Exogenous Ketones in Cardiovascular Disease and Diabetes: From Bench to Bedside
Abstract
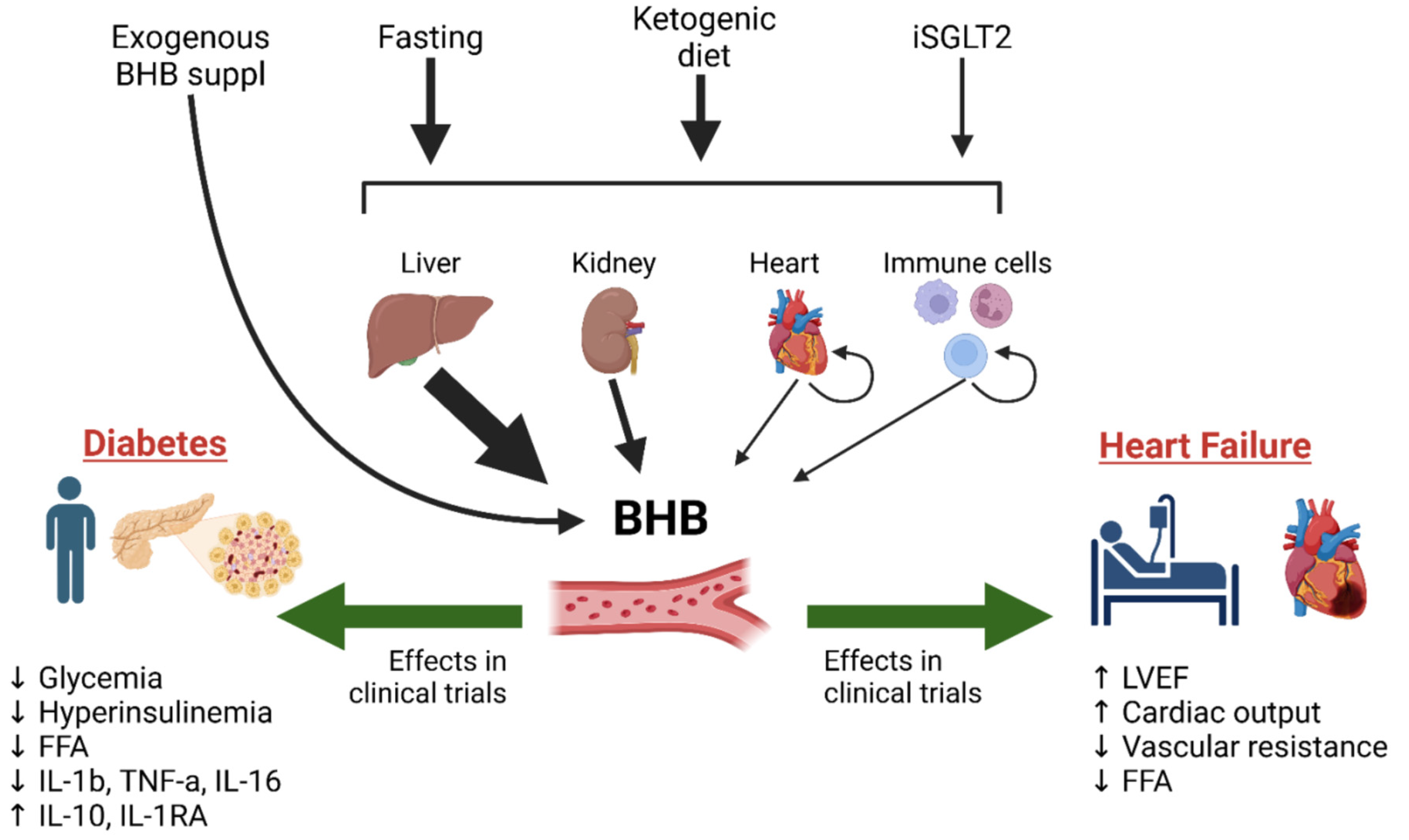
:1. Introduction
2. Ketones as Prognostic Markers in Cardiovascular Disease and Diabetes: Friend or Foe?
3. Ketones and Cardiovascular Disease
3.1. Animal Studies
3.2. Human Studies
4. Ketones and Diabetes
4.1. Animal Studies
4.2. Human Studies
5. Ketones and SGLT2 Inhibitors
5.1. SGLT2 Inhibitors Cardioprotective Action: Animal Studies
5.2. SGLT2 Inhibitors Cardioprotective Action: Human Studies
5.3. SGLT2 Inhibitors and Diabetes: Animal Studies
5.4. SGLT2 Inhibitors and Diabetes: Human Studies
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Newman, J.C.; Verdin, E. beta-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Cheng, X.; He, Y.; Xie, Y.; Xu, F.; Xu, Y.; Huang, W. Function and mechanism of histone beta-hydroxybutyrylation in health and disease. Front. Immunol. 2022, 13, 981285. [Google Scholar] [CrossRef]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Shippy, D.C.; Evered, A.H.; Ulland, T.K. Ketone body metabolism and the NLRP3 inflammasome in Alzheimer’s disease. Immunol. Rev. 2024. [Google Scholar] [CrossRef]
- Goldberg, E.L.; Shchukina, I.; Asher, J.L.; Sidorov, S.; Artyomov, M.N.; Dixit, V.D. Ketogenesis activates metabolically protective gammadelta T cells in visceral adipose tissue. Nat. Metab. 2020, 2, 50–61. [Google Scholar] [CrossRef]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef]
- Melena, I.; Piani, F.; Tommerdahl, K.L.; Severn, C.; Chung, L.T.; MacDonald, A.; Vinovskis, C.; Cherney, D.; Pyle, L.; Roncal-Jimenez, C.A.; et al. Aminoaciduria and metabolic dysregulation during diabetic ketoacidosis: Results from the diabetic kidney alarm (DKA) study. J. Diabetes Complicat. 2022, 36, 108203. [Google Scholar] [CrossRef]
- Hiyoshi, N.; Enomoto, T.; Uefune, F.; Kato, Y.; Wu, Y.; Araki, K.; Sakano, D.; Shiraki, N.; Kume, S. A specific plasma amino acid profile in the Insulin2(Q104del) Kuma mice at the diabetic state and reversal from hyperglycemia. Biochem. Biophys. Res. Commun. 2023, 679, 58–65. [Google Scholar] [CrossRef]
- Pillai, S.; Mahmud, I.; Mahar, R.; Griffith, C.; Langsen, M.; Nguyen, J.; Wojtkowiak, J.W.; Swietach, P.; Gatenby, R.A.; Bui, M.M.; et al. Lipogenesis mediated by OGR1 regulates metabolic adaptation to acid stress in cancer cells via autophagy. Cell Rep. 2022, 39, 110796. [Google Scholar] [CrossRef]
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Kansakar, U.; Varzideh, F.; Wilson, S.; Mone, P.; Lombardi, A.; Gambardella, J.; Santulli, G. Heart failure in diabetes. Metabolism 2021, 125, 154910. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. J. Physiol. 2017, 595, 2857–2871. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.R.; Puchalska, P.; Crawford, P.A.; Kelly, D.P. Ketones and the Heart: Metabolic Principles and Therapeutic Implications. Circ. Res. 2023, 132, 882–898. [Google Scholar] [CrossRef] [PubMed]
- Delles, C.; Rankin, N.J.; Boachie, C.; McConnachie, A.; Ford, I.; Kangas, A.; Soininen, P.; Trompet, S.; Mooijaart, S.P.; Jukema, J.W.; et al. Nuclear magnetic resonance-based metabolomics identifies phenylalanine as a novel predictor of incident heart failure hospitalisation: Results from PROSPER and FINRISK 1997. Eur. J. Heart Fail. 2018, 20, 663–673. [Google Scholar] [CrossRef]
- Brouwers, F.P.; de Boer, R.A.; van der Harst, P.; Voors, A.A.; Gansevoort, R.T.; Bakker, S.J.; Hillege, H.L.; van Veldhuisen, D.J.; van Gilst, W.H. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur. Heart J. 2013, 34, 1424–1431. [Google Scholar] [CrossRef]
- Flores-Guerrero, J.L.; Westenbrink, B.D.; Connelly, M.A.; Otvos, J.D.; Groothof, D.; Shalaurova, I.; Garcia, E.; Navis, G.; de Boer, R.A.; Bakker, S.J.L.; et al. Association of beta-hydroxybutyrate with development of heart failure: Sex differences in a Dutch population cohort. Eur. J. Clin. Investig. 2021, 51, e13468. [Google Scholar] [CrossRef]
- Lommi, J.; Kupari, M.; Koskinen, P.; Naveri, H.; Leinonen, H.; Pulkki, K.; Harkonen, M. Blood ketone bodies in congestive heart failure. J. Am. Coll. Cardiol. 1996, 28, 665–672. [Google Scholar] [CrossRef]
- Stryeck, S.; Gastrager, M.; Degoricija, V.; Trbusic, M.; Potocnjak, I.; Radulovic, B.; Pregartner, G.; Berghold, A.; Madl, T.; Frank, S. Serum Concentrations of Citrate, Tyrosine, 2- and 3- Hydroxybutyrate are Associated with Increased 3-Month Mortality in Acute Heart Failure Patients. Sci. Rep. 2019, 9, 6743. [Google Scholar] [CrossRef]
- Jorsal, A.; Kistorp, C.; Holmager, P.; Tougaard, R.S.; Nielsen, R.; Hanselmann, A.; Nilsson, B.; Moller, J.E.; Hjort, J.; Rasmussen, J.; et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)—A multicentre, double-blind, randomised, placebo-controlled trial. Eur. J. Heart Fail. 2017, 19, 69–77. [Google Scholar] [CrossRef]
- Christensen, K.H.; Nielsen, R.R.; Schou, M.; Gustafsson, I.; Jorsal, A.; Flyvbjerg, A.; Tarnow, L.; Botker, H.E.; Kistorp, C.; Johannsen, M.; et al. Circulating 3-hydroxy butyrate predicts mortality in patients with chronic heart failure with reduced ejection fraction. ESC Heart Fail. 2024, 11, 837–845. [Google Scholar] [CrossRef]
- Song, J.P.; Chen, L.; Chen, X.; Ren, J.; Zhang, N.N.; Tirasawasdichai, T.; Hu, Z.L.; Hua, W.; Hu, Y.R.; Tang, H.R.; et al. Elevated plasma beta-hydroxybutyrate predicts adverse outcomes and disease progression in patients with arrhythmogenic cardiomyopathy. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Monzo, L.; Kovar, J.; Borlaug, B.A.; Benes, J.; Kotrc, M.; Kroupova, K.; Jabor, A.; Franekova, J.; Melenovsky, V. Circulating beta-hydroxybutyrate levels in advanced heart failure with reduced ejection fraction: Determinants and prognostic impact. Eur. J. Heart Fail. 2024, 26, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Lexis, C.P.; van der Horst, I.C.; Lipsic, E.; Wieringa, W.G.; de Boer, R.A.; van den Heuvel, A.F.; van der Werf, H.W.; Schurer, R.A.; Pundziute, G.; Tan, E.S.; et al. Effect of metformin on left ventricular function after acute myocardial infarction in patients without diabetes: The GIPS-III randomized clinical trial. JAMA 2014, 311, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- de Koning, M.L.Y.; Westenbrink, B.D.; Assa, S.; Garcia, E.; Connelly, M.A.; van Veldhuisen, D.J.; Dullaart, R.P.F.; Lipsic, E.; van der Harst, P. Association of Circulating Ketone Bodies With Functional Outcomes After ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 78, 1421–1432. [Google Scholar] [CrossRef]
