Review of Supplements That Patients Commonly Report Using for Dementia
Abstract
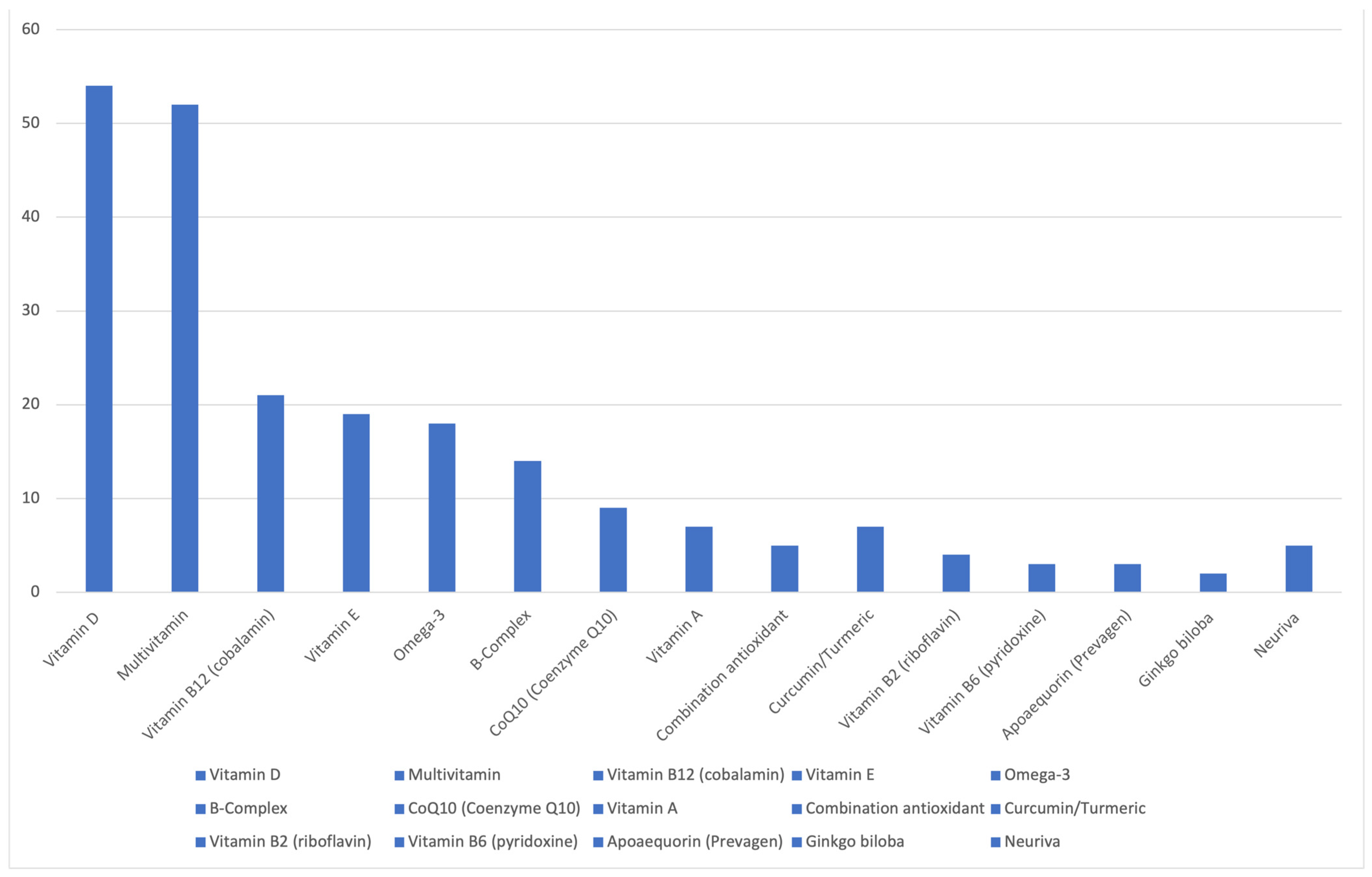
1. Introduction
2. Methods
3. Review of Several Supplements Patients Commonly Reported Using for Cognition
3.1. Apoaequorin (Prevagen®)
3.1.1. Purported Mechanism of Action
3.1.2. Available Study Data
3.1.3. Safety Data and Side Effects
3.1.4. Summary
3.2. Gingko Biloba
3.2.1. Pharmacology and Purported Mechanism of Action
3.2.2. Efficacy Data
3.2.3. Safety Data and Side Effects
3.2.4. Summary
3.3. Curcumin (Turmeric)
3.3.1. Efficacy Data
3.3.2. Safety Data and Side Effects
3.3.3. Summary
3.4. Neuriva®
3.4.1. Efficacy Data
3.4.2. Safety Data and Side Effects
3.4.3. Summary
3.5. B-Vitamins
3.5.1. Safety Data and Side Effects
3.5.2. Recommendations
3.6. Multivitamins
3.6.1. Efficacy Data
3.6.2. Safety Data and Side Effects
3.6.3. Summary
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grand View Research. U.S. Nutritional Supplements Market Trends. Available online: https://www.grandviewresearch.com/industry-analysis/us-nutritional-supplements-market-report (accessed on 24 August 2024).
