Reassessing Pulmonary Hypertension Classification: Utilizing Criteria for Heart Failure with Preserved Ejection Fraction Instead of Pulmonary Arterial Wedge Pressure
Abstract
1. Introduction
2. Methods
2.1. Database
2.2. Patient Selection
2.3. Right Heart Catheterization
2.4. Classification Criteria
2.5. Statistical Analyses
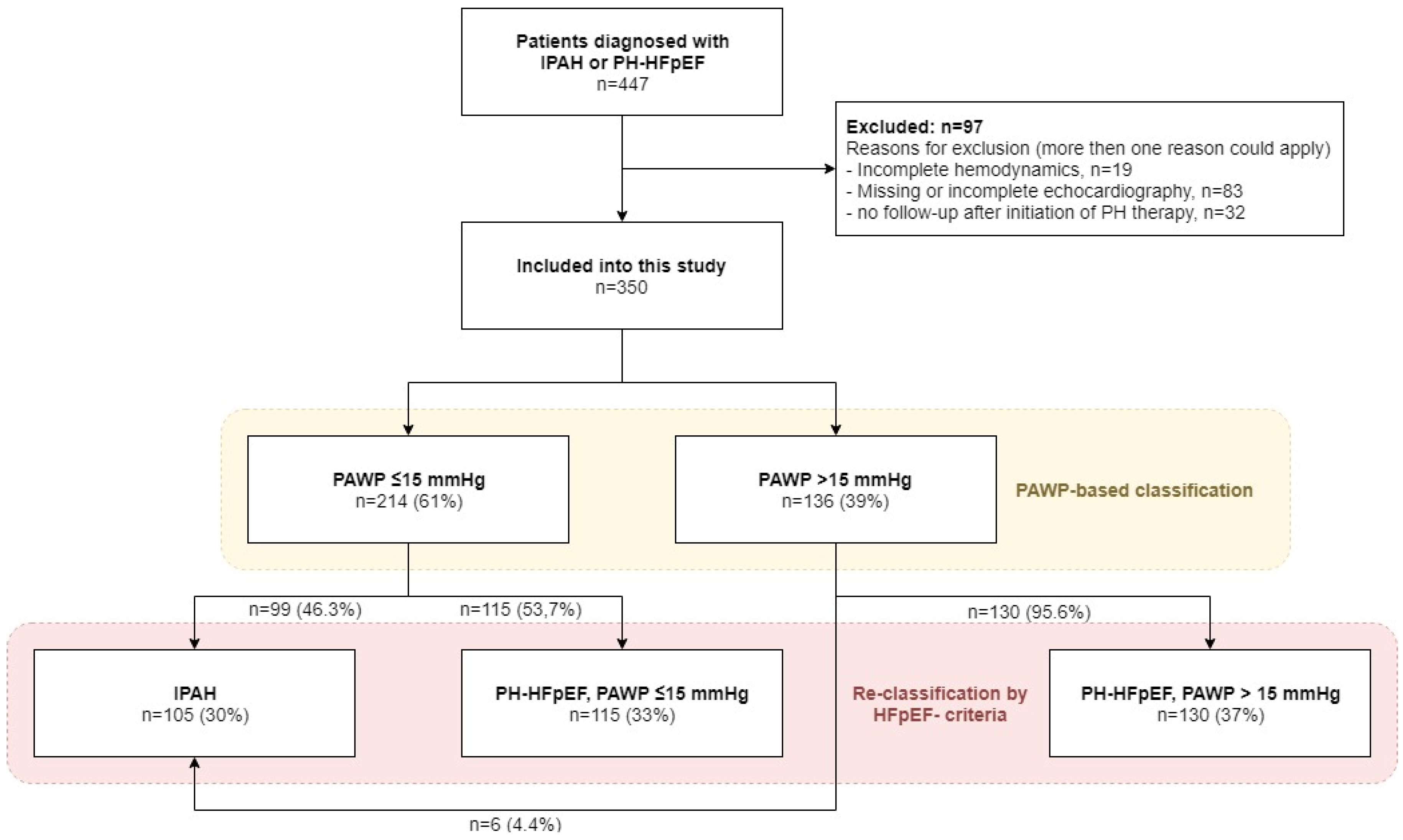
3. Results
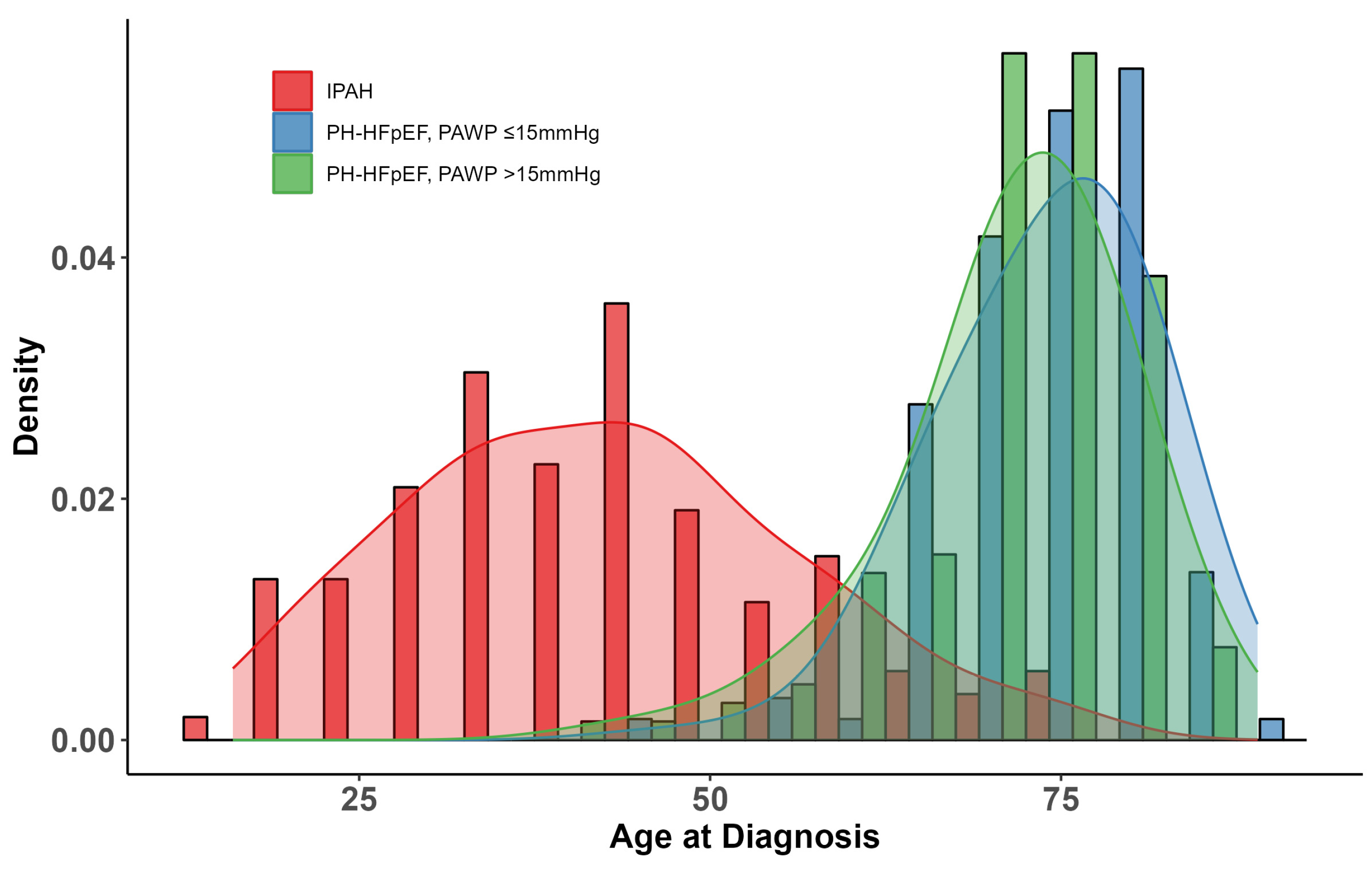
3.1. Patients
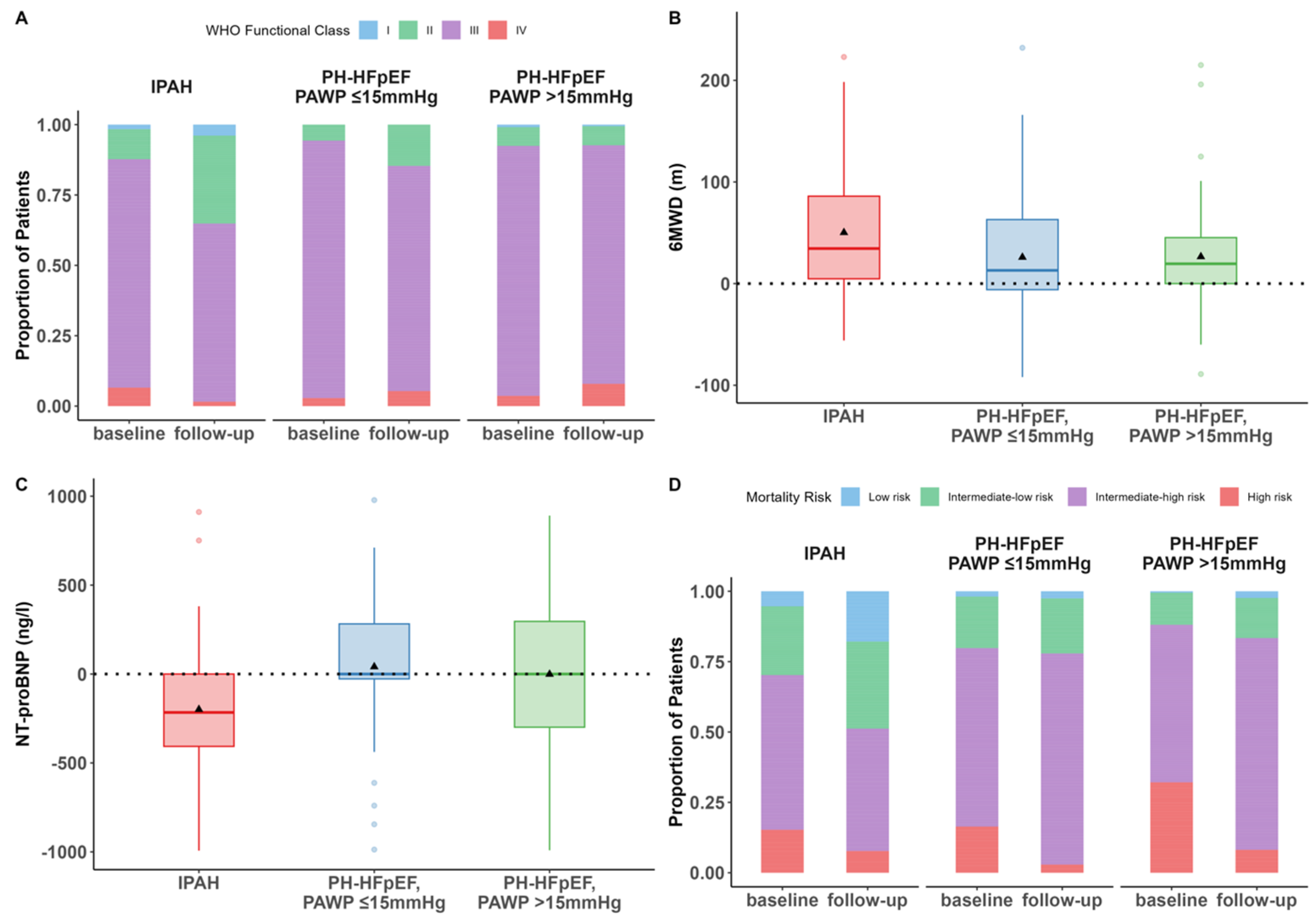
3.2. Changes in WHO-FC, 6MWD, NT-proBNP, and Risk After Initiation of PH Therapy
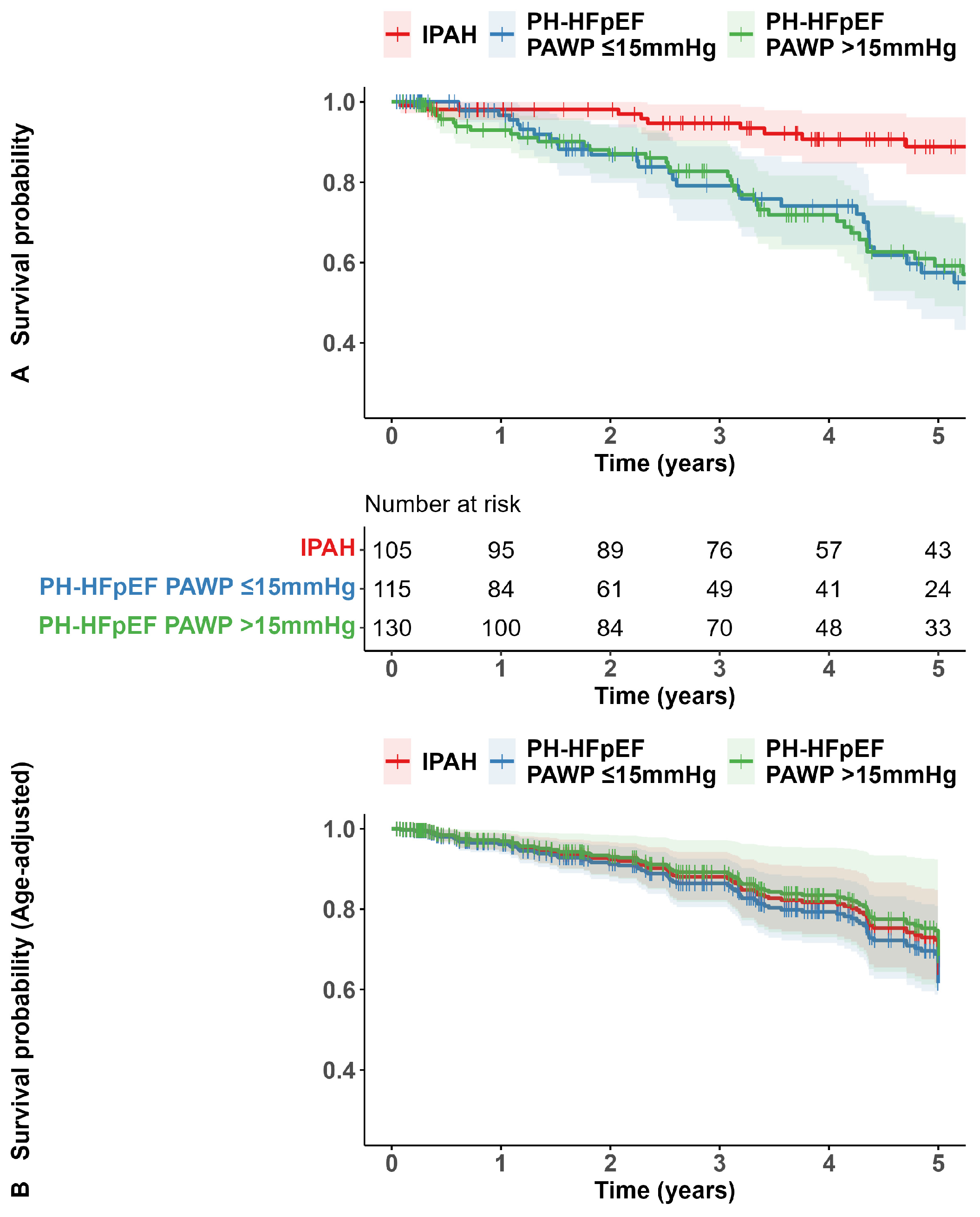
3.3. Survival
3.4. Secondary Analysis Based on PAWP ≤ 12 Versus >12 mmHg
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2022, 61, 2200879. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Sliwa-Hahnle, K.; Jing, Z.-C.; Gibbs, J.S.R. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Rich, S.; Dantzker, D.R.; Ayres, S.M.; Bergofsky, E.H.; Brundage, B.H.; Detre, K.M.; Fishman, A.P.; Goldring, R.M.; Groves, B.M.; Koerner, S.K. Primary pulmonary hypertension. A national prospective study. Ann. Intern. Med. 1987, 107, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Frost, A.E.; Badesch, D.B.; Barst, R.J.; Benza, R.L.; Elliott, C.G.; Farber, H.W.; Krichman, A.; Liou, T.G.; Raskob, G.E.; Wason, P.; et al. The changing picture of patients with pulmonary arterial hypertension in the United States: How REVEAL differs from historic and non-US Contemporary Registries. Chest 2011, 139, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Pausch, C.; Grunig, E.; Klose, H.; Staehler, G.; Huscher, D.; Pittrow, D.; Olsson, K.M.; Vizza, C.D.; Gall, H.; et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J. Heart Lung Transplant. 2020, 39, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Johnson, M.K.; Kiely, D.G.; Condliffe, R.; Elliot, C.A.; Gibbs, J.S.; Howard, L.S.; Pepke-Zaba, J.; Sheares, K.K.; Corris, P.A.; et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: Results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am. J. Respir. Crit. Care Med. 2012, 186, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, S.; Pausch, C.; Coghlan, J.G.; Huscher, D.; Pittrow, D.; Grünig, E.; Staehler, G.; Vizza, C.D.; Gall, H.; Distler, O.; et al. Risk stratification and response to therapy in patients with pulmonary arterial hypertension and comorbidities: A COMPERA analysis. J. Heart Lung Transplant. 2022, 42, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Rutten, F.H.; Lee, M.M.; Hawkins, N.M.; Petrie, M.C. Heart failure with preserved ejection fraction: Everything the clinician needs to know. Lancet 2024, 403, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Avian, A.; Olschewski, A.; Olschewski, H. Zero reference level for right heart catheterisation. Eur. Respir. J. 2013, 42, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Pausch, C.; Olsson, K.M.; Huscher, D.; Pittrow, D.; Grunig, E.; Staehler, G.; Vizza, C.D.; Gall, H.; Distler, O.; et al. COMPERA 2.0: A refined four-stratum risk assessment model for pulmonary arterial hypertension. Eur. Respir. J. 2022, 60, 2103311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hatano, S.; Strasser, T. Primary Pulmonary Hypertension: Report on a WHO Meeting, Geneva 15–17 October 1973; World Health Organization: Geneva, Switzerland, 1975; pp. 1–45.