- Chu, Y.; Hua, Y.; He, L.; He, J.; Chen, Y.; Yang, J.; Mahmoud, I.; Zeng, F.; Zeng, X.; Benavides, G.A.; et al. beta-hydroxybutyrate administered at reperfusion reduces infarct size and preserves cardiac function by improving mitochondrial function through autophagy in male mice. J. Mol. Cell. Cardiol. 2024, 186, 31–44. [Google Scholar] [CrossRef]
- Aziz, F.; Tripolt, N.J.; Pferschy, P.N.; Scharnagl, H.; Abdellatif, M.; Oulhaj, A.; Benedikt, M.; Kolesnik, E.; von Lewinski, D.; Sourij, H. Ketone body levels and its associations with cardiac markers following an acute myocardial infarction: A post hoc analysis of the EMMY trial. Cardiovasc. Diabetol. 2024, 23, 145. [Google Scholar] [CrossRef]
- Lommi, J.; Koskinen, P.; Naveri, H.; Harkonen, M.; Kupari, M. Heart failure ketosis. J. Intern. Med. 1997, 242, 231–238. [Google Scholar] [CrossRef]
- Chowdhury, S.; Faheem, S.M.; Nawaz, S.S.; Siddiqui, K. The role of metabolomics in personalized medicine for diabetes. Pers. Med. 2021, 18, 501–508. [Google Scholar] [CrossRef]
- Ahola-Olli, A.V.; Mustelin, L.; Kalimeri, M.; Kettunen, J.; Jokelainen, J.; Auvinen, J.; Puukka, K.; Havulinna, A.S.; Lehtimaki, T.; Kahonen, M.; et al. Circulating metabolites and the risk of type 2 diabetes: A prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia 2019, 62, 2298–2309. [Google Scholar] [CrossRef]
- Szili-Torok, T.; de Borst, M.H.; Garcia, E.; Gansevoort, R.T.; Dullaart, R.P.F.; Connelly, M.A.; Bakker, S.J.L.; Tietge, U.J.F. Fasting Ketone Bodies and Incident Type 2 Diabetes in the General Population. Diabetes 2023, 72, 1187–1192. [Google Scholar] [CrossRef]
- McMichael, L.E.; Heath, H.; Johnson, C.M.; Fanter, R.; Alarcon, N.; Quintana-Diaz, A.; Pilolla, K.; Schaffner, A.; Jelalian, E.; Wing, R.R.; et al. Metabolites involved in purine degradation, insulin resistance, and fatty acid oxidation are associated with prediction of Gestational diabetes in plasma. Metabolomics 2021, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.; Eckhart, A.; Perichon, R.; Wulff, J.; Mitchell, M.; Adam, K.P.; Wolfert, R.; Button, E.; Lawton, K.; Elverson, R.; et al. A novel test for IGT utilizing metabolite markers of glucose tolerance. J. Diabetes Sci. Technol. 2015, 9, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Fikri, A.M.; Smyth, R.; Kumar, V.; Al-Abadla, Z.; Abusnana, S.; Munday, M.R. Pre-diagnostic biomarkers of type 2 diabetes identified in the UAE’s obese national population using targeted metabolomics. Sci. Rep. 2020, 10, 17616. [Google Scholar] [CrossRef] [PubMed]
- Dudzik, D.; Zorawski, M.; Skotnicki, M.; Zarzycki, W.; Garcia, A.; Angulo, S.; Lorenzo, M.P.; Barbas, C.; Ramos, M.P. GC-MS based Gestational Diabetes Mellitus longitudinal study: Identification of 2-and 3-hydroxybutyrate as potential prognostic biomarkers. J. Pharm. Biomed. Anal. 2017, 144, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, Y.; Vangipurapu, J.; Cederberg, H.; Stancakova, A.; Pihlajamaki, J.; Soininen, P.; Kangas, A.J.; Paananen, J.; Civelek, M.; Saleem, N.K.; et al. Association of ketone body levels with hyperglycemia and type 2 diabetes in 9398 Finnish men. Diabetes 2013, 62, 3618–3626. [Google Scholar] [CrossRef]
- Lee, M.; Cho, Y.; Lee, Y.H.; Kang, E.S.; Cha, B.S.; Lee, B.W. beta-hydroxybutyrate as a biomarker of beta-cell function in new-onset type 2 diabetes and its association with treatment response at 6 months. Diabetes Metab. 2023, 49, 101427. [Google Scholar] [CrossRef]
- Kwon, H.N.; Lee, Y.J.; Kang, J.H.; Choi, J.H.; An, Y.J.; Kang, S.; Lee, D.H.; Suh, Y.J.; Heo, Y.; Park, S. Prediction of glycated hemoglobin levels at 3 months after metabolic surgery based on the 7-day plasma metabolic profile. PLoS ONE 2014, 9, e109609. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, N.; Fu, P.; An, Y.; Sun, F.; Wang, C.; Han, X.; Zhang, Y.; Yu, X.; Liu, Y. Dapagliflozin Attenuates Heart Failure With Preserved Ejection Fraction Remodeling and Dysfunction by Elevating beta-Hydroxybutyrate-activated Citrate Synthase. J. Cardiovasc. Pharmacol. 2023, 82, 375–388. [Google Scholar] [CrossRef]
- Pherwani, S.; Connolly, D.; Sun, Q.; Karwi, Q.G.; Carr, M.; Ho, K.L.; Wagg, C.S.; Zhang, L.; Levasseur, J.; Silver, H.; et al. Ketones provide an extra source of fuel for the failing heart without impairing glucose oxidation. Metabolism 2024, 154, 155818. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, M.; Li, Q.; Xu, X.; Ou, W.; Zhang, Y.; Xiao, H.; Yu, H.; Zheng, Y.; Liang, Y.; et al. Targeting Mitochondria-Inflammation Circuit by beta-Hydroxybutyrate Mitigates HFpEF. Circ. Res. 2021, 128, 232–245. [Google Scholar] [CrossRef]
- Liao, S.; Tang, Y.; Yue, X.; Gao, R.; Yao, W.; Zhou, Y.; Zhang, H. beta-Hydroxybutyrate Mitigated Heart Failure with Preserved Ejection Fraction by Increasing Treg Cells via Nox2/GSK-3beta. J. Inflamm. Res. 2021, 14, 4697–4706. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Jankauskas, S.S.; Kansakar, U.; Varzideh, F.; Avvisato, R.; Prevete, N.; Sidoli, S.; Mone, P.; Wang, X.; Lombardi, A.; et al. Ketone Bodies Rescue Mitochondrial Dysfunction Via Epigenetic Remodeling. JACC Basic Transl. Sci. 2023, 8, 1123–1137. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.L.; Karwi, Q.G.; Wang, F.; Wagg, C.; Zhang, L.; Panidarapu, S.; Chen, B.; Pherwani, S.; Greenwell, A.A.; Oudit, G.Y.; et al. The ketogenic diet does not improve cardiac function and blunts glucose oxidation in ischaemic heart failure. Cardiovasc. Res. 2024, 120, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.L.; Davidson, M.T.; Kurishima, C.; Vega, R.B.; Powers, J.C.; Matsuura, T.R.; Petucci, C.; Lewandowski, E.D.; Crawford, P.A.; Muoio, D.M.; et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yu, Y.; Zhang, Y.; Zhang, Z.; An, W.; Zhao, X. Treatment with D-beta-hydroxybutyrate protects heart from ischemia/reperfusion injury in mice. Eur. J. Pharmacol. 2018, 829, 121–128. [Google Scholar] [CrossRef]
