Multiple Sclerosis-like Lesions Induced by Radiation: A Case Report and Systematic Review of the Literature
Abstract
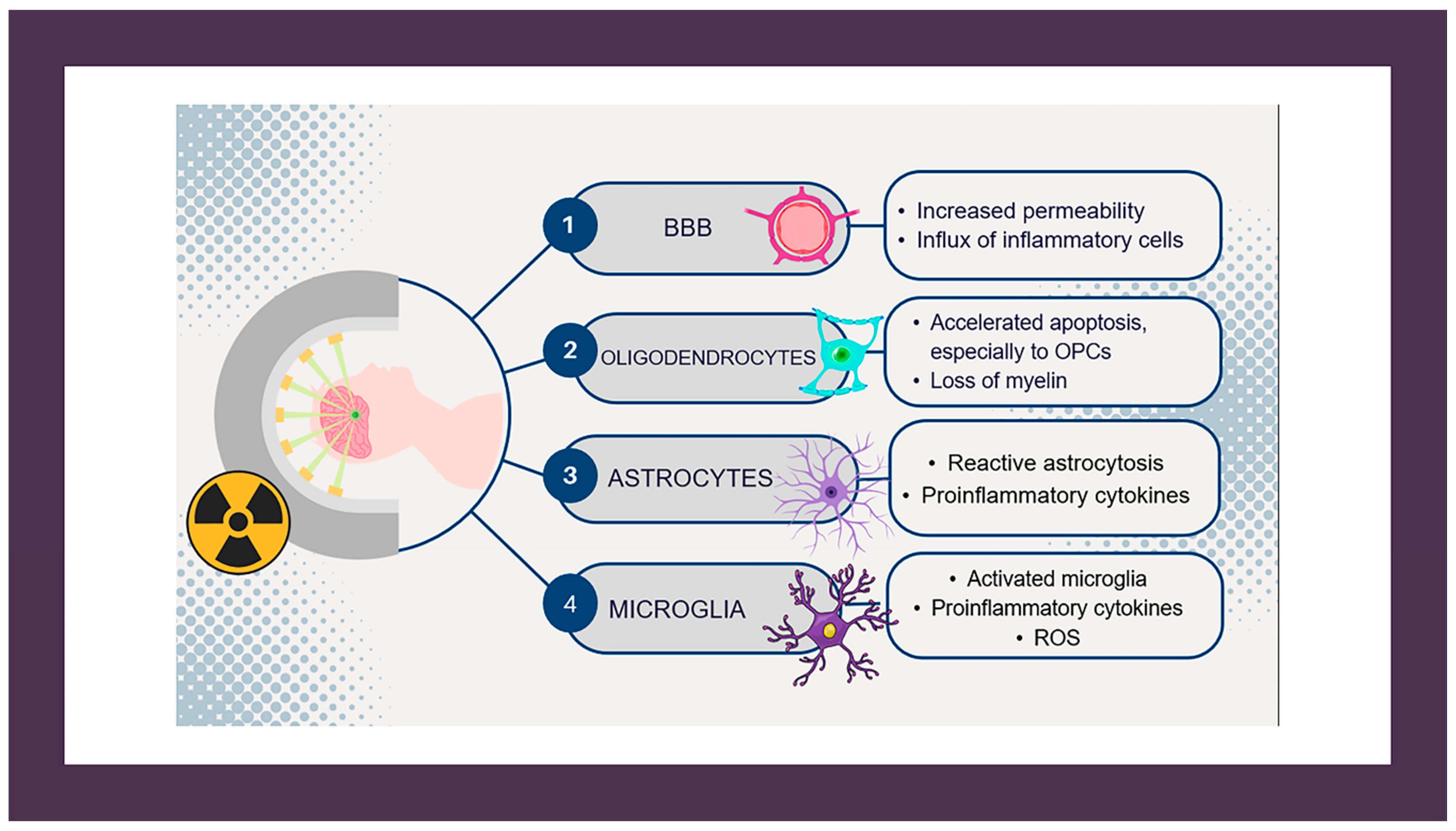
1. Introduction
2. Methods
2.1. Case Report
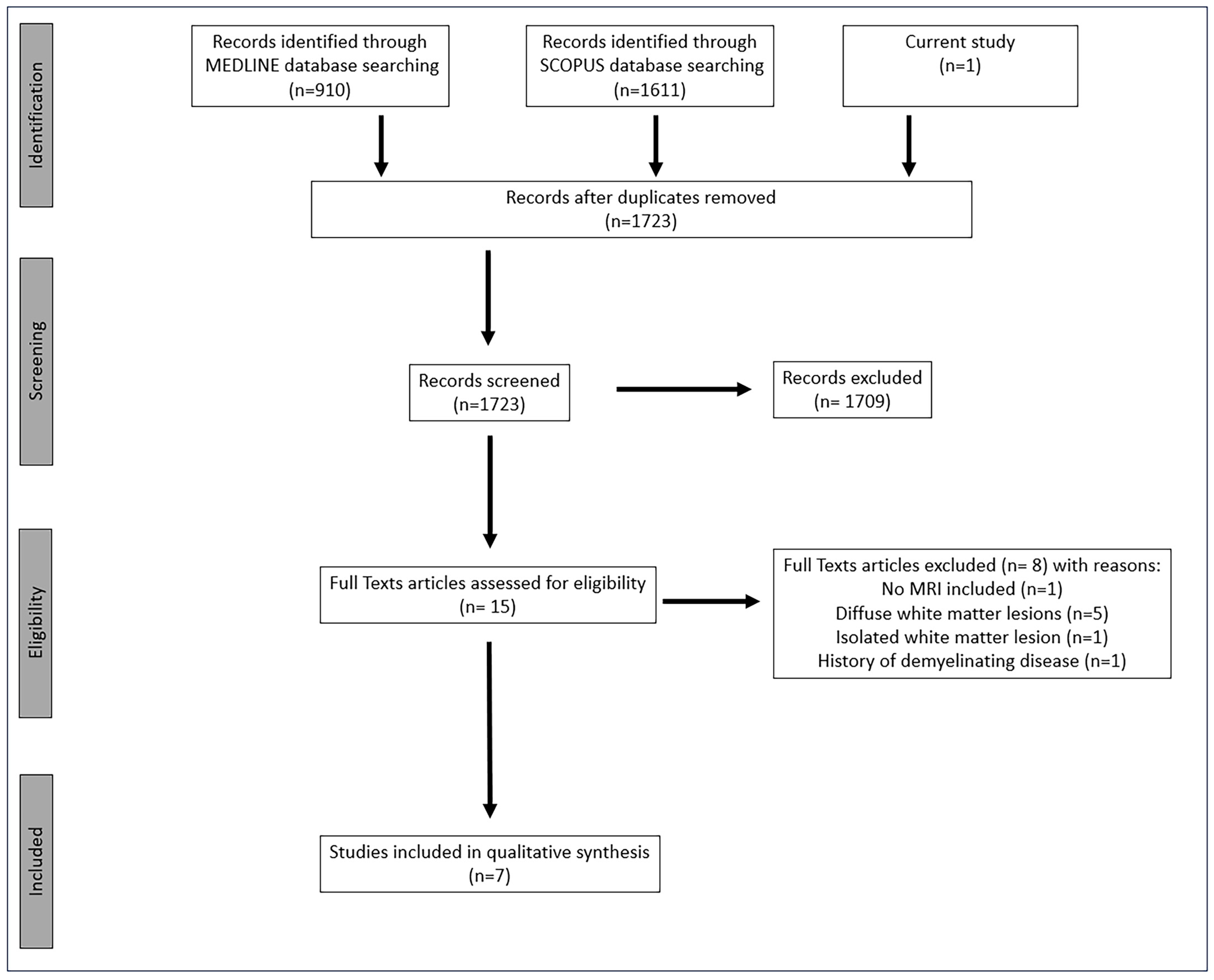
2.2. Systematic Review of the Literature
3. Results
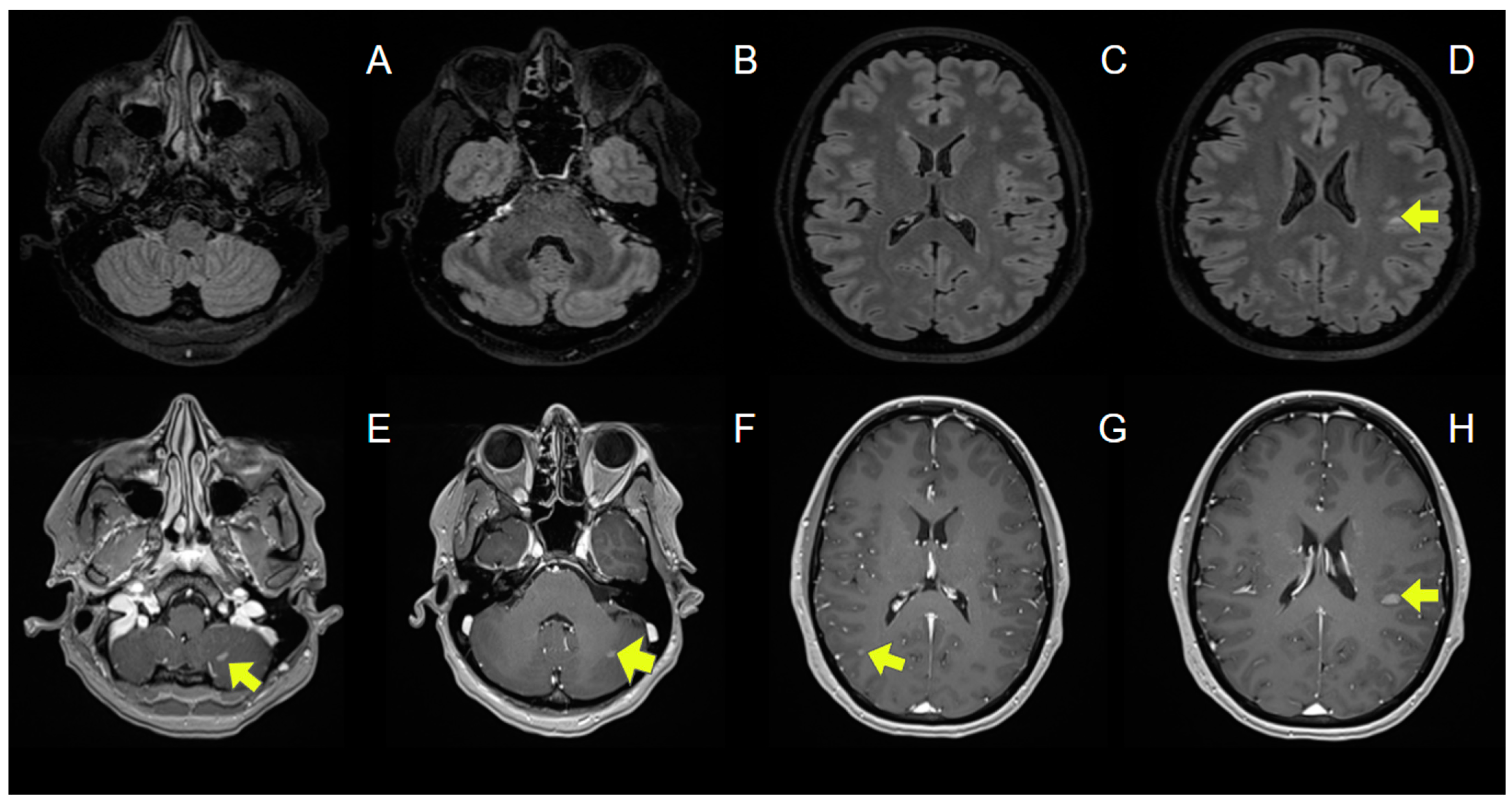
3.1. Case Report
3.2. Systematic Review of the Literature
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tofilon, P.J.; Fike, J.R. The radioresponse of the central nervous system: A dynamic process. Radiat. Res. 2000, 153, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Li, S.; Wang, J.; Qiu, W.; Gao, H. Bibliometric and visualization analysis of radiation brain injury from 2003 to 2023. Front. Neurol. 2023, 14, 1275836. [Google Scholar] [CrossRef] [PubMed]
- Greene-Schloesser, D.; Robbins, M.E.; Peiffer, A.M.; Shaw, E.G.; Wheeler, K.T.; Chan, M.D. Radiation-induced brain injury: A review. Front. Oncol. 2012, 2, 73. [Google Scholar] [CrossRef]
- Jacob, J.; Durand, T.; Feuvret, L.; Mazeron, J.-J.; Delattre, J.-Y.; Hoang-Xuan, K.; Psimaras, D.; Douzane, H.; Ribeiro, M.; Capelle, L.; et al. Cognitive impairment and morphological changes after radiation therapy in brain tumors: A review. Radiother. Oncol. 2018, 128, 221–228. [Google Scholar] [CrossRef]
- Van Der Maazen, R.W.; Kleiboer, B.J.; Verhagen, I.; Van Der Kogel, A. Repair Capacity of Adult Rat Glial Progenitor Cells Determined by an In Vitro Clonogenic Assay after In Vitro or In Vivo Fractionated Irradiation. Int. J. Radiat. Biol. 1993, 63, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Nakkazi, A.; Forster, D.; Whitfield, G.A.; Dyer, D.P.; Dickie, B.R. A systematic review of normal tissue neurovascular unit damage following brain irradiation—Factors affecting damage severity and timing of effects. Neuro-Oncol. Adv. 2024, 6, vdae098. [Google Scholar] [CrossRef]