- GreatGreenWall. Great Green Wall Website. Available online: https://www.greatgreenwall.org/ (accessed on 24 August 2024).
- Nahin, R.L.; Barnes, P.M.; Stussman, B.J. Expenditures on Complementary Health Approaches: United States, 2012; National Health Statistics Reports; National Center for Health Statistics (U.S.): Hyattsville, MD, USA, 2016; pp. 1–11.
- Gahche, J.J.; Bailey, R.L.; Potischman, N.; Dwyer, J.T. Dietary Supplement Use Was Very High among Older Adults in the United States in 2011–2014. J. Nutr. 2017, 147, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.C.K.; Eshetie, T.C.; Gray, S.L.; Marcum, Z.A. Dietary Supplement Use in Middle-aged and Older Adults. J. Nutr. Health Aging 2022, 26, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Fravel, M.A.; Ernst, M.E.; Gilmartin-Thomas, J.; Woods, R.L.; Orchard, S.G.; Owen, A.J. Dietary supplement and complementary and alternative medicine use among older adults in Australia and the United States. J. Am. Geriatr. Soc. 2023, 71, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- AARP_Research. 2019 AARP Brain Health and Dietary Supplements Survey. Available online: https://www.aarp.org/content/dam/aarp/research/surveys_statistics/health/2019/brain-health-and-dietary-supplements-report.doi.10.26419-2Fres.00318.001.pdf (accessed on 24 August 2024).
- Mehegan, L.R.G. Brain Health and Dietary Supplements: Where’s the Evidence? 2019 AARP Brain Health and Dietary Supplements Survey. 2019. Available online: https://www.aarp.org/pri/topics/health/prevention-wellness/brain-health-and-dietary-supplements-survey/ (accessed on 24 August 2024).
- New York State Attorney General. Attorney General James Wins Trial Against Quincy Bioscience for Deceptive and Fraudulent Advertising of “Memory Improvement” Supplement Prevagen. 2024. Available online: https://ag.ny.gov/press-release/2024/attorney-general-james-wins-trial-against-quincy-bioscience-deceptive-and (accessed on 24 August 2024).
- Caldwell, J.A.; McGraw, S.M.; Thompson, L.A.; Lieberman, H.R. A Survey Instrument to Assess Intake of Dietary Supplements, Related Products, and Caffeine in High-Use Populations. J. Nutr. 2018, 148 (Suppl. 2), 1445S–1451S. [Google Scholar] [CrossRef] [PubMed]
- Moran, D.L.; Underwood, M.Y.; Gabourie, T.A.; Lerner, K.C. Effects of a Supplement Containing Apoaequorin on Verbal Learning in Older Adults in the Community. Adv. Mind Body Med. 2016, 30, 4–11. [Google Scholar]
- Detert, J.A.; Adams, E.L.; Lescher, J.D.; Lyons, J.A.; Moyer, J.R., Jr. Pretreatment with apoaequorin protects hippocampal CA1 neurons from oxygen-glucose deprivation. PLoS ONE 2013, 8, e79002. [Google Scholar] [CrossRef][Green Version]
- Schneider, L.S.; DeKosky, S.T.; Farlow, M.R.; Tariot, P.N.; Hoerr, R.; Kieser, M. A randomized, double-blind, placebo-controlled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer’s type. Curr. Alzheimer Res. 2005, 2, 541–551. [Google Scholar] [CrossRef]
- Vellas, B.; Coley, N.; Ousset, P.J.; Berrut, G.; Dartigues, J.F.; Dubois, B.; Grandjean, H.; Pasquier, F.; Piette, F.; Robert, P.; et al. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): A randomised placebo-controlled trial. Lancet Neurol. 2012, 11, 851–859. [Google Scholar] [CrossRef]
- Gavrilova, S.I.; Preuss, U.W.; Wong, J.W.; Hoerr, R.; Kaschel, R.; Bachinskaya, N.; Group, G.I.S. Efficacy and safety of Ginkgo biloba extract EGb 761 in mild cognitive impairment with neuropsychiatric symptoms: A randomized, placebo-controlled, double-blind, multi-center trial. Int. J. Geriatr. Psychiatry 2014, 29, 1087–1095. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Williamson, J.D.; Fitzpatrick, A.L.; Kronmal, R.A.; Ives, D.G.; Saxton, J.A.; Lopez, O.L.; Burke, G.; Carlson, M.C.; Fried, L.P.; et al. Ginkgo biloba for prevention of dementia: A randomized controlled trial. JAMA 2008, 300, 2253–2262. [Google Scholar] [CrossRef]
