Dickkopf 3 as a New Monitoring Tool for Kidney Function After Living Kidney Donation
Abstract
1. Introduction
2. Material and Methods
2.1. Patients’ Baseline Characteristics
2.2. Dickkopf 3 Analysis
2.3. Histological Analysis
2.4. Statistical Analysis
3. Results
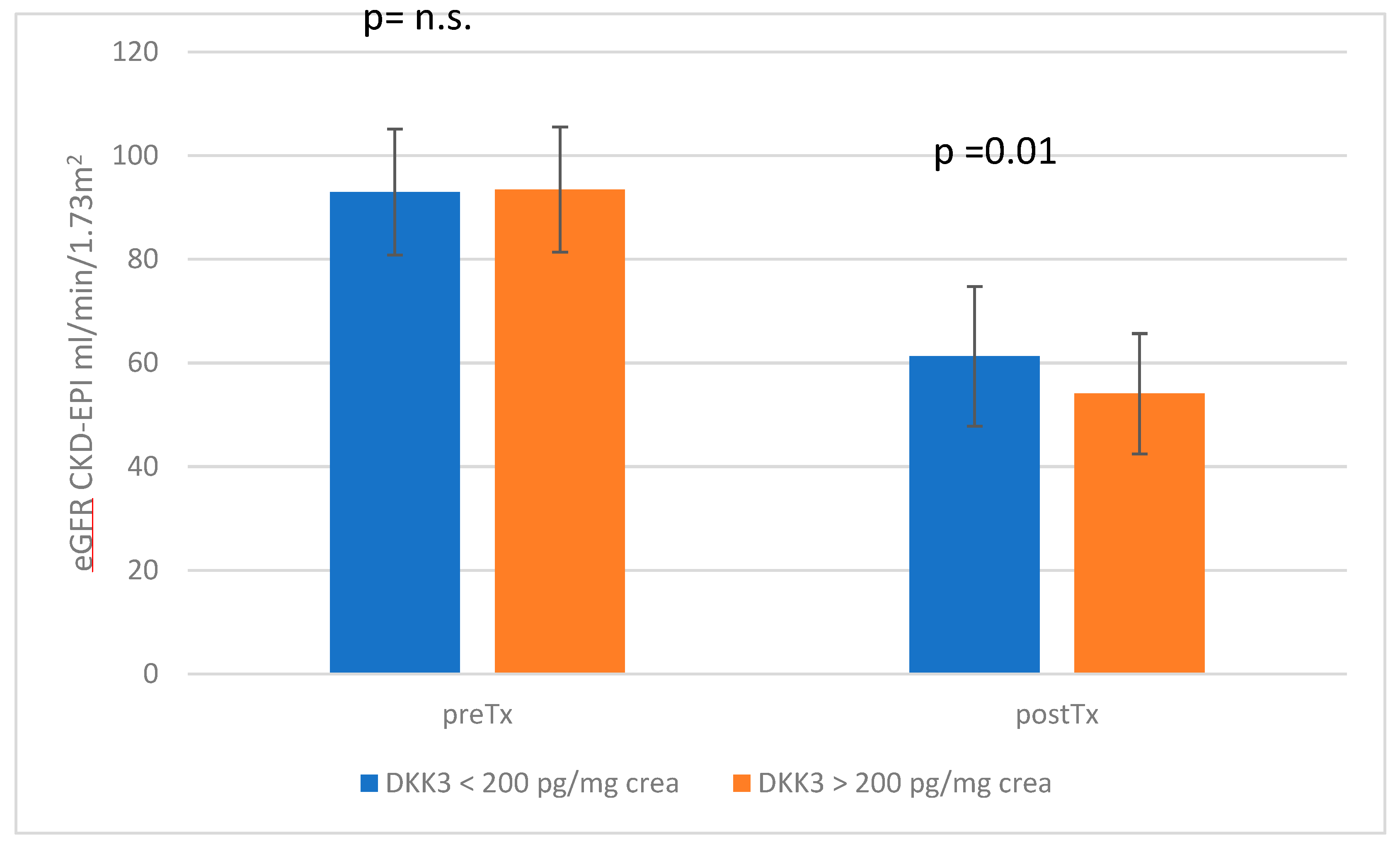
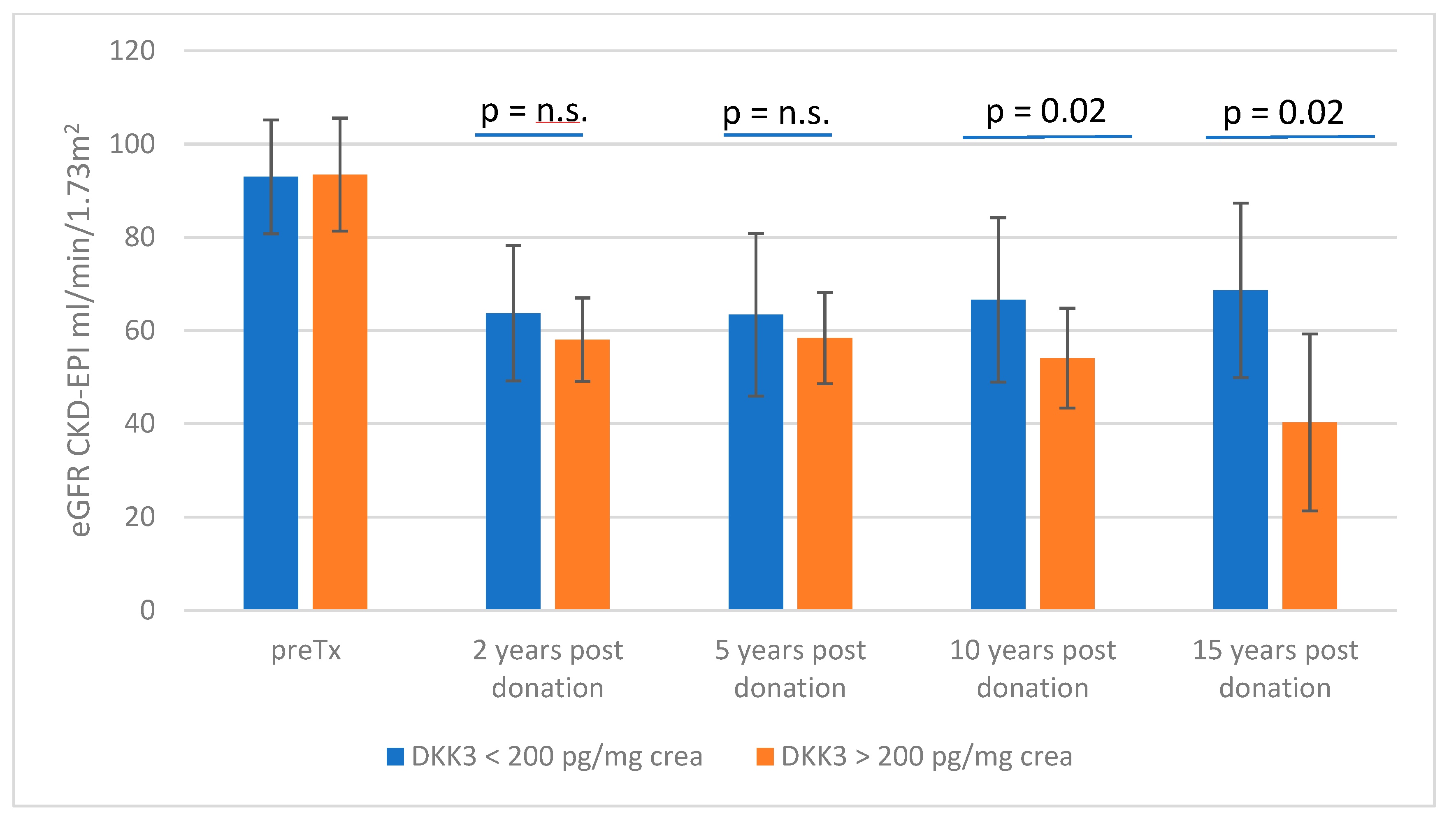
3.1. Stratification According to DKK3 Values </≥ 200 pg/mg Crea
3.2. Results of the Biopsy Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- statistics.eurotransplant.org: 2052P_Germany_kidney: 08.02.2023: Counting Recipient Transplants. Published by Eurotransplant, Netherlands. Available online: https://statistics.eurotransplant.org/reportloader.php?report=11341-33105-33153&format=html&download=0 (accessed on 7 February 2024).
