Effect of Exposure to Blue Light from Electronic Devices and the Mediterranean Diet on Macular Pigment
Abstract
:1. Introduction
2. Materials and Method
2.1. Study Design and Population
2.2. Macular Pigment Optical Density (MPOD) Evaluation
2.3. Screen Time Usage During Daytime
2.4. Adherence to the Mediterranean Diet: The Mediterranean Diet Score (MES)
2.5. Statistical Analyses
3. Results
3.1. Light Sources of the Electronic Devices and Time of Exposure by Age Groups
3.2. Time of Exposure to LEDs and MPOD
3.3. Mediterranean Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| L | Lutein |
| Z | Zeaxanthin |
| BLH | blue light hazard |
| MP | macular pigment |
| MPOD | macular pigment optical density |
| LEDs | light emitting diodes |
| CVS | computer vision syndrome |
| AMD | age-related macular degeneration |
| YW | younger women group |
| OW | older women group |
| HFP | heterochromatic flicker photometer |
| MPS II | Macular Pigment Screener II |
| TTE | Total time of Exposure |
References
- Sliney, D.H.; Bergman, R.; O’Hagan, J. Photobiological Risk Classification of Lamps and Lamp Systems—History and Rationale. LEUKOS—J. Illum. Eng. Soc. N. Am. 2016, 12, 213–234. [Google Scholar] [CrossRef]
- Moyano, D.B.; Sola, Y.; González-Lezcano, R.A. Blue-Light Levels Emitted from Portable Electronic Devices Compared to Sunlight. Energies 2020, 13, 4276. [Google Scholar] [CrossRef]
- Jaadane, I.; Boulenguez, P.; Chahory, S.; Carré, S.; Savoldelli, M.; Jonet, L.; Behar-Cohen, F.; Martinsons, C.; Torriglia, A. Retinal Damage Induced by Commercial Light Emitting Diodes (LEDs). Free Radic. Biol. Med. 2015, 84, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S.A. Computer Vision Syndrome. Workplace Health Saf. 2017, 65, 328. [Google Scholar] [CrossRef]
- Wiggins, N.P.; Daum, K.M. Visual Discomfort and Astigmatic Refractive Errors in VDT Use. J. Am. Optom. Assoc. 1991, 62, 680–684. [Google Scholar] [PubMed]
- Gowrisankaran, S.; Sheedy, J.E. Computer Vision Syndrome: A Review. Work 2015, 52, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.A.; Rosenfield, M.; Portello, J.K. Blink Patterns: Reading from a Computer Screen versus Hard Copy. Optom. Vis. Sci. 2014, 91, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Ferguson, I.; Tsubota, K. Effects of Blue Light on the Circadian System and Eye Physiology. Mol. Visión 2016, 22, 61–72. [Google Scholar]
- Guidolin, C.; Aerts, S.; Agbeshie, G.K.; Akuffo, K.O.; Aydin, S.N.; Baeza-Moyano, D.; Bolte, J.; Broszio, K.; Cantarero-García, G.; Didikoglu, A.; et al. Protocol for a prospective, multicentre, cross-sectional cohort study to assess personal light exposure. BMC Public Health 2024, 24, 3285. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Dhakal, R.; Verkicharla, P.K. Short-Term Exposure to Blue Light Shows an Inhibitory Effect on Axial Elongation in Human Eyes Independent Of Defocus. Investig. Ophthalmol. Vis. Sci. 2021, 62, 22. [Google Scholar] [CrossRef]