- Kovacs, G.; Avian, A.; Pienn, M.; Naeije, R.; Olschewski, H. Reading pulmonary vascular pressure tracings. How to handle the problems of zero leveling and respiratory swings. Am. J. Respir. Crit. Care Med. 2014, 190, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschope, C.; Sanderson, J.E.; Rusconi, C.; Flachskampf, F.A.; Rademakers, F.E.; Marino, P.; Smiseth, O.A.; De Keulenaer, G.; Leite-Moreira, A.F.; et al. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur. Heart J. 2007, 28, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Guglin, M.; Munshi, K.; Volz, E.; Bennett, M.; Benjamin, T.A.; Bhatt, K.; Bhimaraj, A.; Fendler, T.; Fudim, M.; Guha, A.; et al. Misclassification of Pulmonary Hypertension with Current Hemodynamic Criteria. Chest 2024. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Bortman, G.; De Marco, T.; Huston, J.H.; Lang, I.M.; Rosenkranz, S.H.; Vachiéry, J.-L.; Tedford, R.J. Pulmonary hypertension associated with left heart disease. Eur. Respir. J. 2024, 64, 2401344. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.S.; Smiseth, O.A.; Dokainish, H.; Abudiab, M.M.; Schutt, R.C.; Kumar, A.; Sato, K.; Harb, S.; Gude, E.; Remme, E.W.; et al. Estimating Left Ventricular Filling Pressure by Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Nishimura, R.A.; Sorajja, P.; Lam, C.S.; Redfield, M.M. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ. Heart Fail. 2010, 3, 588–595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andersen, M.J.; Olson, T.P.; Melenovsky, V.; Kane, G.C.; Borlaug, B.A. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ. Heart Fail. 2015, 8, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Litwin, S.E.; Komtebedde, J.; Hu, M.; Burkhoff, D.; Hasenfuß, G.; Borlaug, B.A.; Solomon, S.D.; Zile, M.R.; Mohan, R.C.; Khawash, R.; et al. Exercise-Induced Left Atrial Hypertension in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2023, 11, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Kane, G.C.; Melenovsky, V.; Olson, T.P. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur. Heart J. 2016, 37, 3293–3302. [Google Scholar] [CrossRef] [PubMed]
- Gorter, T.M.; Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Borlaug, B.A. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur. Heart J. 2018, 39, 2825–2835. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; Borlaug, B.A.; Lewis, G.D.; Hastings, J.L.; Shafer, K.M.; Bhella, P.S.; Carrick-Ranson, G.; Levine, B.D. Hemodynamic responses to rapid saline loading: The impact of age, sex, and heart failure. Circulation 2013, 127, 55–62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stubbs, H.; MacLellan, A.; McGettrick, M.; Jani, B.; Brewis, M.; Church, C.; Johnson, M. Predicting Group II pulmonary hypertension: Diagnostic accuracy of the H2FPEF and OPTICS scores in Scotland. Open Heart 2022, 9, e002023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jansen, S.M.A.; Huis In ‘t Veld, A.E.; Jacobs, W.; Grotjohan, H.P.; Waskowsky, M.; van der Maten, J.; van der Weerdt, A.; Hoekstra, R.; Overbeek, M.J.; Mollema, S.A.; et al. Noninvasive Prediction of Elevated Wedge Pressure in Pulmonary Hypertension Patients Without Clear Signs of Left-Sided Heart Disease: External Validation of the OPTICS Risk Score. J. Am. Heart Assoc. 2020, 9, e015992. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Smiseth, O.A.; Morris, D.A.; Cardim, N.; Cikes, M.; Delgado, V.; Donal, E.; Flachskampf, F.A.; Galderisi, M.; Gerber, B.L.; Gimelli, A.; et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e34–e61. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, V.V.; Vachiery, J.L.; Oudiz, R.J.; Rosenkranz, S.; Galie, N.; Barbera, J.A.; Frost, A.E.; Ghofrani, H.A.; Peacock, A.J.; Simonneau, G.; et al. Patients with pulmonary arterial hypertension with and without cardiovascular risk factors: Results from the AMBITION trial. J. Heart Lung Transplant. 2019, 38, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, S.; Channick, R.; Chin, K.M.; Jenner, B.; Gaine, S.; Galie, N.; Ghofrani, H.A.; Hoeper, M.M.; McLaughlin, V.V.; Du Roure, C.; et al. The impact of comorbidities on selexipag treatment effect in patients with pulmonary arterial hypertension: Insights from the GRIPHON study. Eur. J. Heart Fail. 2021, 24, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Kianzad, A.; van Wezenbeek, J.; Celant, L.R.; Oosterveer, F.P.T.; Vonk Noordegraaf, A.; Meijboom, L.J.; de Man, F.S.; Bogaard, H.J.; Handoko, M.L. Idiopathic pulmonary arterial hypertension patients with a high H(2)FPEF-score: Insights from the Amsterdam UMC PAH-cohort. J. Heart Lung Transplant. 2022, 41, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Hoendermis, E.S.; Liu, L.C.; Hummel, Y.M.; van der Meer, P.; de Boer, R.A.; Berger, R.M.; van Veldhuisen, D.J.; Voors, A.A. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: A randomized controlled trial. Eur. Heart J. 2015, 36, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Henderson, A.D.; Lam, C.S.; Pitt, B.; Senni, M.; et al. Finerenone in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2024, 391, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Zile, M.R.; Kramer, C.M.; Baum, S.J.; Litwin, S.E.; Menon, V.; Ge, J.; Weerakkody, G.J.; Ou, Y.; Bunck, M.C.; et al. Tirzepatide for Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Barst, R.J.; Rubin, L.J.; Long, W.A.; McGoon, M.D.; Rich, S.; Badesch, D.B.; Groves, B.M.; Tapson, V.F.; Bourge, R.C.; Brundage, B.H.; et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N. Engl. J. Med. 1996, 334, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Barbera, J.A.; Frost, A.E.; Ghofrani, H.A.; Hoeper, M.M.; McLaughlin, V.V.; Peacock, A.J.; Simonneau, G.; Vachiery, J.L.; Grunig, E.; et al. Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N. Engl. J. Med. 2015, 373, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.M.; Sitbon, O.; Doelberg, M.; Feldman, J.; Gibbs, J.S.R.; Grunig, E.; Hoeper, M.M.; Martin, N.; Mathai, S.C.; McLaughlin, V.V.; et al. Three- Versus Two-Drug Therapy for Patients with Newly Diagnosed Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 2021, 78, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; McLaughlin, V.; Gibbs, J.S.R.; Gomberg-Maitland, M.; Hoeper, M.M.; Preston, I.R.; Souza, R.; Waxman, A.; Escribano Subias, P.; Feldman, J.; et al. Sotatercept for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021, 384, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Badesch, D.B.; Ghofrani, H.A.; Gibbs, J.S.R.; Gomberg-Maitland, M.; McLaughlin, V.V.; Preston, I.R.; Souza, R.; Waxman, A.B.; Grunig, E.; et al. Phase 3 Trial of Sotatercept for Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2023, 388, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
| Functional | Morphological | Biomarker (Sinus Rhythm) | Biomarker (Atrial Fibrillation) | |
|---|---|---|---|---|
