Elevated miRNA-499 Levels in Early Phase of Non-ST Elevation Acute Coronary Syndromes Predict Increased Long-Term Risk of Major Adverse Cardiac Events
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Routine Clinical Assessment
2.3. Final Diagnosis of Non-ST Myocardial Infarction
2.4. Plasma miRNAs
2.4.1. Isolation of miRNAs
2.4.2. miRNA Quantification
2.4.3. Real-Time PCR Analysis
2.5. Follow-Up and Clinical Endpoints
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Patients
3.2. Levels of miRNAs in NSTEMI, UA and SCAD
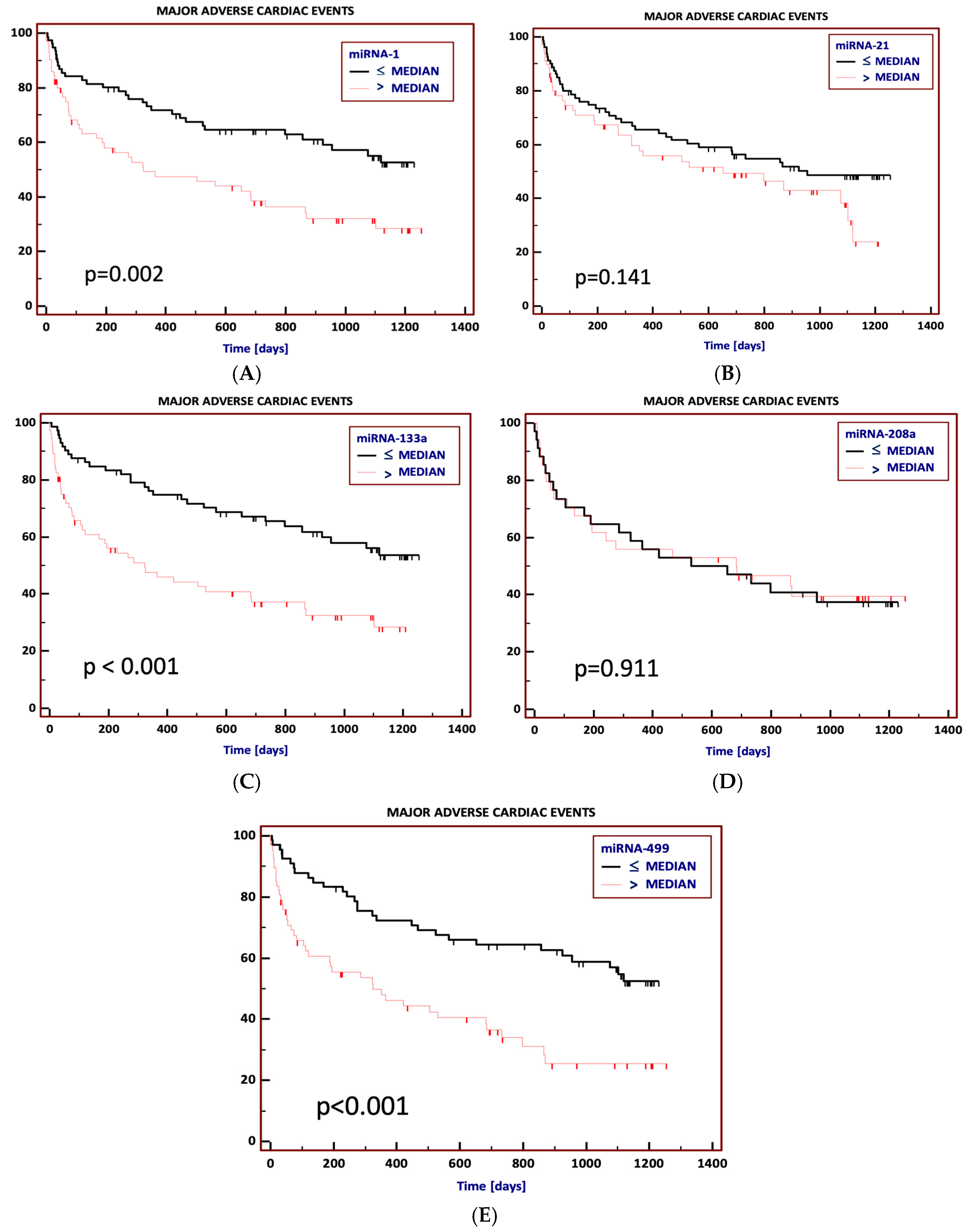
3.3. Prediction of Major Adverse Cardiac Events (MACEs) Using miRNAs
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roffi, M.; Patrono, C.; Collet, J.-P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef]
- Small, E.M.; Olson, E.N. Pervasive Roles of MicroRNAs in Cardiovascular Biology. Nature 2011, 469, 336–342. [Google Scholar] [CrossRef]
- Navickas, R.; Gal, D.; Laucevičius, A.; Taparauskaitė, A.; Zdanytė, M.; Holvoet, P. Identifying Circulating MicroRNAs as Biomarkers of Cardiovascular Disease: A Systematic Review. Cardiovasc. Res. 2016, 111, 322–337. [Google Scholar] [CrossRef]
- He, W.; Zhu, L.; Huang, Y.; Zhang, Y.; Shen, W.; Fang, L.; Li, J.; Wang, Z.; Xie, Q. The Relationship of MicroRNA-21 and Plaque Stability in Acute Coronary Syndrome. Medicine 2019, 98, e18049. [Google Scholar] [CrossRef]
- Wu, J.; Yue, B.; Lan, X.; Wang, Y.; Fang, X.; Ma, Y.; Bai, Y.; Qi, X.; Zhang, C.; Chen, H. MiR-499 Regulates Myoblast Proliferation and Differentiation by Targeting Transforming Growth Factor β Receptor 1. J. Cell. Physiol. 2019, 234, 2523–2536. [Google Scholar] [CrossRef]
- Townley-Tilson, W.H.D.; Callis, T.E.; Wang, D. MicroRNAs 1, 133, and 206: Critical Factors of Skeletal and Cardiac Muscle Development, Function, and Disease. Int. J. Biochem. Cell Biol. 2010, 42, 1252–1255. [Google Scholar] [CrossRef]
- Kura, B.; Kalocayova, B.; Devaux, Y.; Bartekova, M. Potential Clinical Implications of MiR-1 and MiR-21 in Heart Disease and Cardioprotection. Int. J. Mol. Sci. 2020, 21, 700. [Google Scholar] [CrossRef]
- Creemers, E.E.; Tijsen, A.J.; Pinto, Y.M. Circulating MicroRNAs Novel Biomarkers and Extracellular Communicators in Cardiovascular Disease? Circ. Res. 2012, 110, 483–495. [Google Scholar] [CrossRef]
- Olivieri, F.; Antonicelli, R.; Capogrossi, M.C.; Procopio, A.D. Circulating MicroRNAs (MiRs) for Diagnosing Acute Myocardial Infarction: An Exciting Challenge. Int. J. Cardiol. 2013, 167, 3028–3029. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Ono, K.; Horie, T.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Baba, O.; Kojima, Y.; Shizuta, S.; et al. Increased MicroRNA-1 and MicroRNA-133a Levels in Serum of Patients with Cardiovascular Disease Indicate Myocardial Damage. Circ. Cardiovasc. Genet. 