chem. 2001, 276, 20610–20616. [Google Scholar] [CrossRef]
- Mizukami, H.; Young, N.S.; Brown, K.E. Adeno-Associated Virus Type 2 Binds to a 150-Kilodalton Cell Membrane Glycoprotein. Virology 1996, 217, 124–130. [Google Scholar] [CrossRef]
- Pillay, S.; Meyer, N.L.; Puschnik, A.S.; Davulcu, O.; Diep, J.; Ishikawa, Y.; Jae, L.T.; Wosen, J.E.; Nagamine, C.M.; Chapman, M.S.; et al. An Essential Receptor for Adeno-Associated Virus Infection. Nature 2016, 530, 108–112. [Google Scholar] [CrossRef]
- Dudek, A.; Pillay, S.; Puschnik, A.; Nagamine, C.; Cheng, F.; Qiu, J.; Carette, J.; Vandenberghe, L. An Alternate Route for Adeno-Associated Virus Entry Independent of AAVR. J. Virol. 2018, 92, e.02213-17. [Google Scholar] [CrossRef]
- Nonnenmacher, M.E.; Cintrat, J.-C.; Gillet, D.; Weber, T. Syntaxin 5-Dependent Retrograde Transport to the Trans-Golgi Network Is Required for Adeno-Associated Virus Transduction. J. Virol. 2015, 89, 1673–1687. [Google Scholar] [CrossRef]
- Bantel-Schaal, U.; Hub, B.; Kartenbeck, J. Endocytosis of Adeno-Associated Virus Type 5 Leads to Accumulation of Virus Particles in the Golgi Compartment. J. Virol. 2002, 76, 2340–2349. [Google Scholar] [CrossRef]
- Pajusola, K.; Gruchala, M.; Joch, H.; Luüscher, T.F.; Ylä-Herttuala, S.; Buüeler, H. Cell-Type-Specific Characteristics Modulate the Transduction Efficiency of Adeno-Associated Virus Type 2 and Restrain Infection of Endothelial Cells. J. Virol. 2002, 76, 11530–11540. [Google Scholar] [CrossRef]
- Saidkasimova, S.; Williamson, T.H. Suprachoroidal Space Interventions; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; ISBN 978-3-030-76852-2. [Google Scholar]
- Testa, F.; Maguire, A.; Rossi, S.; Pierce, E.; Melillo, P.; Marshall, K.; Banfi, S.; Surace, E.; Sun, J.; Acerra, C.; et al. Three Year Follow-Up after Unilateral Subretinal Delivery of Adeno-Associated Virus in Patients with Leber Congenital Amaurosis Type 2. Ophthalmology 2013, 120, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Groppe, M.; Salvetti, A.P.; MacLaren, R. Technique of Retinal Gene Therapy: Delivery of Viral Vector into the Subretinal Space. Eye 2017, 31, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Hauswirth, W.W.; Aleman, T.S.; Kaushal, S.; Cideciyan, A.V.; Schwartz, S.B.; Wang, L.; Conlon, T.J.; Boye, S.L.; Flotte, T.R.; Byrne, B.J.; et al. Treatment of Leber Congenital Amaurosis Due to RPE65 Mutations by Ocular Subretinal Injection of Adeno-Associated Virus Gene Vector: Short-Term Results of a Phase I Trial. Hum. Gene Ther. 2008, 19, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Kansara, V.; Muya, L.; Wan, C.; Ciulla, T.A. Suprachoroidal Delivery of Viral and Nonviral Gene Therapy for Retinal Diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 384–392. [Google Scholar] [CrossRef]
- Spencer, R.; Fisher, S.; Lewis, G.; Malone, T. Epiretinal Membrane in a Subject after Transvitreal Delivery of Palucorcel (CNTO 2476). Clin. Ophthalmol. 2017, 11, 1797–1803. [Google Scholar] [CrossRef]
- Hancock, S.E.; Wan, C.-R.; Fisher, N.E.; Andino, R.V.; Ciulla, T.A. Biomechanics of Suprachoroidal Drug Delivery: From Benchtop to Clinical Investigation in Ocular Therapies. Expert. Opin. Drug Deliv. 2021, 18, 777–788. [Google Scholar] [CrossRef]
- Naftali Ben Haim, L.; Moisseiev, E. Drug Delivery via the Suprachoroidal Space for the Treatment of Retinal Diseases. Pharmaceutics 2021, 13, 967. [Google Scholar] [CrossRef]
- Rodrigues, G.A.; Shalaev, E.; Karami, T.K.; Cunningham, J.; Slater, N.K.H.; Rivers, H.M. Pharmaceutical Development of AAV-Based Gene Therapy Products for the Eye. Pharm. Res. 2019, 36, 29. [Google Scholar] [CrossRef]