- Johannessen, S.; Høgsbro, M. Eclipse Retinopathy. Ugeskr. Laeger 2014, 176, V05140268. [Google Scholar]
- Alaimo, A.; Liñares, G.G.; Bujjamer, J.M.; Gorojod, R.M.; Alcon, S.P.; Martínez, J.H.; Baldessari, A.; Grecco, H.E.; Kotler, M.L. Toxicity of Blue Led Light and A2E Is Associated to Mitochondrial Dynamics Impairment in ARPE-19 Cells: Implications for Age-Related Macular Degeneration. Arch. Toxicol. 2019, 93, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, R.; Calvo, C.M.; Conrady, C.D.; Bernstein, P.S. What Do We Know about the Macular Pigment in AMD: The Past, the Present, and the Future. Eye 2018, 32, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Thurnham, D.I. Macular Zeaxanthins and Lutein—A Review of Dietary Sources and Bioavailability and Some Relationships with Macular Pigment Optical Density and Age-Related Macular Disease. Nutr. Res. Rev. 2007, 20, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.M.; Tharmarajah, S.; Jia, Y.; Semba, R.D.; Schaumberg, D.A.; Robinson, K.A. The Effect of Lutein/Zeaxanthin Intake on Human Macular Pigment Optical Density: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 2244–2254. [Google Scholar] [CrossRef]
- Widomska, J.; Paul Sangiovanni, J.; Subczynski, W.K. Why Is Zeaxanthin the Most Concentrated Xanthophyll in the Central Fovea? Nutrients 2020, 12, 1333. [Google Scholar] [CrossRef]
- Wilson, M.R.; Sandberg, K.A.; Foutch, B.K. Macular Pigment Optical Density and Visual Quality of Life. J. Optom. 2021, 14, 92–99. [Google Scholar] [CrossRef]
- Dietzel, M.; Zeimer, M.; Heimes, B.; Claes, B.; Pauleikhoff, D.; Hense, H.W. Determinants of Macular Pigment Optical Density and Its Relation to Age-Related Maculopathy: Results from the Muenster Aging and Retina Study (MARS). Investig. Ophthalmol. Vis. Sci. 2011, 52, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.D.; Agrón, E.; Mares, J.; Clemons, T.E.; van Asten, F.; Swaroop, A.; Chew, E.Y. Adherence to the Mediterranean Diet and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2020, 127, 1515–1528. [Google Scholar] [CrossRef]
- Lima, V.C.; Rosen, R.B.; Prata, T.S.; Dorairaj, S.; Spielberg, L.; Maia, M.; Sallum, J.M. Association of Age and Macular Pigment Optical Density Using Dual-Wavelength Autofluorescence Imaging. Clin. Ophthalmol. 2013, 7, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.; O’Donovan, O.; Kavanagh, H.; Stack, J.; Harrison, M.; Muldoon, A.; Mellerio, J.; Beatty, S. Macular Pigment and Percentage of Body Fat. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3940–3950. [Google Scholar] [CrossRef]