- Snitz, B.E.; O’Meara, E.S.; Carlson, M.C.; Arnold, A.M.; Ives, D.G.; Rapp, S.R.; Saxton, J.; Lopez, O.L.; Dunn, L.O.; Sink, K.M.; et al. Ginkgo biloba for preventing cognitive decline in older adults: A randomized trial. JAMA 2009, 302, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Ihl, R.; Tribanek, M.; Bachinskaya, N.; Group, G.S. Efficacy and tolerability of a once daily formulation of Ginkgo biloba extract EGb 761(R) in Alzheimer’s disease and vascular dementia: Results from a randomised controlled trial. Pharmacopsychiatry 2012, 45, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, X.; Fang, Q.; Zhou, J.; Zhang, M.; Wang, H.; Chen, Y.; Xu, B.; Wu, Y.; Qian, L.; et al. Ginkgo biloba extract improved cognitive and neurological functions of acute ischaemic stroke: A randomised controlled trial. Stroke Vasc. Neurol. 2017, 2, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.; Grimley Evans, J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef] [PubMed]
- Amri, H.; Ogwuegbu, S.O.; Boujrad, N.; Drieu, K.; Papadopoulos, V. In vivo regulation of peripheral-type benzodiazepine receptor and glucocorticoid synthesis by Ginkgo biloba extract EGb 761 and isolated ginkgolides. Endocrinology 1996, 137, 5707–5718. [Google Scholar] [CrossRef]
- Amri, H.; Drieu, K.; Papadopoulos, V. Use of ginkgolide B and a ginkgolide-activated response element to control gene transcription: Example of the adrenocortical peripheral-type benzodiazepine receptor. Cell. Mol. Biol. 2002, 48, 633–639. [Google Scholar]
- Oyama, Y.; Chikahisa, L.; Ueha, T.; Kanemaru, K.; Noda, K. Ginkgo biloba extract protects brain neurons against oxidative stress induced by hydrogen peroxide. Brain Res. 1996, 712, 349–352. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, J.; Wang, Y.; Zhao, X.N.; Zhang, Z.X. Neuron degeneration induced by verapamil and attenuated by EGb761. J. Basic Clin. Physiol. Pharmacol. 1997, 8, 301–314. [Google Scholar] [CrossRef]
- Klein, J.; Chatterjee, S.S.; Loffelholz, K. Phospholipid breakdown and choline release under hypoxic conditions: Inhibition by bilobalide, a constituent of Ginkgo biloba. Brain Res. 1997, 755, 347–350. [Google Scholar] [CrossRef]
- Bastianetto, S.; Ramassamy, C.; Dore, S.; Christen, Y.; Poirier, J.; Quirion, R. The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. Eur. J. Neurosci. 2000, 12, 1882–1890. [Google Scholar] [CrossRef]
- Zhou, L.J.; Song, W.; Zhu, X.Z.; Chen, Z.L.; Yin, M.L.; Cheng, X.F. Protective effects of bilobalide on amyloid beta-peptide 25-35-induced PC12 cell cytotoxicity. Acta Pharmacol. Sin. 2000, 21, 75–79. [Google Scholar] [PubMed]
- Song, W.; Guan, H.J.; Zhu, X.Z.; Chen, Z.L.; Yin, M.L.; Cheng, X.F. Protective effect of bilobalide against nitric oxide-induced neurotoxicity in PC12 cells. Acta Pharmacol. Sin. 2000, 21, 415–420. [Google Scholar] [PubMed]
- Yao, Z.X.; Han, Z.; Drieu, K.; Papadopoulos, V. Ginkgo biloba extract (Egb 761) inhibits beta-amyloid production by lowering free cholesterol levels. J. Nutr. Biochem. 2004, 15, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Longpre, F.; Garneau, P.; Christen, Y.; Ramassamy, C. Protection by EGb 761 against beta-amyloid-induced neurotoxicity: Involvement of NF-kappaB, SIRT1, and MAPKs pathways and inhibition of amyloid fibril formation. Free Radic. Biol. Med. 2006, 41, 1781–1794. [Google Scholar] [CrossRef]
- Gargouri, B.; Carstensen, J.; Bhatia, H.S.; Huell, M.; Dietz, G.P.H.; Fiebich, B.L. Anti-neuroinflammatory effects of Ginkgo biloba extract EGb761 in LPS-activated primary microglial cells. Phytomedicine 2018, 44, 45–55. [Google Scholar] [CrossRef]
- Schindowski, K.; Leutner, S.; Kressmann, S.; Eckert, A.; Muller, W.E. Age-related increase of oxidative stress-induced apoptosis in mice prevention by Ginkgo biloba extract (EGb761). J. Neural Transm. 2001, 108, 969–978. [Google Scholar] [CrossRef]
- Drieu, K.; Vranckx, R.; Benassayad, C.; Haourigi, M.; Hassid, J.; Yoa, R.G.; Rapin, J.R.; Nunez, E.A. Effect of the extract of Ginkgo biloba (EGb 761) on the circulating and cellular profiles of polyunsaturated fatty acids: Correlation with the anti-oxidant properties of the extract. Prostaglandins Leukot. Essent. Fat. Acids 2000, 63, 293–300. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Z.; Butko, P.; Christen, Y.; Lambert, M.P.; Klein, W.L.; Link, C.D.; Luo, Y. Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J. Neurosci. 2006, 26, 13102–13113. [Google Scholar] [CrossRef]
- Tunali-Akbay, T.; Sener, G.; Salvarli, H.; Sehirli, O.; Yarat, A. Protective effects of Ginkgo biloba extract against mercury(II)-induced cardiovascular oxidative damage in rats. Phytother. Res. 2007, 21, 26–31. [Google Scholar] [CrossRef]