- Kotterman, M.A.; Yin, L.; Strazzeri, J.M.; Flannery, J.G.; Merigan, W.H.; Schaffer, D.V. Antibody Neutralization Poses a Barrier to Intravitreal Adeno-Associated Viral Vector Gene Delivery to Non-Human Primates. Gene Ther. 2015, 22, 116–126. [Google Scholar] [CrossRef]
- Reichel, F.; Peters, T.; Wilhelm, B.; Biel, M.; Ueffing, M.; Wissinger, B.; Bartz-Schmidt, K.; Klein, R.; Michalakis, S.; Fischer, M.D.; et al. Humoral Immune Response After Intravitreal But Not After Subretinal AAV8 in Primates and Patients. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1910–1915. [Google Scholar] [CrossRef]
- Timmers, A.; Newmark, J.; Turunen, H.; Farivar, T.; Liu, J.; Song, C.; Ye, G.; Pennock, S.; Gaskin, C.; Knop, D.; et al. Ocular Inflammatory Response to Intravitreal Injection of AAV Vector: Relative Contribution of Genome and Capsid. Hum. Gene Ther. 2019, 31, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Y.; Johnson, T.V. The Internal Limiting Membrane: Roles in Retinal Development and Implications for Emerging Ocular Therapies. Exp. Eye Res. 2021, 206, 108545. [Google Scholar] [CrossRef] [PubMed]
- Molinari, E.; Sayer, J.A. Gene and Epigenetic Editing in the Treatment of Primary Ciliopathies. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2021; Volume 182, pp. 353–401. ISBN 978-0-323-85301-9. [Google Scholar]
- Cehajic-Kapetanovic, J.; Le Goff, M.; Allen, A.; Lucas, R.; Bishop, P. Glycosidic Enzymes Enhance Retinal Transduction Following Intravitreal Delivery of AAV2. Mol. Vis. 2011, 17, 1771–1783. [Google Scholar] [PubMed]
- Schnichels, S.; Paquet-Durand, F.; Löscher, M.; Tsai, T.; Hurst, J.; Joachim, S.C.; Klettner, A. Retina in a Dish: Cell Cultures, Retinal Explants and Animal Models for Common Diseases of the Retina. Prog. Retin. Eye Res. 2021, 81, 100880. [Google Scholar] [CrossRef]
- Stalmans, P.; Benz, M.; Gandorfer, A.; Kampik, A.; Girach, A.; Pakola, S.; Haller, J. Enzymatic Vitreolysis with Ocriplasmin for Vitreomacular Traction and Macular Holes. New Engl. J. Med. 2012, 367, 606–615. [Google Scholar] [CrossRef]
- Maurya, S.; Jayandharan, G.R. Exosome-Associated SUMOylation Mutant AAV Demonstrates Improved Ocular Gene Transfer Efficiency in Vivo. Virus Res. 2020, 283, 197966. [Google Scholar] [CrossRef]
- Wassmer, S.J.; Carvalho, L.S.; György, B.; Vandenberghe, L.H.; Maguire, C.A. Exosome-Associated AAV2 Vector Mediates Robust Gene Delivery into the Murine Retina upon Intravitreal Injection. Sci. Rep. 2017, 7, 45329. [Google Scholar] [CrossRef]
- Kay, C.; Ryals, R.; Aslanidi, G.; Min, S.; Ruan, Q.; Sun, J.; Dyka, F.; Kasuga, D.; Ayala, A.; Vliet, K.; et al. Targeting Photoreceptors via Intravitreal Delivery Using Novel, Capsid-Mutated AAV Vectors. PLoS ONE 2013, 8, e62097. [Google Scholar] [CrossRef]
- Dalkara, D.; Byrne, L.; Lee, T.; Hoffmann, N.; Schaffer, D.; Flannery, J.G. Enhanced Gene Delivery to the Neonatal Retina through Systemic Administration of Tyrosine-Mutated AAV9. Gene Ther. 2012, 19, 176–181. [Google Scholar] [CrossRef]
- Murali, A.; Ramlogan-Steel, C.A.; Andrzejewski, S.; Steel, J.C.; Layton, C.J. Retinal Explant Culture: A Platform to Investigate Human Neuro-retina. Clin. Exper Ophthalmol. 2019, 47, 274–285. [Google Scholar] [CrossRef]