2011, 4, 446–454. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.; Sun, Q. Diagnostic and Prognostic Value of Circulating MiRNA-499 and MiRNA-22 in Acute Myocardial Infarction. J. Clin. Lab. Anal. 2020, 34, e23332. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Hao, X.; Yang, J.; Han, X.; Li, H.; Li, T.; Wang, D.; Teng, Y.; Ma, L.; et al. Comparison of the Clinical Value of MiRNAs and Conventional Biomarkers in AMI: A Systematic Review. Front. Genet 2021, 12, 668324. [Google Scholar] [CrossRef]
- Adachi, T.; Nakanishi, M.; Otsuka, Y.; Nishimura, K.; Hirokawa, G.; Goto, Y.; Nonogi, H.; Iwai, N. Plasma MicroRNA 499 as a Biomarker of Acute Myocardial Infarction. Clin. Chem. 2010, 56, 1183–1185. [Google Scholar] [CrossRef]
- Białek, S.G.; Górko, D.; Zajkowska, A.; Kołtowski, Ł.; Grabowski, M.; Stachurska, A.; Kochman, J.; Sygitowicz, G.; Małecki, M.; Opolski, G.; et al. Release Kinetics of Circulating MiRNA-208a in Early Phase of Myocardial Infarction. Kardiol. Pol. 2015, 73, 613–619. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, Q.; You, W.; Chen, M.; Xia, J. MiRNAs as Biomarkers of Myocardial Infarction: A Meta-Analysis. PLoS ONE 2014, 9, e88566. [Google Scholar] [CrossRef]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating MicroRNAs Are New and Sensitive Biomarkers of Myocardial Infarction. Eur. Heart J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef]
- Boon, R.A.; Dimmeler, S. MicroRNAs in Myocardial Infarction. Nat. Rev. Cardiol. 2015, 12, 135–142. [Google Scholar] [CrossRef]
- Gacoń, J.; Kabłak-Ziembicka, A.; Stępień, E.; Enguita, F.J.; Karch, I.; Derlaga, B.; Żmudka, K.; Przewłocki, T. Decision-Making MicroRNAs (MiR-124, -133a/b, -34a and -134) in Patients with Occluded Target Vessel in Acute Coronary Syndrome. Kardiol. Pol. 2016, 74, 280–288. [Google Scholar] [CrossRef]
- Olivieri, F.; Antonicelli, R.; Lorenzi, M.; D’Alessandra, Y.; Lazzarini, R.; Santini, G.; Spazzafumo, L.; Lisa, R.; La Sala, L.; Galeazzi, R.; et al. Diagnostic Potential of Circulating MiR-499-5p in Elderly Patients with Acute Non ST-Elevation Myocardial Infarction. Int. J. Cardiol. 2013, 167, 531–536. [Google Scholar] [CrossRef]
- Jakob, P.; Kacprowski, T.; Briand-Schumacher, S.; Heg, D.; Klingenberg, R.; Stähli, B.E.; Jaguszewski, M.; Rodondi, N.; Nanchen, D.; Räber, L.; et al. Profiling and Validation of Circulating MicroRNAs for Cardiovascular Events in Patients Presenting with ST-Segment Elevation Myocardial Infarction. Eur. Heart J. 2017, 38, 511–515. [Google Scholar] [CrossRef]