- Hori, S.; Tomizawa, M.; Inoue, K.; Yoneda, T.; Nakahama, T.; Onishi, K.; Morizawa, Y.; Gotoh, D.; Nakai, Y.; Miyake, M.; et al. Follow-up After Donor Nephrectomy in Living Kidney Donors: How to Manage Living Kidney Donors Postoperatively. Vivo 2024, 38, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Kasiske, B.L.; Levey, A.S.; Adams, P.L.; Alberú, J.; Bakr, M.A.; Gallon, L.; Garvey, C.A.; Guleria, S.; Li, P.K.-T.; et al. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation 2017, 101 (Suppl. S1), S1–S109. [Google Scholar] [CrossRef] [PubMed]
- Zewinger, S.; Rauen, T.; Rudnicki, M.; Federico, G.; Wagner, M.; Triem, S.; Schunk, S.J.; Petrakis, I.; Schmit, D.; Wagenpfeil, S.; et al. Dickkopf-3 (DKK3) in Urine Identifies Patients with Short-Term Risk of eGFR Loss. J. Am. Soc. Nephrol. 2018, 29, 2722–2733. [Google Scholar] [CrossRef] [PubMed]
- Gröne, E.F.; Federico, G.; Nelson, P.J.; Arnold, B.; Gröne, H.-J. The hormetic functions of Wnt pathways in tubular injury. Pflug. Arch. 2017, 469, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, C.J.; Liu, Y. Wnt Signaling in Kidney Development and Disease. Prog. Mol. Biol. Transl. Sci. 2018, 153, 181–207. [Google Scholar] [CrossRef] [PubMed]
- Federico, G.; Meister, M.; Mathow, D.; Heine, G.H.; Moldenhauer, G.; Popovic, Z.V.; Nordström, V.; Kopp Schneider, A.; Hielscher, T.; Nelson, P.J.; et al. Tubular Dickkopf-3 promotes the development of renal atrophy and fibrosis. JCI Insight 2016, 1, e84916. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.; Steines, L.; Müller, K.; Zeman, F.; Findeisen, P.; Banas, B.; Bergler, T. Dickkopf 3-A New Indicator for the Deterioration of Allograft Function After Kidney Transplantation. Front. Med. 2022, 9, 885018. [Google Scholar] [CrossRef] [PubMed]
- de Fallois, J.; Günzel, A.; Daniel, C.; Stumpf, J.; Busch, M.; Pein, U.; Paliege, A.; Amann, K.; Wiech, T.; Hantmann, E.; et al. Deceased donor urinary Dickkopf-3 associates with future allograft function following kidney transplantation. Am. J. Transplant. in press. 2024. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Tanaka, N.; Tomizawa, M.; Yoneda, T.; Shimada, K.; Nishimura, N.; Nakai, Y.; Miyake, M.; Torimoto, K.; Itami, H.; et al. Clinical Impact of Subclinical Interstitial Fibrosis or Tubular Atrophy in 1-Hour Allograft Biopsy for Remnant Renal Function in Living Kidney Donors: A Prospective Observational Study. Transplant. Proc. 2021, 53, 2833–2840. [Google Scholar] [CrossRef] [PubMed]