- Katsura, M.; Sato, J.; Akahane, M.; Furuta, T.; Mori, H.; Abe, O. Recognizing Radiation-induced Changes in the Central Nervous System: Where to Look and What to Look For. RadioGraphics 2021, 41, 224–248. [Google Scholar] [CrossRef]
- Valk, P.E.; Dillon, W.P. Radiation injury of the brain. AJNR Am. J. Neuroradiol. 1991, 12, 45–62. [Google Scholar]
- Zhong, X.; Huang, B.; Feng, J.; Yang, W.; Liu, H. Delayed leukoencephalopathy of non-small cell lung cancer patients with brain metastases underwent whole brain radiation therapy. J. Neuro-Oncol. 2015, 125, 177–181. [Google Scholar] [CrossRef]
- Barkhof, F.; Koeller, K.K. Demyelinating Diseases of the CNS (Brain and Spine). In Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; IDKD Springer Series; Springer: Cham, Switzerland, 2020; pp. 165–176. [Google Scholar]
- Wattjes, M.P.; Ciccarelli, O.; Reich, D.S.; Banwell, B.; de Stefano, N.; Enzinger, C.; Fazekas, F.; Filippi, M.; Frederiksen, J.; Gasperini, C.; et al. 2021 MAGNIMS–CMSC–NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021, 20, 653–670. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0; The Cochrane Collaboration: Oxford, UK, 2013. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Giannopapas, V.; Kitsos, D.; Tsogka, A.; Tzartos, J.S.; Paraskevas, G.; Tsivgoulis, G.; Voumvourakis, K.; Giannopoulos, S.; Bakalidou, D. Sexual dysfunction therapeutic approaches in patients with multiple sclerosis: A systematic review. Neurol. Sci. 2023, 44, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Stefanou, M.-I.; Giannopapas, V.; Kitsos, D.K.; Chondrogianni, M.; Theodorou, A.; Kosmidou, M.; Vlotinou, P.; Bakirtzis, C.; Andreadou, E.; Tzartos, J.S.; et al. Prevalence and epidemiology of stroke in patients with multiple sclerosis: A systematic review and meta-analysis. J. Neurol. 