- Baum, L.; Lam, C.W.; Cheung, S.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Rainey-Smith, S.R.; Brown, B.M.; Sohrabi, H.R.; Shah, T.; Goozee, K.G.; Gupta, V.B.; Martins, R.N. Curcumin and cognition: A randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br. J. Nutr. 2016, 115, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.P.; Liu, J.; Merrill, D.A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.M.; White, D.J.; Pipingas, A.; Poorun, K.; Scholey, A. Further Evidence of Benefits to Mood and Working Memory from Lipidated Curcumin in Healthy Older People: A 12-Week, Double-Blind, Placebo-Controlled, Partial Replication Study. Nutrients 2020, 12, 1678. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.P.; Chiam, P.C.; Lee, T.; Chua, H.C.; Lim, L.; Kua, E.H. Curry consumption and cognitive function in the elderly. Am. J. Epidemiol. 2006, 164, 898–906. [Google Scholar] [CrossRef]
- Hishikawa, N.; Takahashi, Y.; Amakusa, Y.; Tanno, Y.; Tuji, Y.; Niwa, H.; Murakami, N.; Krishna, U.K. Effects of turmeric on Alzheimer’s disease with behavioral and psychological symptoms of dementia. Ayu 2012, 33, 499–504. [Google Scholar] [CrossRef]
- Frautschy, S.A.; Hu, W.; Kim, P.; Miller, S.A.; Chu, T.; Harris-White, M.E.; Cole, G.M. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol. Aging 2001, 22, 993–1005. [Google Scholar] [CrossRef]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208. [Google Scholar] [CrossRef]
- Doma, K.M.; Lewis, E.D.; Barracato, J.M.; Brink, L.R.; Gratson, A.A.; Pandey, N.; Crowley, D.C.; Evans, M. A Randomized, Double-Blind, Placebo-Controlled, Parallel Study Investigating the Efficacy of a Whole Coffee Cherry Extract and Phosphatidylserine Formulation on Cognitive Performance of Healthy Adults with Self-Perceived Memory Problems. Neurol. Ther. 2023, 12, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, A.W.; Denton, D.A.; Di Nisio, M.; Chong, L.Y.; Abraham, R.P.; Al-Assaf, A.S.; Anderson, J.L.; Malik, M.A.; Vernooij, R.W.; Martínez, G.; et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst. Rev. 2018, 12, Cd011906. [Google Scholar] [CrossRef] [PubMed]
- Riggs, K.M.; Spiro, A., 3rd; Tucker, K.; Rush, D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am. J. Clin. Nutr. 1996, 63, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Cook, N.; Manson, J.; Buring, J.E.; Albert, C.M.; Grodstein, F. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2008, 88, 1602–1610. [Google Scholar] [CrossRef]
- Malouf, R.; Grimley Evans, J. The effect of vitamin B6 on cognition. Cochrane Database Syst. Rev. 2003, Cd004393. [Google Scholar] [CrossRef]
- Malouf, R.; Grimley Evans, J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst. Rev. 2008, CD004514. [Google Scholar] [CrossRef]
- Bailey, R.L.; Jun, S.; Murphy, L.; Green, R.; Gahche, J.J.; Dwyer, J.T.; Potischman, N.; McCabe, G.P.; Miller, J.W. High folic acid or folate combined with low vitamin B-12 status: Potential but inconsistent association with cognitive function in a nationally representative cross-sectional sample of US older adults participating in the NHANES. Am. J. Clin. Nutr. 2020, 112, 1547–1557. [Google Scholar] [CrossRef]
- Eussen, S.J.; de Groot, L.C.; Joosten, L.W.; Bloo, R.J.; Clarke, R.; Ueland, P.M.; Schneede, J.; Blom, H.J.; Hoefnagels, W.H.; van Staveren, W.A. Effect of oral vitamin B-12 with or without folic acid on cognitive function in older people with mild vitamin B-12 deficiency: A randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006, 84, 361–370. [Google Scholar] [CrossRef]
- Malouf, R.; Areosa Sastre, A. Vitamin B12 for Cognition. Cochrane Database Syst. Rev. 2003, CD004394. [Google Scholar] [CrossRef]
- Vyas, C.M.; Manson, J.E.; Sesso, H.D.; Cook, N.R.; Rist, P.M.; Weinberg, A.; Moorthy, M.V.; Baker, L.D.; Espeland, M.A.; Yeung, L.K.; et al. Effect of multivitamin-mineral supplementation versus placebo on cognitive function: Results from the clinic subcohort of the COcoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial and meta-analysis of 3 cognitive studies within COSMOS. Am. J. Clin. Nutr. 2024, 119, 692–701. [Google Scholar] [CrossRef]
- Mehegan, L.R.G. A Look at Brain Health and Dietary Supplements: 2021 AARP Omni Survey of Supplement Use Among Adults Age 50 and Older. 2021. Available online: https://www.aarp.org/content/dam/aarp/research/surveys_statistics/health/2021/brain-health-and-dietary-supplements-fact-sheet-omni.doi.10.26419-2Fres.00318.003.pdf (accessed on 24 August 2024).