- Seefeldt, J.M.; Libai, Y.; Berg, K.; Jespersen, N.R.; Lassen, T.R.; Dalsgaard, F.F.; Ryhammer, P.; Pedersen, M.; Ilkjaer, L.B.; Hu, M.A.; et al. Effects of ketone body 3-hydroxybutyrate on cardiac and mitochondrial function during donation after circulatory death heart transplantation. Sci. Rep. 2024, 14, 757. [Google Scholar] [CrossRef]
- Ma, X.; Dong, Z.; Liu, J.; Ma, L.; Sun, X.; Gao, R.; Pan, L.; Zhang, J.; An, J.; Hu, K.; et al. beta-Hydroxybutyrate Exacerbates Hypoxic Injury by Inhibiting HIF-1alpha-Dependent Glycolysis in Cardiomyocytes-Adding Fuel to the Fire? Cardiovasc. Drugs Ther. 2022, 36, 383–397. [Google Scholar] [CrossRef]
- Lan, Z.; Chen, A.; Li, L.; Ye, Y.; Liang, Q.; Dong, Q.; Wang, S.; Fu, M.; Li, Y.; Liu, X.; et al. Downregulation of HDAC9 by the ketone metabolite beta-hydroxybutyrate suppresses vascular calcification. J. Pathol. 2022, 258, 213–226. [Google Scholar] [CrossRef]
- Place, D.E.; Kanneganti, T.D. Fueling Ketone Metabolism Quenches Salt-Induced Hypertension. Trends Endocrinol. Metab. 2019, 30, 145–147. [Google Scholar] [CrossRef]
- Trang, N.N.; Lee, T.W.; Kao, Y.H.; Chao, T.F.; Lee, T.I.; Chen, Y.J. Ketogenic diet modulates cardiac metabolic dysregulation in streptozocin-induced diabetic rats. J. Nutr. Biochem. 2023, 111, 109161. [Google Scholar] [CrossRef]
- Oneglia, A.P.; Young, B.E.; Cipher, D.J.; Zaha, V.; Nelson, M.D. Acute effects of beta-hydroxybutyrate on left ventricular function in young, healthy adults. J. Appl. Physiol. 2023, 135, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Hu, R.; Vidula, M.K.; Dugyala, S.; Tierney, A.; Ky, B.; Margulies, K.B.; Shah, S.H.; Kelly, D.P.; Bravo, P.E. Acute Echocardiographic Effects of Exogenous Ketone Administration in Healthy Participants. J. Am. Soc. Echocardiogr. 2022, 35, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Gormsen, L.C.; Svart, M.; Thomsen, H.H.; Sondergaard, E.; Vendelbo, M.H.; Christensen, N.; Tolbod, L.P.; Harms, H.J.; Nielsen, R.; Wiggers, H.; et al. Ketone Body Infusion with 3-Hydroxybutyrate Reduces Myocardial Glucose Uptake and Increases Blood Flow in Humans: A Positron Emission Tomography Study. J. Am. Heart Assoc. 2017, 6, e005066. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.; Moller, N.; Gormsen, L.C.; Tolbod, L.P.; Hansson, N.H.; Sorensen, J.; Harms, H.J.; Frokiaer, J.; Eiskjaer, H.; Jespersen, N.R.; et al. Cardiovascular Effects of Treatment With the Ketone Body 3-Hydroxybutyrate in Chronic Heart Failure Patients. Circulation 2019, 139, 2129–2141. [Google Scholar] [CrossRef]
- Kim, I.S.; Izawa, H.; Sobue, T.; Ishihara, H.; Somura, F.; Nishizawa, T.; Nagata, K.; Iwase, M.; Yokota, M. Prognostic value of mechanical efficiency in ambulatory patients with idiopathic dilated cardiomyopathy in sinus rhythm. J. Am. Coll. Cardiol. 2002, 39, 1264–1268. [Google Scholar] [CrossRef]
- Gopalasingam, N.; Christensen, K.H.; Berg Hansen, K.; Nielsen, R.; Johannsen, M.; Gormsen, L.C.; Boedtkjer, E.; Norregaard, R.; Moller, N.; Wiggers, H. Stimulation of the Hydroxycarboxylic Acid Receptor 2 With the Ketone Body 3-Hydroxybutyrate and Niacin in Patients With Chronic Heart Failure: Hemodynamic and Metabolic Effects. J. Am. Heart Assoc. 2023, 12, e029849. [Google Scholar] [CrossRef]
- Tunaru, S.; Kero, J.; Schaub, A.; Wufka, C.; Blaukat, A.; Pfeffer, K.; Offermanns, S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 2003, 9, 352–355. [Google Scholar] [CrossRef]
- Taggart, A.K.; Kero, J.; Gan, X.; Cai, T.Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.J.; et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652. [Google Scholar] [CrossRef]
- McKenney, J. Niacin for dyslipidemia: Considerations in product selection. Am. J. Health Syst. Pharm. 2003, 60, 995–1005. [Google Scholar] [CrossRef]
- Kurihara, M.; Akama, Y.; Kimura, J. Inhibitory effect of beta-hydroxybutyric acid on L-type Ca2+ current under beta-adrenergic stimulation in guinea pig cardiac ventricular myocytes. Fukushima J. Med. Sci. 2012, 58, 144–150. [Google Scholar] [CrossRef]
- Benitah, J.P.; Alvarez, J.L.; Gomez, A.M. L-type Ca2+ current in ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2010, 48, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.; Gille, A.; Offermanns, S. Role of HCA(2) (GPR109A) in nicotinic acid and fumaric acid ester-induced effects on the skin. Pharmacol. Ther. 2012, 136, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Califf, R.M.; Adams, K.F.; McKenna, W.J.; Gheorghiade, M.; Uretsky, B.F.; McNulty, S.E.; Darius, H.; Schulman, K.; Zannad, F.; Handberg-Thurmond, E.; et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The Flolan International Randomized Survival Trial (FIRST). Am. Heart J. 1997, 134, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Punzengruber, C.; Stanek, B.; Sinzinger, H.; Silberbauer, K. Bicyclo-prostaglandin E2 metabolite in congestive heart failure and relation to vasoconstrictor neurohumoral principles. Am. J. Cardiol. 1986, 57, 619–623. [Google Scholar] [CrossRef]
- Berg-Hansen, K.; Gopalasingam, N.; Christensen, K.H.; Ladefoged, B.; Andersen, M.J.; Poulsen, S.H.; Borlaug, B.A.; Nielsen, R.; Moller, N.; Wiggers, H. Cardiovascular Effects of Oral Ketone Ester Treatment in Patients With Heart Failure With Reduced Ejection Fraction: A Randomized, Controlled, Double-Blind Trial. Circulation 2024, 149, 1474–1489. [Google Scholar] [CrossRef]
- Gopalasingam, N.; Berg-Hansen, K.; Christensen, K.H.; Ladefoged, B.T.; Poulsen, S.H.; Andersen, M.J.; Borlaug, B.; Nielsen, R.; Moller, N.; Wiggers, H. Randomized Crossover Trial of 2-Week Ketone Ester Treatment in Patients With Type 2 Diabetes and Heart Failure With Preserved Ejection Fraction. Circulation 2024, 150, 1570–1583. [Google Scholar] [CrossRef]