2024, 271, 4075–4085. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Kihlstrom, L.; Guo, W.-Y.; Karlsson, B.; Lindquist, C.; Lindqvist, M. Magnetic resonance imaging of obliterated arteriovenous malformations up to 23 years after radiosurgery. J. Neurosurg. 1997, 86, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.M.; Ben-Porat, L.S.; Panageas, K.S.; Kim, A.K.; Correa, D.D.; Yahalom, J.; DeAngelis, L.M.; Abrey, L.E. Delayed neurotoxicity in primary central nervous system lymphoma. Arch. Neurol. 2005, 62, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Redjal, N.; Agarwalla, P.K.; Dietrich, J.; Dinevski, N.; Stemmer-Rachamimov, A.; Nahed, B.V.; Loeffler, J.S. Remote acute demyelination after focal proton radiation therapy for optic nerve meningioma. J. Clin. Neurosci. 2015, 22, 1367–1369. [Google Scholar] [CrossRef]
- Lai, R.; Abrey, L.E.; Rosenblum, M.K.; DeAngelis, L.M. Treatment-induced leukoencephalopathy in primary CNS lymphoma: A clinical and autopsy study. Neurology 2004, 62, 451–456. [Google Scholar] [CrossRef]
- Tsuruda, J.S.; Kortman, K.E.; Bradley, W.G.; Wheeler, D.; Van Dalsem, W.; Bradley, T.; Tsuruda, K.K.J.; Curnes, J.; Laster, D.; Ball; et al. Radiation effects on cerebral white matter: MR evaluation. Am. J. Roentgenol. 1987, 149, 165–171. [Google Scholar] [CrossRef]
- Zhong, G.; Zhang, J.; Liu, X.; Yang, S.B.; Gu, H.B. Astrocytoma with myelin oligodendrocyte glycoprotein antibody associated encephalomyelitis: A case report. Medicine 2022, 101, e31003. [Google Scholar] [CrossRef] [PubMed]