- Ke-Gang, J.; Zhi-Wei, L.; Xin, Z.; Jing, W.; Ping, S.; Xue-Jing, H.; Hong-Xia, T.; Xin, T.; Xiao-Cheng, L. Evaluating Diagnostic and Prognostic Value of Plasma MiRNA133a in Acute Chest Pain Patients Undergoing Coronary Angiography. Medicine 2016, 95, e3412. [Google Scholar] [CrossRef]
- Miśkowiec, D.; Lipiec, P.; Wierzbowska-Drabik, K.; Kupczyńska, K.; Michalski, B.; Wdowiak-Okrojek, K.; Wejner-Mik, P.; Kasprzak, J.D. Association between MicroRNA-21 Concentration and Lipid Profile in Patients with Acute Coronary Syndrome without Persistent ST-Segment Elevation. Pol. Arch. Med. Wewn. 2016, 126, 48–57. [Google Scholar] [CrossRef]
- Agiannitopoulos, K.; Pavlopoulou, P.; Tsamis, K.; Bampali, K.; Samara, P.; Nasioulas, G.; Mertzanos, G.; Babalis, D.; Lamnissou, K. Expression of MiR-208b and MiR-499 in Greek Patients with Acute Myocardial Infarction. In Vivo 2018, 32, 313–318. [Google Scholar] [CrossRef]
- Velu, K.; Ramesh, R.; Medha, R.; Sweta, K.; Hanifa, M. Role of Serum MicroRNA-499 as a Diagnostic Marker in Acute Myocardial Infarction (AMI). Cor Vasa 2018, 61, e272–e276. [Google Scholar] [CrossRef]
- Zhao, C.H.; Cheng, G.-C.; He, R.L.; Hong, Y.; Wan, Q.L.; Wang, Z.-Z.; Pan, Z.-Y. Analysis and Clinical Significance of MicroRNA-499 Expression Levels in Serum of Patients with Acute Myocardial Infarction. Genet. Mol. Res. 2015, 14, 4027–4034. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, H.; Yan, P.; Zhou, X.; Wang, Y.; Yao, Y. Circulating MicroRNA-499 as a Diagnostic Biomarker for Acute Myocardial Infarction: A Meta-Analysis. Dis. Markers 2019, 2019, 6121696. [Google Scholar] [CrossRef]
- Li, P.; Li, S.-Y.; Liu, M.; Ruan, J.-W.; Wang, Z.-D.; Xie, W.-C. Value of the Expression of MiR-208, MiR-494, MiR-499 and MiR-1303 in Early Diagnosis of Acute Myocardial Infarction. Life Sci. 2019, 232, 116547. [Google Scholar] [CrossRef]
- Oerlemans, M.I.F.J.; Mosterd, A.; Dekker, M.S.; de Vrey, E.A.; van Mil, A.; Pasterkamp, G.; Doevendans, P.A.; Hoes, A.W.; Sluijter, J.P.G. Early Assessment of Acute Coronary Syndromes in the Emergency Department: The Potential Diagnostic Value of Circulating MicroRNAs. EMBO Mol. Med. 2012, 4, 1176–1185. [Google Scholar] [CrossRef]
- Wang, G.-K.; Zhu, J.-Q.; Zhang, J.-T.; Li, Q.; Li, Y.; He, J.; Qin, Y.-W.; Jing, Q. Circulating MicroRNA: A Novel Potential Biomarker for Early Diagnosis of Acute Myocardial Infarction in Humans. Eur. Heart J. 2010, 31, 659–666. [Google Scholar] [CrossRef]
- Widera, C.; Gupta, S.K.; Lorenzen, J.M.; Bang, C.; Bauersachs, J.; Bethmann, K.; Kempf, T.; Wollert, K.C.; Thum, T. Diagnostic and Prognostic Impact of Six Circulating MicroRNAs in Acute Coronary Syndrome. J. Mol. Cell. Cardiol. 2011, 51, 872–875. [Google Scholar] [CrossRef]