- Berg-Hansen, K.; Christensen, K.H.; Gopalasingam, N.; Nielsen, R.; Eiskjaer, H.; Moller, N.; Birkelund, T.; Christensen, S.; Wiggers, H. Beneficial Effects of Ketone Ester in Patients With Cardiogenic Shock: A Randomized, Controlled, Double-Blind Trial. JACC Heart Fail. 2023, 11, 1337–1347. [Google Scholar] [CrossRef]
- Takahara, S.; Soni, S.; Phaterpekar, K.; Kim, T.T.; Maayah, Z.H.; Levasseur, J.L.; Silver, H.L.; Freed, D.H.; Ferdaoussi, M.; Dyck, J.R.B. Chronic exogenous ketone supplementation blunts the decline of cardiac function in the failing heart. ESC Heart Fail. 2021, 8, 5606–5612. [Google Scholar] [CrossRef]
- Yurista, S.R.; Matsuura, T.R.; Sillje, H.H.W.; Nijholt, K.T.; McDaid, K.S.; Shewale, S.V.; Leone, T.C.; Newman, J.C.; Verdin, E.; van Veldhuisen, D.J.; et al. Ketone Ester Treatment Improves Cardiac Function and Reduces Pathologic Remodeling in Preclinical Models of Heart Failure. Circ. Heart Fail. 2021, 14, e007684. [Google Scholar] [CrossRef]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef]
- Ferrannini, E.; Baldi, S.; Scozzaro, T.; Tsimihodimos, V.; Tesfaye, F.; Shaw, W.; Rosenthal, N.; Figtree, G.A.; Neal, B.; Mahaffey, K.W.; et al. Fasting Substrate Concentrations Predict Cardiovascular Outcomes in the CANagliflozin cardioVascular Assessment Study (CANVAS). Diabetes Care 2022, 45, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Veelen, A.; Andriessen, C.; Op den Kamp, Y.; Erazo-Tapia, E.; de Ligt, M.; Mevenkamp, J.; Jorgensen, J.A.; Moonen-Kornips, E.; Schaart, G.; Esterline, R.; et al. Effects of the sodium-glucose cotransporter 2 inhibitor dapagliflozin on substrate metabolism in prediabetic insulin resistant individuals: A randomized, double-blind crossover trial. Metabolism 2023, 140, 155396. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, S.G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.J.; Linder, B.A.; Hester, S.; Fontes, M.; Pernomian, L.; Wenceslau, C.F.; Robinson, A.T.; McCarthy, C.G. The janus face of ketone bodies in hypertension. J. Hypertens. 2022, 40, 2111–2119. [Google Scholar] [CrossRef]
- Nielsen, R.; Christensen, K.H.; Gopalasingam, N.; Berg-Hansen, K.; Seefeldt, J.; Homilius, C.; Boedtkjer, E.; Andersen, M.J.; Wiggers, H.; Moller, N.; et al. Hemodynamic Effects of Ketone Bodies in Patients With Pulmonary Hypertension. J. Am. Heart Assoc. 2023, 12, e028232. [Google Scholar] [CrossRef]
- Myette-Cote, E.; Caldwell, H.G.; Ainslie, P.N.; Clarke, K.; Little, J.P. A ketone monoester drink reduces the glycemic response to an oral glucose challenge in individuals with obesity: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 1491–1501. [Google Scholar] [CrossRef]
- Maechler, P.; Wollheim, C.B. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature 1999, 402, 685–689. [Google Scholar] [CrossRef]
- Yang, X.J.; Kow, L.M.; Funabashi, T.; Mobbs, C.V. Hypothalamic glucose sensor: Similarities to and differences from pancreatic beta-cell mechanisms. Diabetes 1999, 48, 1763–1772. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Rohling, M.; Lenzen-Schulte, M.; Schloot, N.C.; Martin, S. Ketone bodies: From enemy to friend and guardian angel. BMC Med. 2021, 19, 313. [Google Scholar] [CrossRef]
- Pujol, J.B.; Christinat, N.; Ratinaud, Y.; Savoia, C.; Mitchell, S.E.; Dioum, E.H.M. Coordination of GPR40 and Ketogenesis Signaling by Medium Chain Fatty Acids Regulates Beta Cell Function. Nutrients 2018, 10, 473. [Google Scholar] [CrossRef]
- Blackard, J.T.; Kong, L.; Lombardi, A.; Homann, D.; Hammerstad, S.S.; Tomer, Y. A preliminary analysis of hepatitis C virus in pancreatic islet cells. Virol. J. 2017, 14, 237. [Google Scholar] [CrossRef] [PubMed]
- Ohneda, M.; Inman, L.R.; Unger, R.H. Caloric restriction in obese pre-diabetic rats prevents beta-cell depletion, loss of beta-cell GLUT 2 and glucose incompetence. Diabetologia 1995, 38, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Liu, X.; Chen, X.; Zhang, S.; Chen, Y.; Chen, J.; Chen, J.; Wu, F.; Chen, G.Q. 3-Hydroxybutyrate ameliorates insulin resistance by inhibiting PPARgamma Ser273 phosphorylation in type 2 diabetic mice. Signal Transduct. Target. Ther. 2023, 8, 190. [Google Scholar] [CrossRef]
- Park, S.B.; Yang, S.J. Ketogenic diet preserves muscle mass and strength in a mouse model of type 2 diabetes. PLoS ONE 2024, 19, e0296651. [Google Scholar] [CrossRef]
- Trotta, M.C.; Maisto, R.; Guida, F.; Boccella, S.; Luongo, L.; Balta, C.; D’Amico, G.; Herman, H.; Hermenean, A.; Bucolo, C.; et al. The activation of retinal HCA2 receptors by systemic beta-hydroxybutyrate inhibits diabetic retinal damage through reduction of endoplasmic reticulum stress and the NLRP3 inflammasome. PLoS ONE 2019, 14, e0211005. [Google Scholar] [CrossRef]
- Sahin, E.; Bektur Aykanat, N.E.; Kacar, S.; Bagci, R.; Sahinturk, V. beta-Hydroxybutyrate, One of the Three Main Ketone Bodies, Ameliorates Acute Pancreatitis in Rats by Suppressing the NLRP3 Inflammasome Pathway. Turk. J. Gastroenterol. 2021, 32, 702–711. [Google Scholar] [CrossRef]
- Pan, A.; Sun, X.M.; Huang, F.Q.; Liu, J.F.; Cai, Y.Y.; Wu, X.; Alolga, R.N.; Li, P.; Liu, B.L.; Liu, Q.; et al. The mitochondrial beta-oxidation enzyme HADHA restrains hepatic glucagon response by promoting beta-hydroxybutyrate production. Nat. Commun. 2022, 13, 386. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, B.; Gong, A.Y.; Malhotra, D.K.; Gupta, R.; Dworkin, L.D.; Gong, R. The ketone body beta-hydroxybutyrate mitigates the senescence response of glomerular podocytes to diabetic insults. Kidney Int. 2021, 100, 1037–1053. [Google Scholar] [CrossRef]
- Wan, S.R.; Teng, F.Y.; Fan, W.; Xu, B.T.; Li, X.Y.; Tan, X.Z.; Guo, M.; Gao, C.L.; Zhang, C.X.; Jiang, Z.Z.; et al. BDH1-mediated betaOHB metabolism ameliorates diabetic kidney disease by activation of NRF2-mediated antioxidative pathway. Aging 2023, 15, 13384–13410. [Google Scholar] [CrossRef]