- Wolf-Schnurrbusch, U.E.K.; Röösli, N.; Weyermann, E.; Heldner, M.R.; Höhne, K.; Wolf, S. Ethnic Differences in Macular Pigment Density and Distribution. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3783–3787. [Google Scholar] [CrossRef]
- Isaac, D.L.C.; de Ávila, M.P.; Cialdini, A.P. Comparison of the Original Amsler Grid with the Preferential Hyperacuity Perimeter for Detecting Choroidal Neovascularization in Age-Related Macular Degeneration. Arq. De Oftalmol. 2007, 70, 771–776. [Google Scholar] [CrossRef] [PubMed]
- WHO. Consultation on Obesity. In Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO technical report series; 894; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Beatty, S.; Koh, H.H.; Carden, D.; Murray, I.J. Macular pigment optical density measurement: A novel compact instrument. Ophthalmic Physiol Opt. 2000, 20, 105–111. [Google Scholar] [CrossRef]
- Abell, R.G.; Hewitt, A.W.; Andric, M.; Allen, P.L.; Verma, N. The Use of Heterochromatic Flicker Photometry to Determine Macular Pigment Optical Density in a Healthy Australian Population. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 417–421. [Google Scholar] [CrossRef]
- Van Der Veen, R.L.P.; Berendschot, T.T.J.M.; Hendrikse, F.; Carden, D.; Makridaki, M.; Murray, I.J. A New Desktop Instrument for Measuring Macular Pigment Optical Density Based on a Novel Technique for Setting Flicker Thresholds. Ophthalmic Physiol. 2009, 29, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-Item Mediterranean Diet Assessment Tool and Obesity Indexes among High-Risk Subjects: The PREDIMED Trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla-Alonso, B.; Beltrán-de-Miguel, B.; Estévez-Santiago, R.; Cuadrado-Vives, C. Markers of Lutein and Zeaxanthin Status in Two Age Groups of Men and Women: Dietary Intake, Serum Concentrations, Lipid Profile and Macular Pigment Optical Density. Nutr. J. 2014, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, J.; Smith, V.C.; Lutze, M. Aging of the Human Lens. Appl. Opt. 1987, 26, 1437. [Google Scholar] [CrossRef]
- Ozawa, Y.; Shigeno, Y.; Nagai, N.; Suzuki, M.; Kurihara, T.; Minami, S.; Hirano, E.; Shinoda, H.; Kobayashi, S.; Tsubota, K. Absolute and Estimated Values of Macular Pigment Optical Density in Young and Aged Asian Participants with or without Age-Related Macular Degeneration. BMC Ophthalmol. 2017, 17, 161. [Google Scholar] [CrossRef]