- Liu, X.; Fan, Z.; Zhao, T.; Cao, W.; Zhang, L.; Li, H.; Xie, Q.; Tian, Y.; Wang, B. Plasma MiR-1, MiR-208, MiR-499 as Potential Predictive Biomarkers for Acute Myocardial Infarction: An Independent Study of Han Population. Exp. Gerontol. 2015, 72, 230–238. [Google Scholar] [CrossRef]
- Devaux, Y.; Mueller, M.; Haaf, P.; Goretti, E.; Twerenbold, R.; Zangrando, J.; Vausort, M.; Reichlin, T.; Wildi, K.; Moehring, B.; et al. Diagnostic and Prognostic Value of Circulating MicroRNAs in Patients with Acute Chest Pain. J. Intern. Med. 2015, 277, 260–271. [Google Scholar] [CrossRef]
- Wang, F.; Long, G.; Zhao, C.; Li, H.; Chaugai, S.; Wang, Y.; Chen, C.; Wang, D.W. Plasma MicroRNA-133a Is a New Marker for Both Acute Myocardial Infarction and Underlying Coronary Artery Stenosis. J. Transl. Med. 2013, 11, 222. [Google Scholar] [CrossRef]
- Goretti, E.; Vausort, M.; Wagner, D.R.; Devaux, Y. Association between Circulating MicroRNAs, Cardiovascular Risk Factors and Outcome in Patients with Acute Myocardial Infarction. Int. J. Cardiol. 2013, 168, 4548–4550. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Su, T.; Li, H.; Huang, Q.; Wu, D.; Yang, C.; Han, Z. Circulating MiR-499 Are Novel and Sensitive Biomarker of Acute Myocardial Infarction. J. Thorac. Dis. 2015, 7, 303–308. [Google Scholar] [CrossRef]
- Yao, Y.; Du, J.; Cao, X.; Wang, Y.; Huang, Y.; Hu, S.; Zheng, Z. Plasma Levels of MicroRNA-499 Provide an Early Indication of Perioperative Myocardial Infarction in Coronary Artery Bypass Graft Patients. PLoS ONE 2014, 9, e104618. [Google Scholar] [CrossRef]
- Gaber, M.A.; Omar, O.H.M.; El-Deek, S.E.M.; Hassan, A.K.M.; Mahmoud, M.S.; Meki, A.-R.M.A. Copeptin, MiRNA-208, and MiRNA-499 as New Biomarkers for Early Detection of Acute Coronary Syndrome. Appl. Biochem. Biotechnol. 2022, 194, 1193–1205. [Google Scholar] [CrossRef]
- Klimczak, D.; Pączek, L.; Jażdżewski, K.; Kuch, M. MicroRNAs: Powerful Regulators and Potential Diagnostic Tools in Cardiovascular Disease. Kardiol. Pol. 2015, 73, 1–6. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Su, T.; Li, H.; Huang, Q.; Wu, D.; Yang, C.; Han, Z. Kinetics of Plasma MicroRNA-499 Expression in Acute Myocardial Infarction. J. Thorac. Dis. 2015, 7, 890–896. [Google Scholar] [CrossRef]
- Venugopal, P.; Balakrishnan, K.; Damal Kandadai, S.; George, M. Usefullness of MicroRNAs in Predicting the Clinical Outcome of Patients with Acute Myocardial Infarction During Follow-Up: A Systematic Review. Genet. Test Mol. Biomark. 2022, 26, 277–289. [Google Scholar] [CrossRef]