- Milic, M.; Rees, J.H. Acute demyelination following radiotherapy for glioma: A cautionary tale. Pract. Neurol. 2017, 17, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Schroth, G.; Zbaren, P.; Delavelle, J.; Greiner, R.; Vock, P.; Allal, A.; Rüfenacht, D.A.; Terrier, F. Long-term changes induced by high-dose irradiation of the head and neck region: Imaging findings. RadioGraphics 1997, 17, 5–26. [Google Scholar] [CrossRef]
- Borges, A.; Garcez, D.; Pedro, C.; Passos, J. Chemoradiation induced multiple sclerosis-like demyelination. eNeurologicalSci 2021, 22, 100315. [Google Scholar] [CrossRef]
- Esakia, T.; Antia, T.; Janelidze, M.; Mariamidze, A.; Okujava, M. Acute Disseminated Encephalomyelitis Following Chemoradiotherapy in an Adult Patient with Nasopharyngeal Cancer. Cureus 2021, 13, 14137. [Google Scholar] [CrossRef]
- Guillemin, F.; Biau, J.; Conde, S.; Clavelou, P.; Dupic, G. Multiple sclerosis as differential diagnosis of radionecrosis for post-irradiation brain lesions: A case report. Clin. Transl. Radiat. Oncol. 2020, 21, 44–48. [Google Scholar] [CrossRef]
- Kemp, S.; Allan, R.S.; Patanjali, N.; Barnett, M.; Jonker, B. Neurological deficit following stereotactic radiosurgery for trigeminal neuralgia. J. Clin. Neurosci. 2016, 34, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Toljan, K.; Kshettry, V.R.; Chao, S.T. Delayed Radiation-Therapy-Induced Cerebral Demyelination. Appl. Radiat. Oncol. 2021, 10, 34–38. [Google Scholar] [CrossRef]
- Wong, O.Y.; Tham, Y.-H.; Lai, M.; Davagnanam, I.; Bremner, F. Radiation-Induced Multiphasic Demyelination. J. Neuro-Ophthalmol. 2019, 39, 111–114. [Google Scholar] [CrossRef]
- Oh, J.; Vidal-Jordana, A.; Montalban, X. Multiple sclerosis: Clinical aspects. Curr. Opin. Neurol. 2018, 31, 752–759. [Google Scholar] [CrossRef]