- Morrill, G.A.; Kostellow, A.B.; Gupta, R.K. Computational comparison of a calcium-dependent jellyfish protein (apoaequorin) and calmodulin-cholesterol in short-term memory maintenance. Neurosci. Lett. 2017, 642, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, V.L.; Smies, C.W.; Moyer, J.R., Jr. Apoaequorin differentially modulates fear memory in adult and aged rats. Brain Behav. 2020, 10, e01832. [Google Scholar] [CrossRef] [PubMed]
- Moran, D.L.; Marone, P.A.; Bauter, M.R.; Soni, M.G. Safety assessment of Apoaequorin, a protein preparation: Subchronic toxicity study in rats. Food Chem. Toxicol. 2013, 57, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prevagen. Prevagen Website. Available online: https://prevagen.com/ (accessed on 4 September 2024).
- Kelley, B.J.; Knopman, D.S. Alternative medicine and Alzheimer disease. Neurologist 2008, 14, 299–306. [Google Scholar] [CrossRef]
- Griggs, B. The outlook for green pharmacy. R. Soc. Health J. 1982, 102, 225–229. [Google Scholar] [CrossRef]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef]
- Bastianetto, S.; Zheng, W.H.; Quirion, R. The Ginkgo biloba extract (EGb 761) protects and rescues hippocampal cells against nitric oxide-induced toxicity: Involvement of its flavonoid constituents and protein kinase C. J. Neurochem. 2000, 74, 2268–2277. [Google Scholar] [CrossRef]
- Poetsch, M.; Dittberner, T.; Woenckhaus, C. Can different genetic changes characterize histogenetic subtypes and biologic behavior in sporadic malignant melanoma of the skin? Cell Mol. Life Sci. 2003, 60, 1923–1932. [Google Scholar] [CrossRef]
- Krieglstein, J.; Ausmeier, F.; El-Abhar, H.; Lippert, K.; Welsch, M.; Rupalla, K.; Henrich-Noack, P. Neuroprotective effects of Ginkgo biloba constituents. Eur. J. Pharm. Sci. 1995, 3, 39–48. [Google Scholar] [CrossRef]
- Krieglstein, J.; Beck, T.; Seibert, A. Influence of an extract of Ginkgo biloba on cerebral blood flow and metabolism. Life Sci. 1986, 39, 2327–2334. [Google Scholar] [CrossRef]
- Jiang, M.; Li, J.; Peng, Q.; Liu, Y.; Liu, W.; Luo, C.; Peng, J.; Li, J.; Yung, K.K.L.; Mo, Z. Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation. J. Neuroinflamm. 2014, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Habtemariam, S.; Daglia, M.; Braidy, N.; Loizzo, M.R.; Tundis, R.; Nabavi, S.F. Neuroprotective Effects of Ginkgolide B Against Ischemic Stroke: A Review of Current Literature. Curr. Top. Med. Chem. 2015, 15, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Montes, P.; Ruiz-Sanchez, E.; Rojas, C.; Rojas, P. Ginkgo biloba Extract 761: A Review of Basic Studies and Potential Clinical Use in Psychiatric Disorders. CNS Neurol. Disord. Drug Targets 2015, 14, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Bajenaru, O.; Prada, G.; Antochi, F.; Jianu, C.; Tudose, C.; Cuciureanu, A.; Docu, A.A.; Perrot, V.; Avram, M.; Tiu, C. Effectiveness and Safety Profile of Ginkgo biloba Standardized Extract (EGb761(R)) in Patients with Amnestic Mild Cognitive Impairment. CNS Neurol. Disord. Drug Targets 2021, 20, 378–384. [Google Scholar] [CrossRef]
- Nature’s Bounty Website—Ginkgo biloba. Available online: https://www.naturesbounty.com/our-products/specialty/diet-supplements/ginkgo-biloba-120-mg-100-capsules/ (accessed on 4 September 2024).
- Life Extension Website—Ginkgo Biloba. Available online: https://www.lifeextension.com/vitamins-supplements/item01658/ginkgo-biloba-certified-extract (accessed on 4 September 2024).
- Natrol Website—Ginkgo Biloba. Available online: https://www.natrol.com/products/ginkgo-biloba-brain-health-capsules (accessed on 4 September 2024).