- Karakas, M.; Schulte, C.; Appelbaum, S.; Ojeda, F.; Lackner, K.J.; Münzel, T.; Schnabel, R.B.; Blankenberg, S.; Zeller, T. Circulating MicroRNAs Strongly Predict Cardiovascular Death in Patients with Coronary Artery Disease-Results from the Large AtheroGene Study. Eur. Heart J. 2017, 38, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Corsten, M.F.; Dennert, R.; Jochems, S.; Kuznetsova, T.; Devaux, Y.; Hofstra, L.; Wagner, D.R.; Staessen, J.A.; Heymans, S.; Schroen, B. Circulating MicroRNA-208b and MicroRNA-499 Reflect Myocardial Damage in Cardiovascular Disease. Circ. Cardiovasc. Genet. 2010, 3, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-K.; Hsieh, Y.-P.; Hsu, S.-W.; Lan, S.-J. Exploring Diagnostic and Prognostic Predictive Values of MicroRNAs for Acute Myocardial Infarction. Medicine 2021, 100, e26627. [Google Scholar] [CrossRef] [PubMed]
- de Ronde, M.W.J.; Pinto, Y.M.; Pinto-Sietsma, S.-J. Circulating MicroRNA Biomarkers for Cardiovascular Risk Prediction: Are We Approaching Clinical Application? Ann. Transl. Med. 2016, 4, 490. [Google Scholar] [CrossRef]
- Mompeón, A.; Ortega-Paz, L.; Vidal-Gómez, X.; Costa, T.J.; Pérez-Cremades, D.; Garcia-Blas, S.; Brugaletta, S.; Sanchis, J.; Sabate, M.; Novella, S.; et al. Disparate miRNA expression in serum and plasma of patients with acute myocardial infarction: A systematic and paired comparative analysis. Sci. Rep. 2020, 10, 5373. [Google Scholar] [CrossRef]

| Variable | Stable Coronary Artery Disease (n = 47) | NSTE-ACS (n = 103) | p-Value | |
|---|---|---|---|---|
| NSTEMI (n = 52) | UA (n = 51) | |||
| Male, n (%) | 16 (34%) | 14 (27%) | 19 (37%) | 0.52 |
| Age, years | 65.3 (10.6) | 66.8 (11.1) | 67.7 (12.2) | 0.47 |
| BMI, kg/m2 | 28.6 (24.8–30.5) | 28.2 (26.0–30.3) | 28.4 (25.0–30.5) | 0.92 |
| Cardiovascular risk factors | ||||
| Diabetes mellitus, n (%) | 19 (40%) | 20 (38%) | 14 (27%) | 0.34 |
| Hypercholesterolemia, n (%) | 40 (85%) | 38 (73%) | 44 (86%) | 0.17 |
| Hypertension, n (%) | 40 (85%) | 44 (85%) | 46 (90%) | 0.66 |
| Smoking, n (%) | 15 (32%) | 27 (52%) | 17 (33%) | 0.07 |
| Positive CAD family history a, n (%) | 2 (4%) | 8 (15%) | 8 (16%) | 0.14 |
| Stroke, n (%) | 5 (11%) | 5 (10%) | 10 (20%) | 0.26 |
| TIMI Risk Score, median (IQR) | - | 4.5 (3–6) | 4 (3–5) | 0.06 |
| GRACE Risk Score | - | 159.0 (33.8) | 139.4 (29.1) | 0.002 |
| AF, n (%) | 5 (11%) | 6 (12%) | 7 (14%) | 0.89 |
| Prior MI, n (%) | 13 (28%) | 11 (21%) | 18 (35%) | 0.28 |
| Prior PCI, n (%) | 21 (45%) | 9 (17%) | 23 (45%) | 0.003 |
| Prior CABG, n (%) | 3 (6%) | 3 (8%) | 4 (8%) | 0.91 |
| Lung disease b, n (%) | 6 (13%) | 2 (4%) | 0 (0%) | 0.02 |
| Laboratory values on admission | ||||
| eGFR, mL × min−1 × 1.73 m−2 | 81.5 (26.2) | 83.5 (24.3) | 74.7 (29.1) | 0.18 |
| NT-proBNP, ng/L | 403 (98–890) | 1192 (257–2813) | 336 (110–661) | 0.01 |
| CRP, mg/dL | 2.5 (1.0–5.0) | 4.0 (1.7–26.4) | 2.4 (0.9–8.2) | 0.06 |
| TC, mg/dL | 155 (130–184) | 194 (154–214) | 160 (147–199) | 0.03 |
| LDL, mg/dL | 81 (67–115) | 116 (81–141) | 84 (71–108) | 0.01 |