- Andersson, M.; Alvarez-Cermeno, J.; Bernardi, G.; Cogato, I.; Fredman, P.; Frederiksen, J.; Fredrikson, S.; Gallo, P.; Grimaldi, L.M.; Gronning, M.; et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: A consensus report. J. Neurol. Neurosurg. Psychiatry 1994, 57, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Tanguturi, S.K.; Alexander, B.M. Neurologic Complications of Radiation Therapy. Neurol. Clin. 2018, 36, 599–625. [Google Scholar] [CrossRef] [PubMed]
- Newton, H.B. Neurological complications of chemotherapy to the central nervous system. Handb. Clin. Neurol. 2012, 105, 903–916. [Google Scholar] [PubMed]
- Ali Mohamed, D.; Semedo, A.; Adeyemi, B.; Hessissen, L.; El Kababri, M.; Allali, N.; Chat, L.; El Haddad, S. Reversible Encepahlopathy Induced by Ifosfamide with Brain Imaging. Glob. Pediatr. Health 2021, 8, 1–5. [Google Scholar] [CrossRef]
- Hemachudha, P.; Rattanawong, W.; Pongpitakmetha, T.; Phuenpathom, W. Fluorouracil-induced leukoencephalopathy mimicking neuroleptic malignant syndrome: A case report. J. Med. Case Rep. 2023, 17, 86. [Google Scholar] [CrossRef]

| Reference | Age/Sex | Initial Diagnosis | Medical History | Social/ Family History | Medi- Cation (Last 2 Years) | RT Type | Total RT Dose (Gy) | Total RT Duration (Days) | Onset of Symptoms After RT | Symptoms | CSF Studies | CSF Specific OCBs | Infection Panel | Auto- Immunity Panel | Regions Affected | Gd + | Treatment | Clinical Response | Recurrence (Time After Onset—Symptoms) | Imaging Follow-Up (Time After Onset) | Treatment After Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sterpi AE, et al. (2024) | 37/F | Breast Cancer | No | No | OLP, PLB, TRP, BVZ, LTR | IMRT | 30 | 10 | 2 months | Ataxia | Normal | No | Negative | Negative | Periventricular WM, corpus callosum, brainstem, cerebellum | - | IVMP with gradual tapering | Yes | No (6 months) | No new lesions (6 months) | N/A |