- Dominguez, L.J.; Barbagallo, M. Nutritional prevention of cognitive decline and dementia. Acta Biomed. 2018, 89, 276–290. [Google Scholar] [CrossRef]
- Ganguli, M.; Chandra, V.; Kamboh, M.I.; Johnston, J.M.; Dodge, H.H.; Thelma, B.K.; Juyal, R.C.; Pandav, R.; Belle, S.H.; DeKosky, S.T. Apolipoprotein E polymorphism and Alzheimer disease: The Indo-US Cross-National Dementia Study. Arch. Neurol. 2000, 57, 824–830. [Google Scholar] [CrossRef]
- Burgos-Moron, E.; Calderon-Montano, J.M.; Salvador, J.; Robles, A.; Lopez-Lazaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef]
- Alghzawi, F.; Jones, R.; Haas, C.J. Turmeric-induced Liver Injury. J. Community Hosp. Intern. Med. Perspect. 2024, 14, 55–59. [Google Scholar] [CrossRef]
- Likhitsup, A.; Chen, V.L.; Fontana, R.J. Estimated Exposure to 6 Potentially Hepatotoxic Botanicals in US Adults. JAMA Netw. Open 2024, 7, e2425822. [Google Scholar] [CrossRef]
- Luma Nutrition Website—Turmeric Curcumin. Available online: https://lumanutrition.com/products/turmeric-curcumin/ (accessed on 4 September 2024).
- Delvin, M. It’s Time to Brain Better: RB Launches Neuriva™, a Dietary Supplement and Holistic Approach to Support Brain Health. Available online: https://www.prnewswire.com/news-releases/its-time-to-brain-better-rb-launches-neuriva-a-dietary-supplement-and-holistic-approach-to-support-brain-health-300837523.html (accessed on 4 September 2024).
- Schiff—Neuriva Website. Available online: https://www.schiffvitamins.com/pages/neuriva-brain-health-supplement-research (accessed on 6 September 2024).
- Glade, M.J.; Smith, K. Phosphatidylserine and the human brain. Nutrition 2015, 31, 781–786. [Google Scholar] [CrossRef]
- Snow, A.D.; Cummings, J.A.; Tanzi, R.E.; Lake, T. In vitro comparison of major memory-support dietary supplements for their effectiveness in reduction/inhibition of beta-amyloid protein fibrils and tau protein tangles: Key primary targets for memory loss. Sci. Rep. 2021, 11, 3001. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Izquierdo, T.; Nemzer, B.; Shu, C.; Huynh, L.; Argumedo, R.; Keller, R.; Pietrzkowski, Z. Modulatory effect of coffee fruit extract on plasma levels of brain-derived neurotrophic factor in healthy subjects. Br. J. Nutr. 2013, 110, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. The Effects of Phosphatidylserine and Omega-3 Fatty Acid-Containing Supplement on Late Life Depression. Ment. Illn. 2015, 7, 5647. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Schneider, J.A.; Tangney, C.; Tremblay-Mercier, J.; Fortier, M.; Bennett, D.A.; Morris, M.C. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2012, 29, 691–697. [Google Scholar] [CrossRef]
- Office of Dietary Supplements (ODS) at the National Institutes of Health (NIH). Dietary Supplement Label Database (DSLD). Available online: https://ods.od.nih.gov/Research/Dietary_Supplement_Label_Database.aspx (accessed on 4 September 2024).
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B(6), Folate, Vitamin B(12), Pantothenic Acid, Biotin, and Choline; National Academies Press (US) Copyright© 1998; National Academy of Sciences: Washington, DC, USA, 1998. [Google Scholar]
- Aronson, J.K. (Ed.) Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Srivastava, S.; Mennemeier, M.; Pimple, S. Effect of Alpinia galanga on Mental Alertness and Sustained Attention with or Without Caffeine: A Randomized Placebo-Controlled Study. J. Am. Coll. Nutr. 2017, 36, 631–639. [Google Scholar] [CrossRef]
- Li, T.; Steibel, J.P.; Willette, A.A. Vitamin B6, B12, and Folate’s Influence on Neural Networks in the UK Biobank Cohort. Nutrients 2024, 16, 2050. [Google Scholar] [CrossRef]
- Collaboration, H.L.T. Lowering blood homocysteine with folic acid based supplements: Meta-analysis of randomised trials. Homocysteine Lowering Trialists’ Collaboration. BMJ 1998, 316, 894–898. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H. Homocysteine, B Vitamins, and Cognitive Impairment. Annu. Rev. Nutr. 2016, 36, 211–239. [Google Scholar] [CrossRef]
- Bleie, O.; Refsum, H.; Ueland, P.M.; Vollset, S.E.; Guttormsen, A.B.; Nexo, E.; Schneede, J.; Nordrehaug, J.E.; Nygard, O. Changes in basal and postmethionine load concentrations of total homocysteine and cystathionine after B vitamin intervention. Am. J. Clin. Nutr. 2004, 80, 641–648. [Google Scholar] [CrossRef][Green Version]
- Quadri, P.; Fragiacomo, C.; Pezzati, R.; Zanda, E.; Forloni, G.; Tettamanti, M.; Lucca, U. Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am. J. Clin. Nutr. 2004, 80, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, W.; Xing, Y.; Jia, J.; Tang, Y. B vitamins and prevention of cognitive decline and incident dementia: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Vitamin B6 Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminB6-HealthProfessional/ (accessed on 7 September 2024).