- Oka, S.I.; Tang, F.; Chin, A.; Ralda, G.; Xu, X.; Hu, C.; Yang, Z.; Abdellatif, M.; Sadoshima, J. beta-Hydroxybutyrate, a Ketone Body, Potentiates the Antioxidant Defense via Thioredoxin 1 Upregulation in Cardiomyocytes. Antioxidants 2021, 10, 1153. [Google Scholar] [CrossRef]
- Tomita, I.; Tsuruta, H.; Yasuda-Yamahara, M.; Yamahara, K.; Kuwagata, S.; Tanaka-Sasaki, Y.; Chin-Kanasaki, M.; Fujita, Y.; Nishi, E.; Katagiri, H.; et al. Ketone bodies: A double-edged sword for mammalian life span. Aging Cell 2023, 22, e13833. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sun, W.; Shan, C.; Li, B.; Liu, J.; Xing, H.; Xu, Q.; Cui, B.; Zhu, W.; Chen, J.; et al. beta-hydroxybutyrate inhibits ferroptosis-mediated pancreatic damage in acute liver failure through the increase of H3K9bhb. Cell Rep. 2022, 41, 111847. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Zhu, Y.; Brownrigg, G.P.; Moravcova, R.; Rogalski, J.C.; Foster, L.J.; Johnson, J.D.; Kolic, J. Beta-Hydroxybutyrate Promotes Basal Insulin Secretion While Decreasing Glucagon Secretion in Mouse and Human Islets. Endocrinology 2024, 165, bqae079. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Myette-Cote, E.; Neudorf, H.; Little, J.P. Potential Therapeutic Effects of Exogenous Ketone Supplementation for Type 2 Diabetes: A Review. Curr. Pharm. Des. 2020, 26, 958–969. [Google Scholar] [CrossRef]
- Cooper, I.D.; Kyriakidou, Y.; Edwards, K.; Petagine, L.; Seyfried, T.N.; Duraj, T.; Soto-Mota, A.; Scarborough, A.; Jacome, S.L.; Brookler, K.; et al. Ketosis Suppression and Ageing (KetoSAge): The Effects of Suppressing Ketosis in Long Term Keto-Adapted Non-Athletic Females. Int. J. Mol. Sci. 2023, 24, 15621. [Google Scholar] [CrossRef]
- Yu, Q.; Falkenhain, K.; Little, J.P.; Wong, K.K.; Nie, J.; Shi, Q.; Kong, Z. Effects of ketone supplements on blood beta-hydroxybutyrate, glucose and insulin: A systematic review and three-level meta-analysis. Complement. Ther. Clin. Pract. 2023, 52, 101774. [Google Scholar] [CrossRef]
- Myette-Cote, E.; Neudorf, H.; Rafiei, H.; Clarke, K.; Little, J.P. Prior ingestion of exogenous ketone monoester attenuates the glycaemic response to an oral glucose tolerance test in healthy young individuals. J. Physiol. 2018, 596, 1385–1395. [Google Scholar] [CrossRef]
- Neudorf, H.; Islam, H.; Falkenhain, K.; Oliveira, B.; Jackson, G.S.; Moreno-Cabanas, A.; Madden, K.; Singer, J.; Walsh, J.J.; Little, J.P. Effect of the ketone beta-hydroxybutyrate on markers of inflammation and immune function in adults with type 2 diabetes. Clin. Exp. Immunol. 2024, 216, 89–103. [Google Scholar] [CrossRef]
- Falkenhain, K.; Daraei, A.; Little, J.P. The Effect of Novel Exogenous Ketone Supplements on Blood Beta-Hydroxybutyrate and Glucose. J. Diet. Suppl. 2024, 21, 38–52. [Google Scholar] [CrossRef]
- Soto-Mota, A.; Norwitz, N.G.; Evans, R.D.; Clarke, K. Exogenous d-beta-hydroxybutyrate lowers blood glucose in part by decreasing the availability of L-alanine for gluconeogenesis. Endocrinol. Diabetes Metab. 2022, 5, e00300. [Google Scholar] [CrossRef]
- Gouirand, V.; Gicquel, T.; Lien, E.C.; Jaune-Pons, E.; Da Costa, Q.; Finetti, P.; Metay, E.; Duluc, C.; Mayers, J.R.; Audebert, S.; et al. Ketogenic HMG-CoA lyase and its product beta-hydroxybutyrate promote pancreatic cancer progression. EMBO J. 2022, 41, e110466. [Google Scholar] [CrossRef] [PubMed]
- Alsereidi, F.R.; Khashim, Z.; Marzook, H.; Gupta, A.; Al-Rawi, A.M.; Ramadan, M.M.; Saleh, M.A. Targeting inflammatory signaling pathways with SGLT2 inhibitors: Insights into cardiovascular health and cardiac cell improvement. Curr. Probl. Cardiol. 2024, 49, 102524. [Google Scholar] [CrossRef] [PubMed]
- Zugner, E.; Yang, H.C.; Kotzbeck, P.; Boulgaropoulos, B.; Sourij, H.; Hagvall, S.; Elmore, C.S.; Esterline, R.; Moosmang, S.; Oscarsson, J.; et al. Differential In Vitro Effects of SGLT2 Inhibitors on Mitochondrial Oxidative Phosphorylation, Glucose Uptake and Cell Metabolism. Int. J. Mol. Sci. 2022, 23, 7966. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Lin, K.D.; Hsieh, C.F.; Wang, J.Y. SGLT2 Inhibitor Canagliflozin Alleviates High Glucose-Induced Inflammatory Toxicity in BV-2 Microglia. Biomedicines 2023, 12, 36. [Google Scholar] [CrossRef]
- Uthman, L.; Kuschma, M.; Romer, G.; Boomsma, M.; Kessler, J.; Hermanides, J.; Hollmann, M.W.; Preckel, B.; Zuurbier, C.J.; Weber, N.C. Novel Anti-inflammatory Effects of Canagliflozin Involving Hexokinase II in Lipopolysaccharide-Stimulated Human Coronary Artery Endothelial Cells. Cardiovasc. Drugs Ther. 2021, 35, 1083–1094. [Google Scholar] [CrossRef]
- Shah, P.A.; Shrivastav, P.S.; Sharma, V.; Yadav, M.S. Challenges in simultaneous extraction and chromatographic separation of metformin and three SGLT-2 inhibitors in human plasma using LC-MS/MS. J. Pharm. Biomed. Anal. 2019, 175, 112790. [Google Scholar] [CrossRef]
- Varzideh, F.; Kansakar, U.; Santulli, G. SGLT2 inhibitors in cardiovascular medicine. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, e67–e68. [Google Scholar] [CrossRef]
- Kshirsagar, R.P.; Kulkarni, A.A.; Chouthe, R.S.; Pathan, S.K.; Une, H.D.; Reddy, G.B.; Diwan, P.V.; Ansari, S.A.; Sangshetti, J.N. SGLT inhibitors as antidiabetic agents: A comprehensive review. RSC Adv. 2020, 10, 1733–1756. [Google Scholar] [CrossRef]
- Han, L.; Qu, Q.; Aydin, D.; Panova, O.; Robertson, M.J.; Xu, Y.; Dror, R.O.; Skiniotis, G.; Feng, L. Structure and mechanism of the SGLT family of glucose transporters. Nature 2022, 601, 274–279. [Google Scholar] [CrossRef]
- Isaji, M. SGLT2 inhibitors: Molecular design and potential differences in effect. Kidney Int. Suppl. 2011, 79, S14–S19. [Google Scholar] [CrossRef]