- Hudry, E.; Martin, C.; Gandhi, S.; György, B.; Scheffer, D.I.; Mu, D.; Merkel, S.F.; Mingozzi, F.; Fitzpatrick, Z.; Dimant, H.; et al. Exosome-associated AAV Vector as a Robust and Convenient Neuroscience Tool. Gene Ther. 2016, 23, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, M.; Chen, F.; Goldberg, J.L.; Sabel, B.A. Nanomedicine and Drug Delivery to the Retina: Current Status and Implications for Gene Therapy. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 1477–1507. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Trenholm, S.; Balint, K.; Kosche, G.; Cowan, C.S.; Mohr, M.A.; Munz, M.; Martinez-Martin, D.; Fläschner, G.; Newton, R.; et al. Virus Stamping for Targeted Single-Cell Infection in Vitro and in Vivo. Nat. Biotechnol. 2018, 36, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Siontas, O.; Koester, J.; Krol, J.; Fauser, S.; Müller, D.J. Magnetically Guided Adeno-Associated Virus Delivery for the Spatially Targeted Transduction of Retina in Eyes. Adv. Healthc. Mater. 2024, 13, 2401577. [Google Scholar] [CrossRef]
- Plank, C.; Zelphati, O.; Mykhaylyk, O. Magnetically Enhanced Nucleic Acid Delivery. Ten Years of Magnetofection—Progress and Prospects. Adv. Drug Deliv. Rev. 2011, 63, 1300–1331. [Google Scholar] [CrossRef]
- Kami, D.; Takeda, S.; Itakura, Y.; Gojo, S.; Watanabe, M.; Toyoda, M. Application of Magnetic Nanoparticles to Gene Delivery. Int. J. Mol. Sci. 2011, 12, 3705–3722. [Google Scholar] [CrossRef]
- Sapet, C.; Pellegrino, C.; Laurent, N.; Sicard, F.; Zelphati, O. Magnetic Nanoparticles Enhance Adenovirus Transduction In Vitro and In Vivo. Pharm. Res. 2012, 29, 1203–1218. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hisano, Y. Directional Gene-Transfer into the Brain by an Adenoviral Vector Tagged with Magnetic Nanoparticles. J. Neurosci. Methods 2011, 194, 316–320. [Google Scholar] [CrossRef]
- Mah, C.; Fraites, T.; Zolotukhin, I.; Song, S.; Flotte, T.; Dobson, J.; Batich, C.; Byrne, B. Improved Method of Recombinant AAV2 Delivery for Systemic Targeted Gene Therapy. Mol. Ther. J. Am. Soc. Gene Ther. 2002, 6, 106–112. [Google Scholar] [CrossRef]
- Pandori, M.W.; Hobson, D.A.; Sano, T. Adenovirus–Microbead Conjugates Possess Enhanced Infectivity: A New Strategy for Localized Gene Delivery. Virology 2002, 299, 204–212. [Google Scholar] [CrossRef]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Krüger, A.; Gänsbacher, B.; Plank, C. Magnetofection: Enhancing and Targeting Gene Delivery by Magnetic Force in Vitro and in Vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Bassetto, M.; Sen, M.; Poulhes, F.; Arango-Gonzalez, B.; Bonvin, E.; Sapet, C.; Ueffing, M.; Zelphati, O. New Method for Efficient siRNA Delivery in Retina Explants: Reverse Magnetofection. Bioconjug. Chem. 2021, 32, 1078–1093. [Google Scholar] [CrossRef] [PubMed]
- Bassetto, M.; Ajoy, D.; Poulhes, F.; Obringer, C.; Walter, A.; Messadeq, N.; Sadeghi, A.; Puranen, J.; Ruponen, M.; Kettunen, M.; et al. Magnetically Assisted Drug Delivery of Topical Eye Drops Maintains Retinal Function In Vivo in Mice. Pharmaceutics 2021, 13, 1650. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Bassetto, M.; Poulhes, F.; Zelphati, O.; Ueffing, M.; Arango-Gonzalez, B. Efficient Ocular Delivery of VCP siRNA via Reverse Magnetofection in RHO P23H Rodent Retina Explants. Pharmaceutics 2021, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Yanai, A.; Häfeli, U.O.; Metcalfe, A.L.; Soema, P.; Addo, L.; Gregory-Evans, C.Y.; Po, K.; Shan, X.; Moritz, O.L.; Gregory-Evans, K. Focused Magnetic Stem Cell Targeting to the Retina Using Superparamagnetic Iron Oxide Nanoparticles. Cell Transpl. 2012, 21, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Tresilwised, N.; Pithayanukul, P.; Mykhaylyk, O.; Holm, P.S.; Holzmüller, R.; Anton, M.; Thalhammer, S.; Adigüzel, D.; Döblinger, M.; Plank, C. Boosting Oncolytic Adenovirus Potency with Magnetic Nanoparticles and Magnetic Force. Mol. Pharm. 2010, 7, 1069–1089. [Google Scholar] [CrossRef]