- Mackey, A.; Davis, S.; Gregory, J. Vitamin B6. In Modern Nutrition in Health and Disease; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2006; pp. 194–210. [Google Scholar]
- Balk, E.M.; Raman, G.; Tatsioni, A.; Chung, M.; Lau, J.; Rosenberg, I.H. Vitamin B6, B12, and folic acid supplementation and cognitive function: A systematic review of randomized trials. Arch. Intern. Med. 2007, 167, 21–30. [Google Scholar] [CrossRef] [PubMed]
- McCleery, J.; Abraham, R.P.; Denton, D.A.; Rutjes, A.W.; Chong, L.Y.; Al-Assaf, A.S.; Griffith, D.J.; Rafeeq, S.; Yaman, H.; Malik, M.A.; et al. Vitamin and mineral supplementation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2018, 11, CD011905. [Google Scholar] [CrossRef]
- Bendich, A.; Cohen, M. Vitamin B6 safety issues. Ann. N. Y. Acad. Sci. 1990, 585, 321–330. [Google Scholar] [CrossRef]
- Mason, J.B. Unraveling the complex relationship between folate and cancer risk. Biofactors 2011, 37, 253–260. [Google Scholar] [CrossRef]
- Knopman, D.S.; DeKosky, S.T.; Cummings, J.L.; Chui, H.; Corey–Bloom, J.; Relkin, N.; Small, G.W.; Miller, B.; Stevens, J.C. Practice parameter: Diagnosis of dementia (an evidence-based review). Neurology 2001, 56, 1143–1153. [Google Scholar] [CrossRef]
- Keenan, T.D.; Agron, E.; Mares, J.A.; Clemons, T.E.; van Asten, F.; Swaroop, A.; Chew, E.Y.; Areds; Groups, A.R. Adherence to a Mediterranean diet and cognitive function in the Age-Related Eye Disease Studies 1 & 2. Alzheimers Dement. 2020, 16, 831–842. [Google Scholar] [CrossRef]
- Keenan, T.D.L.; Agron, E.; Chew, E.Y.; Areds; Groups, A.R. Dietary nutrient intake and cognitive function in the Age-Related Eye Disease Studies 1 and 2. Alzheimers Dement. 2023, 19, 4311–4324. [Google Scholar] [CrossRef]
- Sachs, B.C.; Williams, B.J.; Gaussoin, S.A.; Baker, L.D.; Manson, J.E.; Espeland, M.A.; Sesso, H.D.; Shumaker, S.A.; Rapp, S.R.; Group, C.O.-M.R. Impact of multivitamin-mineral and cocoa extract on incidence of mild cognitive impairment and dementia: Results from the COcoa Supplement and Multivitamin Outcomes Study for the Mind (COSMOS-Mind). Alzheimers Dement. 2023, 19, 4863–4871. [Google Scholar] [CrossRef]
- Loftfield, E.; O’Connell, C.P.; Abnet, C.C.; Graubard, B.I.; Liao, L.M.; Beane Freeman, L.E.; Hofmann, J.N.; Freedman, N.D.; Sinha, R. Multivitamin Use and Mortality Risk in 3 Prospective US Cohorts. JAMA Netw. Open 2024, 7, e2418729. [Google Scholar] [CrossRef] [PubMed]
- Jedrejko, K.; Catlin, O.; Stewart, T.; Anderson, A.; Muszynska, B.; Catlin, D.H. Unauthorized ingredients in “nootropic” dietary supplements: A review of the history, pharmacology, prevalence, international regulations, and potential as doping agents. Drug Test. Anal. 2023, 15, 803–839. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse Effects of Nutraceuticals and Dietary Supplements. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.; Parr, M.K.; Koehler, K.; Mareck, U.; Schanzer, W.; Thevis, M. Nutritional supplements cross-contaminated and faked with doping substances. J. Mass Spectrom. 2008, 43, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.J.; Khan, I.; Bjornsson, E.; Seeff, L.B.; Serrano, J.; Hoofnagle, J.H. Liver injury from herbal and dietary supplements. Hepatology 2017, 65, 363–373. [Google Scholar] [CrossRef]
- Hellmuth, J.; Rabinovici, G.D.; Miller, B.L. Dietary Supplements for Brain Health-Reply. JAMA 2019, 321, 2467–2468. [Google Scholar] [CrossRef]
- Hellmuth, J.; Rabinovici, G.D.; Miller, B.L. The Rise of Pseudomedicine for Dementia and Brain Health. JAMA 2019, 321, 543–544. [Google Scholar] [CrossRef]
- Stanford. Stanford Center on Longevity Expert Consensus Statement on Brain Health. Available online: https://longevity.stanford.edu/expert-consensus-on-brain-health/ (accessed on 5 September 2024).
- Alzheimer_Association. Alzheimer’s Association Website Regarding Alternative Treatments. Available online: https://www.alz.org/alzheimers-dementia/treatments/alternative-treatments (accessed on 5 September 2024).
- ResearchAndMarkets.com. Global Brain Health Supplements Market Report. 2023. Available online: https://finance.yahoo.com/news/global-brain-health-supplements-market-111800756.html (accessed on 4 September 2024).