| Wong OY, et al. (2019) [31] | 31/F | Pituitary Macroadenoma | No | N/A | CBR | IMRT | 50.4 | 28 | 4 months | Decreased visual acuity, diplopia, facial numbness, tongue hemianesthesia | Normal | No | N/A | N/A | Subcortical WM, periventricular WM, brainstem, cerebellum | N/A | PSL | Yes | No (12 months) | No new lesions (12 months) | N/A |
| Borges A, et al. (2021) [26] | 28/M | Suprasellar Germinoma | No | No | CRB, ETP, IFS | WVRT | 24 | 15 | 4 months | Subjective cognitive impairment | Normal | Yes | Negative | Negative | Periventricular WM, subcortical WM, brainstem, cerebellum | + | No | Yes | No (12 months) | No new lesions (10 months) | N/A |
| Kemp S, et al. (2016) [29] | 65/F | Trigeminal Neuralgia | N/A | N/A | GBP | SRS | 90 | 1 | 3 months | Facial numbness, hemiparaesthesia, ataxia | Normal | Yes | N/A | N/A | TREZ | + | IVMP | Yes | Yes (10 months—left lower limb weakness, gait ataxia) | Periventricular WM, cervical spinal cord (10 months) | Fingolimod |
| Guillemin F, et al. (2020) [28] | 36/F | Pituitary Macroadenoma | No | N/A | N/A | SRS | 50.4 | 28 | 3 months | Dizziness, ataxia, nystagmus, left arm hypoesthesia | Normal | Yes | Negative | Negative | Brainstem, cerebellum, subcortical WM | + | IVMP | Yes | Yes (6 months—weakness Nystagmus) | Infratentorial lesions (6 months) | Beta-1A- Interferon |
| Esakia T, et al. (2021) [27] | 34/M | Nasophary-ngeal Carcinoma | No | N/A | CSP, 5FU | Focal RT | 66 | 33 | 2 months | Headache, ataxia, nystagmus | N/A | N/A | N/A | N/A | Cerebellum, periventricular WM | + | IVDEX with gradual tapering | Yes | No (24 months) | No new lesions (24 months) | N/A |