- Hofmann, A.; Wenzel, D.; Becher, U.M.; Freitag, D.F.; Klein, A.M.; Eberbeck, D.; Schulte, M.; Zimmermann, K.; Bergemann, C.; Gleich, B.; et al. Combined Targeting of Lentiviral Vectors and Positioning of Transduced Cells by Magnetic Nanoparticles. Proc. Natl. Acad. Sci. 2009, 106, 44–49. [Google Scholar] [CrossRef]
- Bhattarai, S.; Kc, R.; Kim, S.; Sharma, M.; Khil, M.; Hwang, P.; Chung, G.; Kim, H. N-Hexanoyl Chitosan Stabilized Magnetic Nanoparticles: Implication for Cellular Labeling and Magnetic Resonance Imaging. J. Nanobiotechnol. 2008, 6, 1. [Google Scholar] [CrossRef]
- Wu, Z.; Troll, J.; Jeong, H.-H.; Wei, Q.; Stang, M.; Ziemssen, F.; Wang, Z.; Dong, M.; Schnichels, S.; Qiu, T.; et al. A Swarm of Slippery Micropropellers Penetrates the Vitreous Body of the Eye. Sci. Adv. 2018, 4, eaat4388. [Google Scholar] [CrossRef]
- Zahn, D.; Klein, K.; Radon, P.; Berkov, D.; Erokhin, S.; Nagel, E.; Eichhorn, M.; Wiekhorst, F.; Dutz, S. Investigation of Magnetically Driven Passage of Magnetic Nanoparticles through Eye Tissues for Magnetic Drug Targeting. Nanotechnology 2020, 31, 495101. [Google Scholar] [CrossRef]
- Schneider-Futschik, E.K.; Reyes-Ortega, F. Advantages and Disadvantages of Using Magnetic Nanoparticles for the Treatment of Complicated Ocular Disorders. Pharmaceutics 2021, 13, 1157. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E. Ten Things You Might Not Know about Iron Oxide Nanoparticles. Radiology 2017, 284, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Raju, H.B.; Hu, Y.; Padgett, K.R.; Rodriguez, J.E.; Goldberg, J.L. Investigation of Nanoparticles Using Magnetic Resonance Imaging after Intravitreal Injection. Clin. Exper Ophthalmol. 2012, 40, 100–107. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V.; Rizzello, L. Noble Metals and Soft Bio-Inspired Nanoparticles in Retinal Diseases Treatment: A Perspective. Cells 2020, 9, 679. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Wen, C.; Bai, S.; Wei, P.; Xu, B.; Xu, Y.; Liang, C.; Zhang, Y.; Zhang, G.; et al. Effects of Iron Oxide Nanoparticles as T2-MRI Contrast Agents on Reproductive System in Male Mice. J. Nanobiotechnol. 2022, 20, 98. [Google Scholar] [CrossRef]
- Schubert, R.; Herzog, S.; Trenholm, S.; Roska, B.; Müller, D.J. Magnetically Guided Virus Stamping for the Targeted Infection of Single Cells or Groups of Cells. Nat. Protoc. 2019, 14, 3205–3219. [Google Scholar] [CrossRef]
- Mcrobbie, D.W. Occupational Exposure in MRI. BJR 2012, 85, 293–312. [Google Scholar] [CrossRef]
- Subudhi, S.K.; Chandel, G.R.; Sivasankar, V.S.; Das, S. Magnetic Nanoparticle Aggregation and Complete De-encapsulation of Such Aggregates from a Liquid Drop Interior. ACS Appl. Mater. Interfaces 2024, 16, 64253–64263. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siontas, O.; Ahn, S. Challenges in AAV-Based Retinal Gene Therapies and the Role of Magnetic Nanoparticle Platforms. J. Clin. Med. 2024, 13, 7385. https://doi.org/10.3390/jcm13237385
Siontas O, Ahn S. Challenges in AAV-Based Retinal Gene Therapies and the Role of Magnetic Nanoparticle Platforms. Journal of Clinical Medicine. 2024; 13(23):7385. https://doi.org/10.3390/jcm13237385
Chicago/Turabian StyleSiontas, Oliver, and Seungkuk Ahn. 2024. "Challenges in AAV-Based Retinal Gene Therapies and the Role of Magnetic Nanoparticle Platforms" Journal of Clinical Medicine 13, no. 23: 7385. https://doi.org/10.3390/jcm13237385
APA StyleSiontas, O., & Ahn, S. (2024). Challenges in AAV-Based Retinal Gene Therapies and the Role of Magnetic Nanoparticle Platforms. Journal of Clinical Medicine, 13(23), 7385. https://doi.org/10.3390/jcm13237385