- Scott, T.M.; Rasmussen, H.M.; Chen, O.; Johnson, E.J. Avocado Consumption Increases Macular Pigment Density in Older Adults: A Randomized, Controlled Trial. Nutrients 2017, 9, 919. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.M.; Kenny, R.; O’Regan, C.; Cronin, H.; Loughman, J.; Connolly, E.E.; Kearney, P.; Loane, E.; Beatty, S. Macular Pigment Optical Density in an Ageing Irish Population: The Irish Longitudinal Study on Ageing. Ophthalmic Res. 2010, 44, 131–139. [Google Scholar] [CrossRef]
- Hong, I.H.; Jung, W.H.; Lee, J.H.; Chang, I.B. Macular Pigment Optical Density in the Korean Population: A Cross Sectional Study. J. Korean Med. Sci. 2020, 35, e30. [Google Scholar] [CrossRef]
- Pipis, A.; Touliou, E.; Augustin, A.J. Macular Pigment Optical Density in a Central European Population. Ophthalmic Surg. Lasers Imaging Retin. 2013, 44, 260–267. [Google Scholar] [CrossRef]
- Obana, A.; Gohto, Y.; Asaoka, R.; Gellermann, W.; Bernstein, P.S. Lutein and Zeaxanthin Distribution in the Healthy Macula and Its Association with Various Demographic Factors Examined in Pseudophakic Eyes. Antioxidants 2021, 10, 1857. [Google Scholar] [CrossRef] [PubMed]
- Berendschot, T.T.J.M.; Van Norren, D. On the Age Dependency of the Macular Pigment Optical Density. Exp. Eye Res. 2005, 81, 602–609. [Google Scholar] [CrossRef]
- Hammond, J.; Ciulla, T.A.; Snodderly, D.M. Macular Pigment Density Is Reduced in Obese Subjects. Investig. Ophthalmol. Vis. Sci. 2002, 43, 47–50. [Google Scholar]
- Johnson, E.J.; Hammond, B.R.; Yeum, K.J.; Qin, J.; Wang, X.D.; Castaneda, C.; Snodderly, D.M.; Russell, R.M. Relation among Serum and Tissue Concentrations of Lutein and Zeaxanthin and Macular Pigment Density. Am. J. Clin. Nutr. 2000, 71, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Johnson, E.J.; Shang, F.; Lim, A.; Zhou, H.; Cui, L.; Xu, J.; Snellingen, T.; Liu, X.; Wang, N.; et al. Measurement of Macular Pigment Optical Density in a Healthy Chinese Population Sample. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2106–2111. [Google Scholar] [CrossRef]
- Estévez-Santiago, R.; Olmedilla-Alonso, B.; Beltrán-de-Miguel, B.; Cuadrado-Vives, C. Lutein and Zeaxanthin Supplied by Red/Orange Foods and Fruits Are More Closely Associated with Macular Pigment Optical Density than Those from Green Vegetables in Spanish Subjects. Nutr. Res. 2016, 36, 1210–1221. [Google Scholar] [CrossRef]
- Theruveethi, N.; Bui, B.V.; Joshi, M.B.; Valiathan, M.; Ganeshrao, S.B.; Gopalakrishnan, S.; Kabekkodu, S.P.; Bhat, S.S.; Surendran, S. Blue Light-Induced Retinal Neuronal Injury and Amelioration by Commercially Available Blue Light-Blocking Lenses. Life 2022, 12, 243. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Yun, J.; Yoon, Y.D.; Park, S.I.; Seo, Y.J.; Park, W.S.; Chu, H.Y.; Park, K.H.; Lee, M.Y.; Lee, C.W.; et al. Blue Light Effect on Retinal Pigment Epithelial Cells by Display Devices. Integr. Biol. 2017, 9, 436–443. [Google Scholar] [CrossRef]