| HDL, mg/dL | 53 (44–68) | 42 (34–53) | 49 (40–62) | 0.01 |
| TG, mg/dL | 91 (67–148) | 109 (92–160) | 136 (53–166) | 0.02 |
| Biomarker | Stable Coronary Artery Disease (n = 34) | NSTEMI (n = 20) | UA (n = 16) | p1 | p2 | p3 |
|---|---|---|---|---|---|---|
| miR-1 | 4.68 (2.17–9.37) | 20.04 (7.56–68.88) | 4.49 (2.43–8.02) | <0.001 | <0.001 | 1.0 |
| miR-21 | 1.88 (1.12–2.94) | 2.41 (1.21–4.55) | 2.60 (1.37–4.28) | 0.32 | 1.0 | 0.19 |
| mir-133a | 4.54 (2.25–7.22) | 39.94 (20.02–405.94) | 10.91 (6.09–19.38) | <0.001 | <0.001 | <0.001 |
| miR-208a | 5.03 (3.33–15.49) | 19.15 (6.41–42.05) | 28.45 (5.94–51.27) | 0.03 | 1.0 | 0.03 |
| miR-499 | 1.76 (1.19–2.68) | 12.91 (3.41–63.33) | 3.23 (1.60–4.21) | <0.001 | <0.001 | 0.11 |
| hs-cTnT, ng/mL | 0.013 (0.010–0.021) | 0.405 (0.160–1.60) | 0.015 (0.009–0.033) | <0.001 | <0.001 | 1.0 |
| CK-MB mass, ng/mL | 2.40 (1.80–3.40) | 13.00 (7.88–37.92) | 2.22 (1.82–3.10) | <0.001 | <0.001 | 1.0 |
| h-FABP, ng/mL | - | 52.2 (20.69–108.7) | 21.9 (9.7–57.6) | - | 0.04 | - |
| Variable | HR | 95% CI | p |
|---|---|---|---|
| miRNA-499 > median | 1.80 | 1.05–3.08 | 0.02 |
| hs-cTnT | 1.24 | 1.02–1.50 | 0.04 |
| Diabetes mellitus | 1.72 | 1.06–2.78 | 0.03 |
| PCI at hospital stay | 2.07 | 1.13–3.82 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miśkowiec, D.; Szymczyk, E.; Wejner-Mik, P.; Michalski, B.; Lipiec, P.; Simiera, M.; Kupczyńska, K.; Kasprzak, J.D. Elevated miRNA-499 Levels in Early Phase of Non-ST Elevation Acute Coronary Syndromes Predict Increased Long-Term Risk of Major Adverse Cardiac Events. J. Clin. Med. 2024, 13, 7803. https://doi.org/10.3390/jcm13247803
Miśkowiec D, Szymczyk E, Wejner-Mik P, Michalski B, Lipiec P, Simiera M, Kupczyńska K, Kasprzak JD. Elevated miRNA-499 Levels in Early Phase of Non-ST Elevation Acute Coronary Syndromes Predict Increased Long-Term Risk of Major Adverse Cardiac Events. Journal of Clinical Medicine. 2024; 13(24):7803. https://doi.org/10.3390/jcm13247803
Chicago/Turabian StyleMiśkowiec, Dawid, Ewa Szymczyk, Paulina Wejner-Mik, Błażej Michalski, Piotr Lipiec, Michał Simiera, Karolina Kupczyńska, and Jarosław D. Kasprzak. 2024. "Elevated miRNA-499 Levels in Early Phase of Non-ST Elevation Acute Coronary Syndromes Predict Increased Long-Term Risk of Major Adverse Cardiac Events" Journal of Clinical Medicine 13, no. 24: 7803. https://doi.org/10.3390/jcm13247803
APA StyleMiśkowiec, D., Szymczyk, E., Wejner-Mik, P., Michalski, B., Lipiec, P., Simiera, M., Kupczyńska, K., & Kasprzak, J. D. (2024). Elevated miRNA-499 Levels in Early Phase of Non-ST Elevation Acute Coronary Syndromes Predict Increased Long-Term Risk of Major Adverse Cardiac Events. Journal of Clinical Medicine, 13(24), 7803. https://doi.org/10.3390/jcm13247803