| Major criteria (2 points) | Septal e′ < 7 cm/s or lateral e′ < 10 cm/s or average E/e′ ≥ 15 or TR velocity > 2.8 m/s | LAVI > 34 mL/m2 or LVMI ≥ 149/122 g/m2 (m/w) and RWT > 0.42 | NT-proBNP > 220 pg/mL or BNP > 80 pg/mL | NT-proBNP > 660 pg/mL or BNP > 240 pg/mL |
| Minor criteria (1 point) | Average E/e′ 9–14 or GLS < 16% | LAVI 29–34 mL/m2 or LVMI ≥ 115/95 g/m2 (m/w) or RWT > 0.42 or LV wall thickness ≥ 12 mm | NT-proBNP 125–220 pg/mL or BNP 35–80 pg/mL | NT-proBNP 365–660 pg/mL or BNP 105–240 pg/mL |
| IPAH (i) n = 105 | p-Value (i) vs. (ii) | PH-HFpEF with PAWP ≤ 15 mmHg (ii) n = 115 | p-Value (ii) vs. (iii) | PH-HFpEF with PAWP > 15 mmHg (iii) n = 130 | |
|---|---|---|---|---|---|
| Age, years | 41 [33, 51] | <0.001 | 76 [69, 80] | 0.022 | 73 [69, 77] |
| Female | 76 (72%) | 0.75 | 81 (70%) | 0.332 | 84 (65%) |
| BMI, kg/m2 | 26 [23, 30] | 0.07 | 28 [25, 34] | 0.108 | 30 [26, 35] |
| WHO-FC | 0.045 | 0.432 | |||
| I | 4 (4%) | 0 (0%) | 3 (2%) | ||
| II | 17 (16%) | 11 (10%) | 12 (9%) | ||
| III | 79 (75%) | 101 (88%) | 111 (85%) | ||
| IV | 5 (5%) | 3 (3%) | 4 (3%) | ||
| 6MWD, m | 407 [309, 475] | <0.001 | 278 [213, 372] | 0.328 | 278 [198, 359] |
| NT-proBNP, ng/L | 1049 [260, 2345] | 0.204 | 1142 [516, 2523] | 0.459 | 1694 [664, 3385] |
| Pulmonary function | |||||
| TLC, % pred | 101 [90, 110] | <0.001 | 91 [82, 102] | 0.591 | 89 [78, 98] |
| FVC, % pred | 94 [82, 104] | <0.001 | 87 [74, 101] | 0.784 | 79 [69, 91] |
| FEV1, % pred | 86 [77, 103] | 0.003 | 81 [67, 98] | 0.509 | 74 [62, 86] |
| FEV1/FVC (%) | 80 [76, 85] | 0.008 | 77 [71, 81] | 0.617 | 76 [71, 81] |
| DLCO, % pred | 69 [57, 81] | 0.384 | 63 [54, 80] | 0.066 | 59 [47, 77] |
| PaO2, mmHg | 73 [65, 80] | <0.001 | 67 [60–76] | 0.685 | 67 [58, 75] |
| PaCO2, mmHg | 33 [30, 35] | <0.001 | 38 [34, 41] | 0.324 | 39 [35, 42] |
| Smoking status | |||||
| Never | 41 (39%) | <0.001 | 73 (64%) | 0.09 | 60 (46%) |
| Former | 44 (42%) | 39 (34%) | 66 (51%) | ||
| Active | 20 (19%) | 3 (3%) | 4 (3%) | ||
| Pack years | 11 [4, 20] | 0.033 | 20 [9, 30] | 0.497 | 20 [7, 39] |
| Comorbidities | |||||
| BMI > 30 kg/m2 | 19 (18%) | 0.088 | 32 (28%) | 0.101 | 49 (38%) |
| Hypertension | 36 (34%) | <0.001 | 97 (84%) | 0.669 | 107 (82%) |
| CAD | 3 (3%) | 0.555 | 5 (4%) | 0.386 | 9 (7%) |
| Diabetes mell | 6 (6%) | <0.001 | 45 (39%) | 0.631 | 47 (36%) |
| Atrial fibrillation | 2 (2%) | <0.001 | 76 (66%) | 0.809 | 84 (64%) |
| Hemodynamics | |||||
| RAP, mmHg | 8 [4, 12] | 0.227 | 6 [4, 10] | <0.001 | 12 [9, 15] |
| mPAP, mmHg | 50 [44, 58] | <0.001 | 32 [27, 40] | <0.001 | 43 [36, 50] |
| PAWP, mmHg | 8 [6, 11] | <0.001 | 11 [9, 14] | <0.001 | 20 [17, 23] |
| CI, L/min/m2 | 1.8 [1.5, 2.5] | 0.44 | 2.4 [1.9, 2.7] | 0.688 | 2.1 [1.9, 2.6] |
| PVR, WU | 11.1 [8.5, 16.5] | <0.001 | 4.8 [3.6, 6.7] | 0.306 | 5.3 [3.8, 7.0] |
| SvO2, % | 61 [54, 67] | 0.002 | 68 [61, 71] | 0.001 | 63 [58, 68] |
| Risk (4-strata model) a | 0.096 | 0.019 | |||
| Low | 14 (14%) | 6 (5%) | 2 (2%) | ||
| Intermediate-low | 32 (31%) | 29 (25%) | 22 (17%) | ||
| Intermediate-high | 48 (46%) | 67 (58%) | 72 (57%) | ||
| High | 10 (10%) | 13 (11%) | 31 (24%) | ||
| Diuretics use at baseline | 63 (60%) | 0.002 | 91 (79%) | 0.341 | 109 (84%) |
| Initial PH medication b | |||||
| CCB | 14 (18%) | <0.001 | 0 (0%) | - | 0 (0%) |
| ERA | 70 (67%) | <0.001 | 5 (4%) | 0.37 | 3 (2%) |
| PDE5i | 91 (87%) | <0.001 | 114 (99%) | 0.287 | 130 (100%) |
| sGCs | 10 (10%) | 0.003 | 1 (1%) | 0 (0%) | 0.287 |
| PPA | 21 (20%) | <0.001 | 0 (0%) | 0 (0%) | - |
| Monotherapy | 29 (28%) | 110 (96%) | 127 (98%) | ||