- Emmons, B.R.; Batal, I.; King, K.L.; Yu, M.; Canetta, P.A.; Sandoval, P.R.; Mohan, S.; Tsapepas, D.; Adler, J.T.; Ratner, L.E.; et al. Association of Implantation Biopsy Findings in Living Donor Kidneys with Donor and Recipient Outcomes. Am. J. Kidney Dis. 2024, 83, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Buus, N.H.; Nielsen, C.M.; Skov, K.; Ibsen, L.; Krag, S.; Nyengaard, J.R.M. Prediction of Renal Function in Living Kidney Donors and Recipients of Living Donor Kidneys Using Quantitative Histology. Transplantation 2023, 107, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Janki, S.; Dehghan, A.; van de Wetering, J.; Steyerberg, E.W.; Klop, K.W.J.; Kimenai, H.J.A.N.; Rizopoulos, D.; Hoorn, E.J.; Stracke, S.; Weimar, W.; et al. Long-term prognosis after kidney donation: A propensity score matched comparison of living donors and non-donors from two population cohorts. Eur. J. Epidemiol. 2020, 35, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Huang, Y.-M.; Wang, M.; Shen, J.; Lin, B.-J.; Sui, Y.; Zhao, H.-L. A meta-analysis of renal outcomes in living kidney donors. Medicine 2016, 95, e3847. [Google Scholar] [CrossRef] [PubMed]
- Jehn, U.; Altuner, U.; Henkel, L.; Menke, A.F.; Strauss, M.; Pavenstädt, H.; Reuter, S. Urinary Dickkopf 3 Is Not an Independent Risk Factor in a Cohort of Kidney Transplant Recipients and Living Donors. Int. J. Mol. Sci. 2024, 25, 5376. [Google Scholar] [CrossRef] [PubMed]
- Grams, M.E.; Sang, Y.; Levey, A.S.; Matsushita, K.; Ballew, S.; Chang, A.R.; Chow, E.K.; Kasiske, B.L.; Kovesdy, C.P.; Nadkarni, G.N.; et al. Kidney-Failure Risk Projection for the Living Kidney-Donor Candidate. N. Engl. J. Med. 2016, 374, 411–421. [Google Scholar] [CrossRef] [PubMed]
| Dickkopf 3 < 200 pg/mL | Dickkopf 3 > 200 pg/mL | p-Value | |
|---|---|---|---|
| creatinine (mg/dL) | 0.78 | 0.77 | 0.72 |
| eGFR (mL/min) | 92.95 | 93.46 | 0.84 |
| creatinine clearance (ml/min) | 132.04 | 126.93 | 0.56 |
| body mass index (kg/m2) | 25.73 | 26.40 | 0.35 |
| HbA1c (%) | 5.49 | 5.45 | 0.66 |
| systolic blood pressure—day (mmHg) | 125 | 126 | 0.67 |
| diastolic blood pressure—day (mmHg) | 79 | 80 | 0.64 |
| systolic blood pressure—night (mmHg) | 111 | 112 | 0.95 |
| diastolic blood pressure—night (mmHg) | 69 | 69 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuster, A.; Steines, L.; Banas, B.; Bergler, T. Dickkopf 3 as a New Monitoring Tool for Kidney Function After Living Kidney Donation. J. Clin. Med. 2024, 13, 7454. https://doi.org/10.3390/jcm13237454
Schuster A, Steines L, Banas B, Bergler T. Dickkopf 3 as a New Monitoring Tool for Kidney Function After Living Kidney Donation. Journal of Clinical Medicine. 2024; 13(23):7454. https://doi.org/10.3390/jcm13237454
Chicago/Turabian StyleSchuster, Antonia, Louisa Steines, Bernhard Banas, and Tobias Bergler. 2024. "Dickkopf 3 as a New Monitoring Tool for Kidney Function After Living Kidney Donation" Journal of Clinical Medicine 13, no. 23: 7454. https://doi.org/10.3390/jcm13237454
APA StyleSchuster, A., Steines, L., Banas, B., & Bergler, T. (2024). Dickkopf 3 as a New Monitoring Tool for Kidney Function After Living Kidney Donation. Journal of Clinical Medicine, 13(23), 7454. https://doi.org/10.3390/jcm13237454