- Dardi, I.; Kouvatsos, T.; Jabbour, S.A. SGLT2 inhibitors. Biochem. Pharmacol. 2016, 101, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.K.; Ghosh, S.M.; Chawla, S.; Jasdanwala, S.A. SGLT2 inhibitors: A new emerging therapeutic class in the treatment of type 2 diabetes mellitus. J. Clin. Pharmacol. 2012, 52, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Forzano, I.; Wilson, S.; Lombardi, A.; Jankauskas, S.S.; Kansakar, U.; Mone, P.; Varzideh, F.; Santulli, G. SGLT2 inhibitors: An evidence-based update on cardiovascular implications. Expert. Opin. Investig. Drugs 2023, 32, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Varzideh, F.; Forzano, I.; Wilson, S.; Salemme, L.; de Donato, A.; Lombardi, A.; Rainone, A.; Nunziata, L.; Jankauskas, S.S.; et al. Functional and Clinical Importance of SGLT2-inhibitors in Frailty: From the Kidney to the Heart. Hypertension 2023, 80, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Byrne, N.J.; Parajuli, N.; Levasseur, J.L.; Boisvenue, J.; Beker, D.L.; Masson, G.; Fedak, P.W.M.; Verma, S.; Dyck, J.R.B. Empagliflozin Prevents Worsening of Cardiac Function in an Experimental Model of Pressure Overload-Induced Heart Failure. JACC Basic Transl. Sci. 2017, 2, 347–354. [Google Scholar] [CrossRef]
- Pennig, J.; Scherrer, P.; Gissler, M.C.; Anto-Michel, N.; Hoppe, N.; Funer, L.; Hardtner, C.; Stachon, P.; Wolf, D.; Hilgendorf, I.; et al. Glucose lowering by SGLT2-inhibitor empagliflozin accelerates atherosclerosis regression in hyperglycemic STZ-diabetic mice. Sci. Rep. 2019, 9, 17937. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef]
- Lee, D.M.; Battson, M.L.; Jarrell, D.K.; Hou, S.; Ecton, K.E.; Weir, T.L.; Gentile, C.L. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc. Diabetol. 2018, 17, 62. [Google Scholar] [CrossRef]
- Nambu, H.; Takada, S.; Fukushima, A.; Matsumoto, J.; Kakutani, N.; Maekawa, S.; Shirakawa, R.; Nakano, I.; Furihata, T.; Katayama, T.; et al. Empagliflozin restores lowered exercise endurance capacity via the activation of skeletal muscle fatty acid oxidation in a murine model of heart failure. Eur. J. Pharmacol. 2020, 866, 172810. [Google Scholar] [CrossRef]
- Yokono, M.; Takasu, T.; Hayashizaki, Y.; Mitsuoka, K.; Kihara, R.; Muramatsu, Y.; Miyoshi, S.; Tahara, A.; Kurosaki, E.; Li, Q.; et al. SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high-fat diet-induced obese rats. Eur. J. Pharmacol. 2014, 727, 66–74. [Google Scholar] [CrossRef]
- Hawley, S.A.; Ford, R.J.; Smith, B.K.; Gowans, G.J.; Mancini, S.J.; Pitt, R.D.; Day, E.A.; Salt, I.P.; Steinberg, G.R.; Hardie, D.G. The Na+/Glucose Cotransporter Inhibitor Canagliflozin Activates AMPK by Inhibiting Mitochondrial Function and Increasing Cellular AMP Levels. Diabetes 2016, 65, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Lopaschuk, G.D. Cardiac fatty acid oxidation in heart failure associated with obesity and diabetes. Biochim. Biophys. Acta 2016, 1861, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.E.; Kiel, A.M.; Luebbe, S.T.; Simon, B.R.; Earl, C.C.; Regmi, A.; Roell, W.C.; Mather, K.J.; Tune, J.D.; Goodwill, A.G. Inhibition of sodium-glucose cotransporter-2 preserves cardiac function during regional myocardial ischemia independent of alterations in myocardial substrate utilization. Basic Res. Cardiol. 2019, 114, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Feng, B.; Ma, X.; Sun, K.; Xu, G.; Zhou, Y. Dapagliflozin improves left ventricular remodeling and aorta sympathetic tone in a pig model of heart failure with preserved ejection fraction. Cardiovasc. Diabetol. 2019, 18, 107. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; San Antonio, R.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Flores, E.; Garcia-Ropero, A.; Sanz, J.; Hajjar, R.J.; et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J. Am. Coll. Cardiol. 2019, 73, 1931–1944. [Google Scholar] [CrossRef]
- Baartscheer, A.; Schumacher, C.A.; Wust, R.C.; Fiolet, J.W.; Stienen, G.J.; Coronel, R.; Zuurbier, C.J. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia 2017, 60, 568–573. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, S.J.; Lee, J.J.; Kim, J.S.; Lee, O.H.; Kim, C.K.; Kim, D.; Lee, Y.H.; Oh, J.; Park, S.; et al. Anti-Inflammatory Effect for Atherosclerosis Progression by Sodium-Glucose Cotransporter 2 (SGLT-2) Inhibitor in a Normoglycemic Rabbit Model. Korean Circ. J. 2020, 50, 443–457. [Google Scholar] [CrossRef]
- Shi, X.; Verma, S.; Yun, J.; Brand-Arzamendi, K.; Singh, K.K.; Liu, X.; Garg, A.; Quan, A.; Wen, X.Y. Effect of empagliflozin on cardiac biomarkers in a zebrafish model of heart failure: Clues to the EMPA-REG OUTCOME trial? Mol. Cell. Biochem. 2017, 433, 97–102. [Google Scholar] [CrossRef]
- Rathore, A.; Gupta, N.; Kahn, C.; Kadariya, D. Euglycemic diabetic ketoacidosis caused by empagliflozin complicated by failure to thrive in a geriatric patient. Arch. Clin. Cases 2023, 10, 89–92. [Google Scholar] [CrossRef]
- Miyazaki, M.; Nakano, M.; Takahashi, H.; Isaka, Y.; Hiura, Y. Euglycemic Ketoacidosis in a Patient without Diabetes Taking Sodium-Glucose Cotransporter 2 Inhibitors for Heart Failure. Am. J. Case Rep. 2024, 25, e943945. [Google Scholar] [CrossRef]
- Lee, M.K.H.; Ball, P.A. Euglycemic diabetic ketoacidosis in the setting of acute intracerebral hemorrhage. Surg. Neurol. Int. 2024, 15, 284. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Fu, Z.; Jones, P.; Kwee, L.C.; Windsor, S.L.; Ilkayeva, O.; Newgard, C.B.; Margulies, K.B.; Husain, M.; Inzucchi, S.E.; et al. Metabolomic Profiling of the Effects of Dapagliflozin in Heart Failure With Reduced Ejection Fraction: DEFINE-HF. Circulation 2022, 146, 808–818. [Google Scholar] [CrossRef] [PubMed]
- von Lewinski, D.; Kolesnik, E.; Tripolt, N.J.; Pferschy, P.N.; Benedikt, M.; Wallner, M.; Alber, H.; Berger, R.; Lichtenauer, M.; Saely, C.H.; et al. Empagliflozin in acute myocardial infarction: The EMMY trial. Eur. Heart J. 2022, 43, 4421–4432. [Google Scholar] [CrossRef] [PubMed]
- Tomita, I.; Kume, S.; Sugahara, S.; Osawa, N.; Yamahara, K.; Yasuda-Yamahara, M.; Takeda, N.; Chin-Kanasaki, M.; Kaneko, T.; Mayoux, E.; et al. SGLT2 Inhibition Mediates Protection from Diabetic Kidney Disease by Promoting Ketone Body-Induced mTORC1 Inhibition. Cell Metab. 2020, 32, 404–419.e6. [Google Scholar] [CrossRef]