| Dual combination therapy | 53 (51%) | 5 (4%) | 3 (2%) | ||
| Triple combination therapy | 23 (22%) | <0.001 | 0 (0%) | 0.37 | 0 (0%) |
| PH medication at 1 year | |||||
| CCB | 11 (11%) | <0.001 | 0 (0%) | - | 0 (0%) |
| ERA | 88 (84%) | <0.001 | 9 (8%) | 0.098 | 4 (3%) |
| PDE5i | 83 (79%) | 0.502 | 95 (83%) | 0.828 | 106 (82%) |
| sGCs | 16 (15%) | <0.001 | 1 (1%) | 0.469 * | 0 (0%) |
| PPA | 39 (37%) | <0.001 | 2 (2%) | 0.219 * | 0 (0%) |
| Monotherapy | 10 (10%) | 87 (76%) | 102 (79%) | ||
| Dual combination therapy | 53 (51%) | 7 (6%) | 4 (3%) | ||
| Triple combination therapy | 40 (38%) | <0.001 | 2 (2%) | 0.297 | 0 (0%) |
| IPAH (i) n = 105 | p-Value (i) vs. (ii) | PH-HFpEF with PAWP ≤ 15 mmHg (ii) n = 115 | p-Value (ii) vs. (iii) | PH-HFpEF with PAWP > 15 mmHg (iii) n = 130 | |
|---|---|---|---|---|---|
| TR velocity (m/s) | 3.7 [3.1, 4.2] | 0.007 | 3.4 [3.0, 3.8] | 0.044 | 3.5 [3.1, 3.9] |
| sPAP (mmHg) | 63 [48, 84] | 0.003 | 55 [44, 66] | 0.034 | 60 [49, 75] |
| RAA (cm2) | 22 [17, 28] | 0.359 | 24 [19, 30] | 0.003 | 27 [23, 32] |
| TAPSE (mm) | 20 [17, 24] | 0.272 | 18 [15, 23] | 0.117 | 18 [15, 20] |
| TAPSE/sPAP (mm/mmHg) | 0.31 [0.20, 0.49] | 0.676 | 0.34 [0.22, 0.50] | 0.004 | 0.30 [0.21, 0.4] |
| e′ lateral (cm/s) | 10 [7.3, 12.7] | 0.031 | 7.3 [5.8, 9.0] | 0.804 | 7.8 [5.7, 9.3] |
| e′ septal (cm/s) | 5.8 [4.2, 7.8] | 0.265 | 5.0 [4.0, 6.2] | 0.478 | 5.1 [3.9, 6.5] |
| E/e′ | 7.6 [5.8, 9.4] | <0.001 | 13.1 [10.3, 16.5] | 0.073 | 15.2 [10.9, 21.0] |
| LAVI (mL/m2) | 19 [12, 25] | <0.001 | 45 [34, 62] | 0.898 | 48 [37, 60] |
| LVMI (g/m2) | 72 [57, 81] | 0.017 | 101 [81, 116] | 0.017 | 108 [86, 129] |
| LV posterior wall thickness (mm) | 9 [8, 11] | <0.001 * | 11 [9, 12] | 0.038 * | 12 [10, 13] |
| Interventricular wall thickness (mm) | 10 [9, 12] | <0.001 * | 11 [10, 12] | 0.028 * | 12 [10, 13] |
| RV/LV diameter ratio | 1.13 [0.94, 1.45] | <0.001 | 0.93 [0.81, 1.12] | 0.443 | 0.98 [0.84, 1.13] |
| RWT | 0.43 [0.38, 0.54] | 0.914 | 0.45 [0.39, 0.54] | 0.297 | 0.46 [0.40, 0.54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.-H.; Fuge, J.; Kamp, J.C.; Harrigfeld, B.; Berliner, D.; Hoeper, M.M.; Olsson, K.M. Reassessing Pulmonary Hypertension Classification: Utilizing Criteria for Heart Failure with Preserved Ejection Fraction Instead of Pulmonary Arterial Wedge Pressure. J. Clin. Med. 2024, 13, 7582. https://doi.org/10.3390/jcm13247582
Park D-H, Fuge J, Kamp JC, Harrigfeld B, Berliner D, Hoeper MM, Olsson KM. Reassessing Pulmonary Hypertension Classification: Utilizing Criteria for Heart Failure with Preserved Ejection Fraction Instead of Pulmonary Arterial Wedge Pressure. Journal of Clinical Medicine. 2024; 13(24):7582. https://doi.org/10.3390/jcm13247582
Chicago/Turabian StylePark, Da-Hee, Jan Fuge, Jan Christopher Kamp, Britta Harrigfeld, Dominik Berliner, Marius M. Hoeper, and Karen M. Olsson. 2024. "Reassessing Pulmonary Hypertension Classification: Utilizing Criteria for Heart Failure with Preserved Ejection Fraction Instead of Pulmonary Arterial Wedge Pressure" Journal of Clinical Medicine 13, no. 24: 7582. https://doi.org/10.3390/jcm13247582
APA StylePark, D.-H., Fuge, J., Kamp, J. C., Harrigfeld, B., Berliner, D., Hoeper, M. M., & Olsson, K. M. (2024). Reassessing Pulmonary Hypertension Classification: Utilizing Criteria for Heart Failure with Preserved Ejection Fraction Instead of Pulmonary Arterial Wedge Pressure. Journal of Clinical Medicine, 13(24), 7582. https://doi.org/10.3390/jcm13247582