| Toljan K, et al. (2021) [30] | 41/M | Pituitary Macroade- noma | No | No | N/A | IMRT | 50.4 | 28 | 3 months | Diplopia, facial hemihypoesthesia, dysarthria, tongue numbness, ataxia, hemiparesis, single-sided hypoacusia | 13 cells/μL Protein: 50 mg/dL | No | Negative | Negative | Brainstem, cerebellum | + | IVMP followed by DEX | Yes | No (11 months) | No new lesions (11 months) | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sterpi, A.-E.; Triantafyllou, A.-S.; Tzanetakos, D.; Ampantzi, E.; Kitsos, D.; Theodorou, A.; Koutsouraki, E.; Maili, M.; Stefanou, M.I.; Moschovos, C.; et al. Multiple Sclerosis-like Lesions Induced by Radiation: A Case Report and Systematic Review of the Literature. J. Clin. Med. 2024, 13, 7554. https://doi.org/10.3390/jcm13247554
Sterpi A-E, Triantafyllou A-S, Tzanetakos D, Ampantzi E, Kitsos D, Theodorou A, Koutsouraki E, Maili M, Stefanou MI, Moschovos C, et al. Multiple Sclerosis-like Lesions Induced by Radiation: A Case Report and Systematic Review of the Literature. Journal of Clinical Medicine. 2024; 13(24):7554. https://doi.org/10.3390/jcm13247554
Chicago/Turabian StyleSterpi, Angeliki-Erato, Alexandros-Stavros Triantafyllou, Dimitrios Tzanetakos, Eleni Ampantzi, Dimitrios Kitsos, Aikaterini Theodorou, Effrosyni Koutsouraki, Maria Maili, Maria Ioanna Stefanou, Christos Moschovos, and et al. 2024. "Multiple Sclerosis-like Lesions Induced by Radiation: A Case Report and Systematic Review of the Literature" Journal of Clinical Medicine 13, no. 24: 7554. https://doi.org/10.3390/jcm13247554
APA StyleSterpi, A.-E., Triantafyllou, A.-S., Tzanetakos, D., Ampantzi, E., Kitsos, D., Theodorou, A., Koutsouraki, E., Maili, M., Stefanou, M. I., Moschovos, C., Palaiodimou, L., Tzartos, J., Giannopoulos, S., & Tsivgoulis, G. (2024). Multiple Sclerosis-like Lesions Induced by Radiation: A Case Report and Systematic Review of the Literature. Journal of Clinical Medicine, 13(24), 7554. https://doi.org/10.3390/jcm13247554