| Factors (Mean ± sd) | Absolute MPOD (Mean ± sd) | Estimated MPOD (Mean ± sd) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Sample (n = 82) | Younger Women (YW) (n = 47) | Older Women (OW) (n = 35) | Total Sample | YW | OW | Total Sample | YW | OW | |
| Age (y) | 36.8 ± 16.2 | 23.6 ± 2.2 a | 54.5 ± 7.5 b | 0.42 ± 0.16 | 0.38 ± 0.15 a | 0.47 ± 0.16 b | 0.43 ± 0.16 | 0.39 ± 0.15 a | 0.47 ± 0.15 b |
| Range (min–max) | 20–70 | 20–31 | 42–70 | (0.12–0.89) | (0.12–0.70) | (0.17–0.89) | (0.10–0.86) | (0.12–0.86) | (0.10–0.72) |
| BMI (kg/m2) | 22.9 ± 3.9 | 21.9 ± 3.5 a | 24.1 ± 4.2 b | ||||||
| Range (min–max) | 16.3–35.4 | 16.7–34.3 | 16.3–35.4 | ||||||
| BMI distribution (n (%)) | |||||||||
| Underweight | 7 (8.5%) | 4 (8.7%) | 3 (8.8%) | 0.39 ± 0.16 | 0.31 ± 0.19 | 0.48 ± 0.02 | 0.43 ± 0.14 | 0.37 ± 0.16 | 0.51 ± 0.06 |
| Normal weight | 54 (65.9%) | 36 (78.3%) | 18 (52.9%) | 0.40 ± 0.17 | 0.38 ± 0.15 | 0.46 ± 0.20 | 0.43 ± 0.16 | 0.40 ± 0.16 | 0.49 ± 0.16 |
| Weight excess (overweight + obesity) | 19 (23.2%) | 6 (13.0%) | 13 (23.8%) | 0.48 ± 0.11 | 0.46 ± 0.09 | 0.49 ± 0.11 | 0.43 ± 0.15 | 0.41 ± 0.16 | 0.43 ± 0.16 |
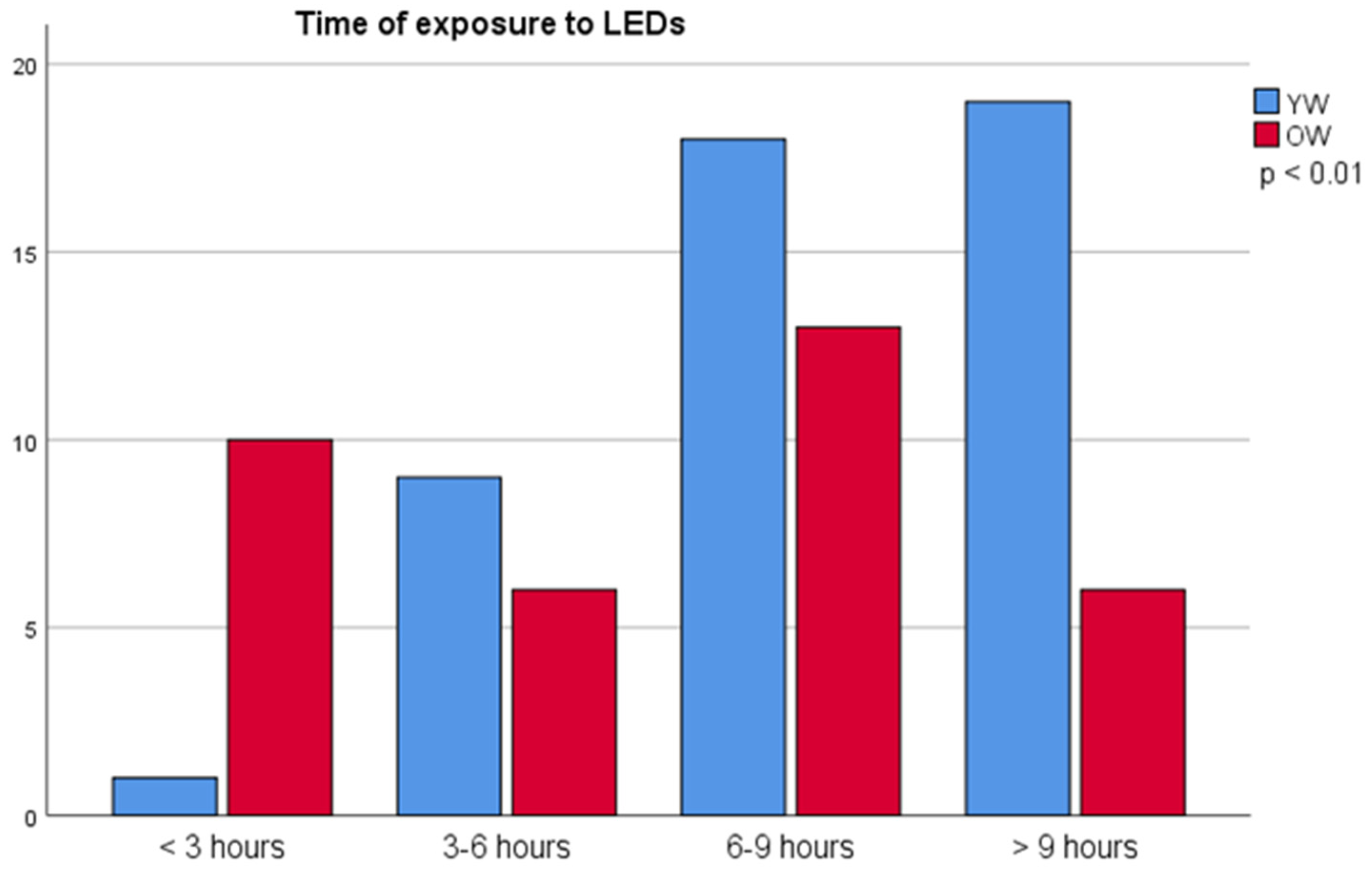
| Time of exposure hour/day (h/d) to Leds | 8.0 ± 4.0 | 9.3 ± 3.7 a | 6.3 ± 3.6 b | ||||||
| <6 h/d | 26 (31.7%) | 10 (21.3%) | 16 (45.7%) | 0.45 ± 0.17 | 0.46 ± 0.15 | 0.45 ± 0.18 | 0.43 ± 0.17 | 0.44 ± 0.15 | 0.43 ± 0.18 |
| >6 h/d | 56 (68.3%) | 37 (78.7%) | 19 (54.3%) | 0.41 ± 0.15 | 0.37 ± 0.14 a | 0.50 ± 0.14 b | 0.42 ± 0.15 | 0.38 ± 0.16 a | 0.50 ± 0.12 b |
| Mediterranean diet score (14 points) | 8.4 ± 2.4 | 7.7 ± 2.1 a | 9.2 ± 2.5 b | ||||||
| Range (min–max) | 1–13 | 2–11 | 1–13 | ||||||
| weak adherence ≤ 5 n, (%) | 9 (11.0%) | 7 (14.9%) | 2 (5.7%) | 0.45 ± 0.181.2 | 0.43 ± 0.18 | 0.53 ± 0.24 | 0.46 ± 0.15 | 0.42 ± 0.15 | 0.57 ± 0.06 |
| moderate to fair adherence 6–9, n (%) | 47 (57.3%) | 31 (66.0%) | 16 (45.7%) | 0.38 ± 0.151 | 0.36 ± 0.15 | 0.43 ± 0.15 | 0.39 ± 0.16 | 0.37 ± 0.16 | 0.42 ± 0.18 |
| good or very good adherence ≥10, n (%) | 26 (31.7%) | 9 (19.1%) | 17 (48.6%) | 0.49 ± 0.142 | 0.44 ± 0.10 | 0.48 ± 0.16 | 0.47 ± 0.13 | 0.44 ± 0.14 | 0.49 ± 0.12 |
| Dietary background (rations per day or week) | |||||||||
| Olive oil (≥4 d) | |||||||||
| YES | 58 (70.7%) | 30 (63.8%) | 28 (80.0%) | 0.42 ± 0.16 | 0.37 ± 0.14 | 0.48 ± 0.16 | 0.43 ± 0.15 | 0.37 ± 0.15 | 0.48 ± 0.15 |
| NO | 24 (29.3%) | 17 (36.2%) | 7 (20.0%) | 0.42 ± 0.15 | 0.40 ± 0.15 | 0.47 ± 0.16 | 0.42 ± 0.16 | 0.44 ± 0.15 | 0.43 ± 0.16 |
| Fruit (≥3 d) | |||||||||