- Su, K.; Zhao, S.L.; Yang, W.X.; Lo, C.S.; Chenier, I.; Liao, M.C.; Pang, Y.C.; Peng, J.Z.; Miyata, K.N.; Cailhier, J.F.; et al. NRF2 Deficiency Attenuates Diabetic Kidney Disease in Db/Db Mice via Down-Regulation of Angiotensinogen, SGLT2, CD36, and FABP4 Expression and Lipid Accumulation in Renal Proximal Tubular Cells. Antioxidants 2023, 12, 1715. [Google Scholar] [CrossRef]
- Kim, M.N.; Moon, J.H.; Cho, Y.M. Sodium-glucose cotransporter-2 inhibition reduces cellular senescence in the diabetic kidney by promoting ketone body-induced NRF2 activation. Diabetes Obes. Metab. 2021, 23, 2561–2571. [Google Scholar] [CrossRef]
- Wu, Q.; Yao, Q.; Hu, T.; Yu, J.; Jiang, K.; Wan, Y.; Tang, Q. Dapagliflozin protects against chronic heart failure in mice by inhibiting macrophage-mediated inflammation, independent of SGLT2. Cell Rep. Med. 2023, 4, 101334. [Google Scholar] [CrossRef]
- Ma, L.; Zou, R.; Shi, W.; Zhou, N.; Chen, S.; Zhou, H.; Chen, X.; Wu, Y. SGLT2 inhibitor dapagliflozin reduces endothelial dysfunction and microvascular damage during cardiac ischemia/reperfusion injury through normalizing the XO-SERCA2-CaMKII-coffilin pathways. Theranostics 2022, 12, 5034–5050. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.; Yang, H.; Gu, L.; Ni, Z.; Mou, S.; Shen, J.; Che, X. Sodium glucose co-transporter 2 (SGLT2) inhibition via dapagliflozin improves diabetic kidney disease (DKD) over time associatied with increasing effect on the gut microbiota in db/db mice. Front. Endocrinol. 2023, 14, 1026040. [Google Scholar] [CrossRef]
- Karlsson, D.; Ahnmark, A.; Sabirsh, A.; Andreasson, A.C.; Gennemark, P.; Sandinge, A.S.; Chen, L.; Tyrberg, B.; Linden, D.; Sorhede Winzell, M. Inhibition of SGLT2 Preserves Function and Promotes Proliferation of Human Islets Cells In Vivo in Diabetic Mice. Biomedicines 2022, 10, 203. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, K.; Liang, L.; Liu, Y.; Song, F.; Ge, Q.; Fang, X.; Yu, T.; Huang, Z.; Jiang, L.; et al. Sodium-Glucose Co-Transporter 2 Inhibition With Empagliflozin Improves Cardiac Function After Cardiac Arrest in Rats by Enhancing Mitochondrial Energy Metabolism. Front. Pharmacol. 2021, 12, 758080. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Xu, Y.; Wang, D.; Chen, F.; Tu, Z.; Qian, J.; Xu, S.; Xu, Y.; Hwa, J.; Li, J.; et al. Cardioprotective mechanism of SGLT2 inhibitor against myocardial infarction is through reduction of autosis. Protein Cell 2022, 13, 336–359. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; De Laurentiis, M.; Rea, D.; Barbieri, A.; Monti, M.G.; Carbone, A.; Paccone, A.; Altucci, L.; Conte, M.; Canale, M.L.; et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc. Diabetol. 2021, 20, 150. [Google Scholar] [CrossRef]
- Radlinger, B.; Ress, C.; Folie, S.; Salzmann, K.; Lechuga, A.; Weiss, B.; Salvenmoser, W.; Graber, M.; Hirsch, J.; Holfeld, J.; et al. Empagliflozin protects mice against diet-induced obesity, insulin resistance and hepatic steatosis. Diabetologia 2023, 66, 754–767. [Google Scholar] [CrossRef]
- Lu, Y.H.; Chang, Y.P.; Li, T.; Han, F.; Li, C.J.; Li, X.Y.; Xue, M.; Cheng, Y.; Meng, Z.Y.; Han, Z.; et al. Empagliflozin Attenuates Hyperuricemia by Upregulation of ABCG2 via AMPK/AKT/CREB Signaling Pathway in Type 2 Diabetic Mice. Int. J. Biol. Sci. 2020, 16, 529–542. [Google Scholar] [CrossRef]
- Soares, R.N.; Ramirez-Perez, F.I.; Cabral-Amador, F.J.; Morales-Quinones, M.; Foote, C.A.; Ghiarone, T.; Sharma, N.; Power, G.; Smith, J.A.; Rector, R.S.; et al. SGLT2 inhibition attenuates arterial dysfunction and decreases vascular F-actin content and expression of proteins associated with oxidative stress in aged mice. Geroscience 2022, 44, 1657–1675. [Google Scholar] [CrossRef]
- Hogan, M.F.; Hackney, D.J.; Aplin, A.C.; Mundinger, T.O.; Larmore, M.J.; Castillo, J.J.; Esser, N.; Zraika, S.; Hull, R.L. SGLT2-i improves markers of islet endothelial cell function in db/db diabetic mice. J. Endocrinol. 2021, 248, 95–106. [Google Scholar] [CrossRef]
- Moellmann, J.; Mann, P.A.; Kappel, B.A.; Kahles, F.; Klinkhammer, B.M.; Boor, P.; Kramann, R.; Ghesquiere, B.; Lebherz, C.; Marx, N.; et al. The sodium-glucose co-transporter-2 inhibitor ertugliflozin modifies the signature of cardiac substrate metabolism and reduces cardiac mTOR signalling, endoplasmic reticulum stress and apoptosis. Diabetes Obes. Metab. 2022, 24, 2263–2272. [Google Scholar] [CrossRef]
- Chae, H.; Augustin, R.; Gatineau, E.; Mayoux, E.; Bensellam, M.; Antoine, N.; Khattab, F.; Lai, B.K.; Brusa, D.; Stierstorfer, B.; et al. SGLT2 is not expressed in pancreatic alpha- and beta-cells, and its inhibition does not directly affect glucagon and insulin secretion in rodents and humans. Mol. Metab. 2020, 42, 101071. [Google Scholar] [CrossRef]
- Scarr, D.; Lovblom, E.; Ye, H.; Liu, H.; Bakhsh, A.; Verhoeff, N.J.; Wolever, T.M.S.; Lawler, P.R.; Sharma, K.; Cherney, D.Z.I.; et al. Ketone production and excretion even during mild hyperglycemia and the impact of sodium-glucose co-transporter inhibition in type 1 diabetes. Diabetes Res. Clin. Pract. 2024, 207, 111031. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wu, M.Z.; Lui, D.T.; Chan, D.S.; Fong, C.H.; Shiu, S.W.; Wong, Y.; Lee, A.C.; Lam, J.K.; Woo, Y.C.; et al. Comparison of Serum Ketone Levels and Cardiometabolic Efficacy of Dapagliflozin versus Sitagliptin among Insulin-Treated Chinese Patients with Type 2 Diabetes Mellitus. Diabetes Metab. J. 2022, 46, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.S.; Van Name, M.A.; Cengiz, E.; Carria, L.R.; Weinzimer, S.A.; Tamborlane, W.V.; Sherr, J.L. Altered Patterns of Early Metabolic Decompensation in Type 1 Diabetes During Treatment with a SGLT2 Inhibitor: An Insulin Pump Suspension Study. Diabetes Technol. Ther. 2017, 19, 618–622. [Google Scholar] [CrossRef]
- Schaub, J.A.; AlAkwaa, F.M.; McCown, P.J.; Naik, A.S.; Nair, V.; Eddy, S.; Menon, R.; Otto, E.A.; Demeke, D.; Hartman, J.; et al. SGLT2 inhibitors mitigate kidney tubular metabolic and mTORC1 perturbations in youth-onset type 2 diabetes. J. Clin. Investig. 2023, 133, e164486. [Google Scholar] [CrossRef]