- Federal Trade Commission. FTC Health Products Compliance Guidance. 2022. Available online: https://www.ftc.gov/system/files/ftc_gov/pdf/Health-Products-Compliance-Guidance.pdf (accessed on 24 August 2024).
- In the Matter of POM Wonderful LLC and Roll Global LLC, as Successor in Interest to Roll International Corporation, Companies, and Stewart A. Resnick, Lynda Rae Resnick, and Matthew Tupper, Individually and as Officers of the Companies. Available online: https://www.ftc.gov/system/files/documents/cases/160614pomorder.pdf (accessed on 7 September 2024).
- Federal Trade Commission Complaint. Available online: https://www.ftc.gov/system/files/documents/cases/2014-03_pomwonderful_dccir_ftcoppbrieffinal.pdf (accessed on 7 September 2024).
- POM Wonderful LLC, et al. Case Timeline. Available online: https://www.ftc.gov/legal-library/browse/cases-proceedings/pom-wonderful-llc-et-al (accessed on 7 September 2024).
| Supplement | Research Reviewed by Study Design Authors (Year) | |||||||
|---|---|---|---|---|---|---|---|---|
| Double-Blind Placebo-Controlled | Randomized Trial | Cochrane Review | Cohort or Longitudinal | Case Study | Association, Cross-Sectional | In Vitro | Animal Models | |
| Apoaequorin | Moran et al. (2016) [11] | Detert et al. (2013) [12] | Ehlers et al. (2020) [11] | |||||
| Ginkgo biloba | Schneider et al. (2005) [13] Vellas et al. (2012) [14] Gavrilova et al. (2014) [15] | DeKosky et al. (2008) [16] Snitz et al. (2009) [17] Ihl et al. (2012) [18] Li et al. (2017) [19] | Birks et al. (2009) [20] | Amri et al. (1996, 2002) [21,22] Oyama et al. (1996) [23] Zhu et al. (1997) [24] Klein et al. (1997) [25] Bastianetto et al. (2000) [26] Zhou et al. (2000) [27] Song et al. (2000) [28] Yao et al. (2004) [29] Longpre et al. (2006) [30] Gargouri et al. (2018) [31] | Schindowski et al. (2001) [32] Drieu et al. (2000) [33] Wu et al. (2006) [34] Tunali-Akbay et al. (2007) [35] | |||
| Curcumin | Baum et al. (2008) [36] Ringman et al. (2012) [37] Rainey-Smith et al. (2016) [38] Small et al. (2018) [39] Cox et al. (2020) [40] | Ng et al. (2006) [41] | Hishikawa et al. (2012) [42] | Frautschy et al. (2001) [43] Lim et al. (2001) [44] Yang et al. (2005) [45] Begum et al. (2008) [46] | ||||
| Neuriva | Doma et al. (2023) [47] | |||||||
| B-complex | Rutjes et al. (2018) [48] | Riggs et al. (1996) [49] | ||||||
| Vitamin B6 | Kang et al. (2008) [50] | Malouf et al. (2003) [51] | Riggs et al. (1996) [49] | |||||
| Vitamin B9 | Kang et al. (2008) [50] | Malouf et al. (2008) [52] | Riggs et al. (1996) [49] Bailey et al. (2020) [53] | |||||
| Vitamin B12 | Kang et al. (2008) [50] | Eussen et al. (2006) [54] Malouf et al. (2003, 2008) [52,55] | Riggs et al. (1996) [49] Bailey et al. (2020) [53] | |||||
| Multivitamin | Sachs et al. (2023) [56] | |||||||
| Neuriva® Original | Neuriva® Plus | Neuriva® Ultra |
|---|---|---|
| NeuroFactor™ | NeuroFactor™ | NeuroFactor™ |
| Phosphatidylserine (PS) | Phosphatidylserine (PS) Vitamin B6 (pyridoxine) | Phosphatidylserine (PS) Vitamin B6 (pyridoxine) |
| Vitamin B9 (folate) | Vitamin B9 (folate) | |
| Vitamin B12 (cyanocobalamin) | Vitamin B12 (cyanocobalamin) Cognivive (alpina galanga extract) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frolov, A.; Wadood, A.; Kelley, B.J. Review of Supplements That Patients Commonly Report Using for Dementia. J. Clin. Med. 2024, 13, 7541. https://doi.org/10.3390/jcm13247541
Frolov A, Wadood A, Kelley BJ. Review of Supplements That Patients Commonly Report Using for Dementia. Journal of Clinical Medicine. 2024; 13(24):7541. https://doi.org/10.3390/jcm13247541
Chicago/Turabian StyleFrolov, Alexander, Audrey Wadood, and Brendan J. Kelley. 2024. "Review of Supplements That Patients Commonly Report Using for Dementia" Journal of Clinical Medicine 13, no. 24: 7541. https://doi.org/10.3390/jcm13247541
APA StyleFrolov, A., Wadood, A., & Kelley, B. J. (2024). Review of Supplements That Patients Commonly Report Using for Dementia. Journal of Clinical Medicine, 13(24), 7541. https://doi.org/10.3390/jcm13247541