| YES | 31 (37.8%) | 14 (29.8%) | 18 (51.4%) | 0.46 ± 0.141 | 0.44 ± 0.121 | 0.48 ± 0.15 | 0.44 ± 0.13 | 0.43 ± 0.11 | 0.45 ± 0.14 |
| NO | 51 (62.2%) | 33 (70.2%) | 17 (48.6%) | 0.38 ± 0.172 | 0.34 ± 0.182 a | 0.46 ± 0.14 b | 0.41 ± 0.17 | 0.38 ± 0.17 a | 0.48 ± 0.16 b |
| Vegetable (≥2 d) | |||||||||
| YES | 46 (56.1%) | 23 (48.9%) | 23 (65.7%) | 0.44 ± 0.15 | 0.39 ± 0.12 | 0.48 ± 0.16 | 0.43 ± 0.13 | 0.40 ± 0.13 | 0.47 ± 0.13 |
| NO | 36 (43.9%) | 24 (51.1%) | 12 (34.3%) | 0.40 ± 0.17 | 0.37 ± 0.17 | 0.46 ± 0.16 | 0.41 ± 0.18 | 0.39 ± 0.18 | 0.46 ± 0.18 |
| Fruit (≥3 d) and vegetable (≥2 d) | |||||||||
| YES | 23 (28%) | 8 (17%) a | 15 (42.9%) b | 0.46 ± 0.15 | 0.45 ± 0.13 | 0.47 ± 0.17 | 0.45 ± 0.17 | 0.46 ± 0.15 | 0.45 ± 0.18 |
| One of two things | 31 (37.8%) | 21 (44.7%) a | 10 (28.6%) b | 0.38 ± 0.17 | 0.33 ± 0.18 a | 0.48 ± 0.10 b | 0.42 ± 0.17 | 0.37 ± 0.15 a | 0.53 ± 0.16 b |
| NO | 28 (34.1%) | 18 (38.3%) a | 10 (28.6%) b | 0.41 ± 0.15 | 0.39 ± 0.16 | 0.44 ± 0.13 | 0.42 ± 0.14 | 0.41 ± 0.15 | 0.45 ± 0.15 |
| Fish consumption (≥3 w) | |||||||||
| YES | 28 (34.1%) | 13 (27.7%) | 15 (42.9%) | 0.44 ± 0.15 | 0.40 ± 0.12 | 0.46 ± 0.14 | 0.46 ± 0.16 | 0.40 ± 0.13 | 0.45 ± 0.13 |
| NO | 54 (65.9%) | 34 (72.3%) | 20 (57.1%) | 0.42 ± 0.16 | 0.37 ± 0.17 | 0.49 ± 0.18 | 0.41 ± 0.15 | 0.39 ± 0.18 | 0.48 ± 0.16 |
| Device | YW | OW |
|---|---|---|
| Mobile phone | 4.60 ± 2.28 | 1.57 ± 1.17 ** |
| Laptop | 2.73 ± 2.32 | 1.08 ± 1.60 ** |
| TV | 1.95 ± 1.24 | 2.20 ± 1.44 |
| Tablet | 0.76 ± 1.21 | 0.31 ± 0.69 |
| PC | 0.59 ± 1.67 | 3.00 ± 3.23 * |
| Total LEDs | 9.31 ± 3.74 | 6.33 ± 3.64 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Romera, M.-C.; Ponce-García, V.; Torres-Parejo, Ú.; López-Muñoz, A. Effect of Exposure to Blue Light from Electronic Devices and the Mediterranean Diet on Macular Pigment. J. Clin. Med. 2024, 13, 7688. https://doi.org/10.3390/jcm13247688
García-Romera M-C, Ponce-García V, Torres-Parejo Ú, López-Muñoz A. Effect of Exposure to Blue Light from Electronic Devices and the Mediterranean Diet on Macular Pigment. Journal of Clinical Medicine. 2024; 13(24):7688. https://doi.org/10.3390/jcm13247688
Chicago/Turabian StyleGarcía-Romera, Marta-C., Víctor Ponce-García, Úrsula Torres-Parejo, and Alfredo López-Muñoz. 2024. "Effect of Exposure to Blue Light from Electronic Devices and the Mediterranean Diet on Macular Pigment" Journal of Clinical Medicine 13, no. 24: 7688. https://doi.org/10.3390/jcm13247688
APA StyleGarcía-Romera, M.-C., Ponce-García, V., Torres-Parejo, Ú., & López-Muñoz, A. (2024). Effect of Exposure to Blue Light from Electronic Devices and the Mediterranean Diet on Macular Pigment. Journal of Clinical Medicine, 13(24), 7688. https://doi.org/10.3390/jcm13247688