- Lupsa, B.C.; Kibbey, R.G.; Inzucchi, S.E. Ketones: The double-edged sword of SGLT2 inhibitors? Diabetologia 2023, 66, 23–32. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, X.; Xu, G. Combination therapy with SGLT2 inhibitors for diabetic kidney disease. Biomed. Pharmacother. 2020, 127, 110192. [Google Scholar] [CrossRef]
- Tsai, K.F.; Chen, Y.L.; Chiou, T.T.; Chu, T.H.; Li, L.C.; Ng, H.Y.; Lee, W.C.; Lee, C.T. Emergence of SGLT2 Inhibitors as Powerful Antioxidants in Human Diseases. Antioxidants 2021, 10, 1166. [Google Scholar] [CrossRef]
- Schonberger, E.; Mihaljevic, V.; Steiner, K.; Saric, S.; Kurevija, T.; Majnaric, L.T.; Bilic Curcic, I.; Canecki-Varzic, S. Immunomodulatory Effects of SGLT2 Inhibitors-Targeting Inflammation and Oxidative Stress in Aging. Int. J. Environ. Res. Public Health 2023, 20, 6671. [Google Scholar] [CrossRef]
- Wei, R.; Cui, X.; Feng, J.; Gu, L.; Lang, S.; Wei, T.; Yang, J.; Liu, J.; Le, Y.; Wang, H.; et al. Dapagliflozin promotes beta cell regeneration by inducing pancreatic endocrine cell phenotype conversion in type 2 diabetic mice. Metabolism 2020, 111, 154324. [Google Scholar] [CrossRef]
- Panchapakesan, U.; Pegg, K.; Gross, S.; Komala, M.G.; Mudaliar, H.; Forbes, J.; Pollock, C.; Mather, A. Effects of SGLT2 inhibition in human kidney proximal tubular cells—Renoprotection in diabetic nephropathy? PLoS ONE 2013, 8, e54442. [Google Scholar] [CrossRef]
- Gaborit, B.; Ancel, P.; Abdullah, A.E.; Maurice, F.; Abdesselam, I.; Calen, A.; Soghomonian, A.; Houssays, M.; Varlet, I.; Eisinger, M.; et al. Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: The EMPACEF study. Cardiovasc. Diabetol. 2021, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, C.; Xu, L.; Li, X.; Sun, H.; Xue, M.; Li, T.; Yu, X.; Sun, B.; Chen, L. Empagliflozin improves diabetic renal tubular injury by alleviating mitochondrial fission via AMPK/SP1/PGAM5 pathway. Metabolism 2020, 111, 154334. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Thorburn, D.R. Mitochondrial dysfunction in diabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Akoumianakis, I.; Badi, I.; Akawi, N.; Kotanidis, C.P.; Polkinghorne, M.; Stadiotti, I.; Sommariva, E.; Antonopoulos, A.S.; Carena, M.C.; et al. Effects of canagliflozin on human myocardial redox signalling: Clinical implications. Eur. Heart J. 2021, 42, 4947–4960. [Google Scholar] [CrossRef]
- Ding, L.; Chen, X.; Zhang, W.; Dai, X.; Guo, H.; Pan, X.; Xu, Y.; Feng, J.; Yuan, M.; Gao, X.; et al. Canagliflozin primes antitumor immunity by triggering PD-L1 degradation in endocytic recycling. J. Clin. Investig. 2023, 133, e154754. [Google Scholar] [CrossRef]
- Matsubayashi, Y.; Yoshida, A.; Suganami, H.; Osawa, T.; Furukawa, K.; Suzuki, H.; Fujihara, K.; Tanaka, S.; Kaku, K.; Sone, H. Association of increased hepatic insulin clearance and change in serum triglycerides or beta-hydroxybutyrate concentration via the sodium/glucose-cotransporter 2 inhibitor tofogliflozin. Diabetes Obes. Metab. 2020, 22, 947–956. [Google Scholar] [CrossRef]
- Shin, J.M.; Son, S.; Jung, K.E.; Kim, C.D.; Lee, Y. Possible role of beta-hydroxybutyrate in inducing inflammation in alopecia areata. Exp. Dermatol. 2024, 33, e15117. [Google Scholar] [CrossRef]
- Dmitrieva-Posocco, O.; Wong, A.C.; Lundgren, P.; Golos, A.M.; Descamps, H.C.; Dohnalova, L.; Cramer, Z.; Tian, Y.; Yueh, B.; Eskiocak, O.; et al. beta-Hydroxybutyrate suppresses colorectal cancer. Nature 2022, 605, 160–165. [Google Scholar] [CrossRef]
- Karagiannis, F.; Peukert, K.; Surace, L.; Michla, M.; Nikolka, F.; Fox, M.; Weiss, P.; Feuerborn, C.; Maier, P.; Schulz, S.; et al. Impaired ketogenesis ties metabolism to T cell dysfunction in COVID-19. Nature 2022, 609, 801–807. [Google Scholar] [CrossRef]
- Chong, D.; Gu, Y.; Zhang, T.; Xu, Y.; Bu, D.; Chen, Z.; Xu, N.; Li, L.; Zhu, X.; Wang, H.; et al. Neonatal ketone body elevation regulates postnatal heart development by promoting cardiomyocyte mitochondrial maturation and metabolic reprogramming. Cell Discov. 2022, 8, 106. [Google Scholar] [CrossRef]
- Nasser, S.; Vialichka, V.; Biesiekierska, M.; Balcerczyk, A.; Pirola, L. Effects of ketogenic diet and ketone bodies on the cardiovascular system: Concentration matters. World J. Diabetes 2020, 11, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. eLife 2016, 5, e15092. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Li, B.; Yu, Y.; Liu, K.; Zhang, Y.; Geng, Q.; Zhang, F.; Li, Y.; Qi, J. beta-Hydroxybutyrate inhibits histone deacetylase 3 to promote claudin-5 generation and attenuate cardiac microvascular hyperpermeability in diabetes. Diabetologia 2021, 64, 226–239. [Google Scholar] [CrossRef]
- Ji, L.; He, Q.; Liu, Y.; Deng, Y.; Xie, M.; Luo, K.; Cai, X.; Zuo, Y.; Wu, W.; Li, Q.; et al. Ketone Body beta-Hydroxybutyrate Prevents Myocardial Oxidative Stress in Septic Cardiomyopathy. Oxidative Med. Cell. Longev. 2022, 2022, 2513837. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kansakar, U.; Nieves Garcia, C.; Santulli, G.; Gambardella, J.; Mone, P.; Jankauskas, S.S.; Lombardi, A. Exogenous Ketones in Cardiovascular Disease and Diabetes: From Bench to Bedside. J. Clin. Med. 2024, 13, 7391. https://doi.org/10.3390/jcm13237391
Kansakar U, Nieves Garcia C, Santulli G, Gambardella J, Mone P, Jankauskas SS, Lombardi A. Exogenous Ketones in Cardiovascular Disease and Diabetes: From Bench to Bedside. Journal of Clinical Medicine. 2024; 13(23):7391. https://doi.org/10.3390/jcm13237391
Chicago/Turabian StyleKansakar, Urna, Crystal Nieves Garcia, Gaetano Santulli, Jessica Gambardella, Pasquale Mone, Stanislovas S. Jankauskas, and Angela Lombardi. 2024. "Exogenous Ketones in Cardiovascular Disease and Diabetes: From Bench to Bedside" Journal of Clinical Medicine 13, no. 23: 7391. https://doi.org/10.3390/jcm13237391
APA StyleKansakar, U., Nieves Garcia, C., Santulli, G., Gambardella, J., Mone, P., Jankauskas, S. S., & Lombardi, A. (2024). Exogenous Ketones in Cardiovascular Disease and Diabetes: From Bench to Bedside. Journal of Clinical Medicine, 13(23), 7391. https://doi.org/10.3390/jcm13237391





