Cardio-Renal Syndrome: Latest Developments in Device-Based Therapy
Abstract
1. Introduction
1.1. Overview of Cardio-Renal Syndrome
- Hemodynamic factors such as increased venous congestion and reduced renal perfusion from elevated central venous pressures, which often accompany heart failure.
- Neurohormonal dysregulation, stemming from activation of the renin–angiotensin–aldosterone system (RAAS) and the sympathetic nervous system, causing further renal vasoconstriction and sodium retention.
- Inflammatory pathways are activated in both organs, promoting fibrotic changes and vascular remodeling.
- Oxidative stress contributes to endothelial dysfunction, exacerbating damage in both cardiac and renal tissues.
1.2. Diuretic Resistance in Cardio-Renal Syndrome
1.3. Sodium Avidity in CRS
1.4. The Concept and Utility of a “Diuretic Holiday”
1.5. Device-Based Therapy in CRS Management
1.6. Forward Failure, Backward Failure, and DRI2P2S Classification
1.7. DRI2P2S Classification and Pathophysiological Mechanisms in CRS
- D—Dilators (splanchnic denervation) or Decongestive (diuretics, aquapheresis, peritoneal dialysis).
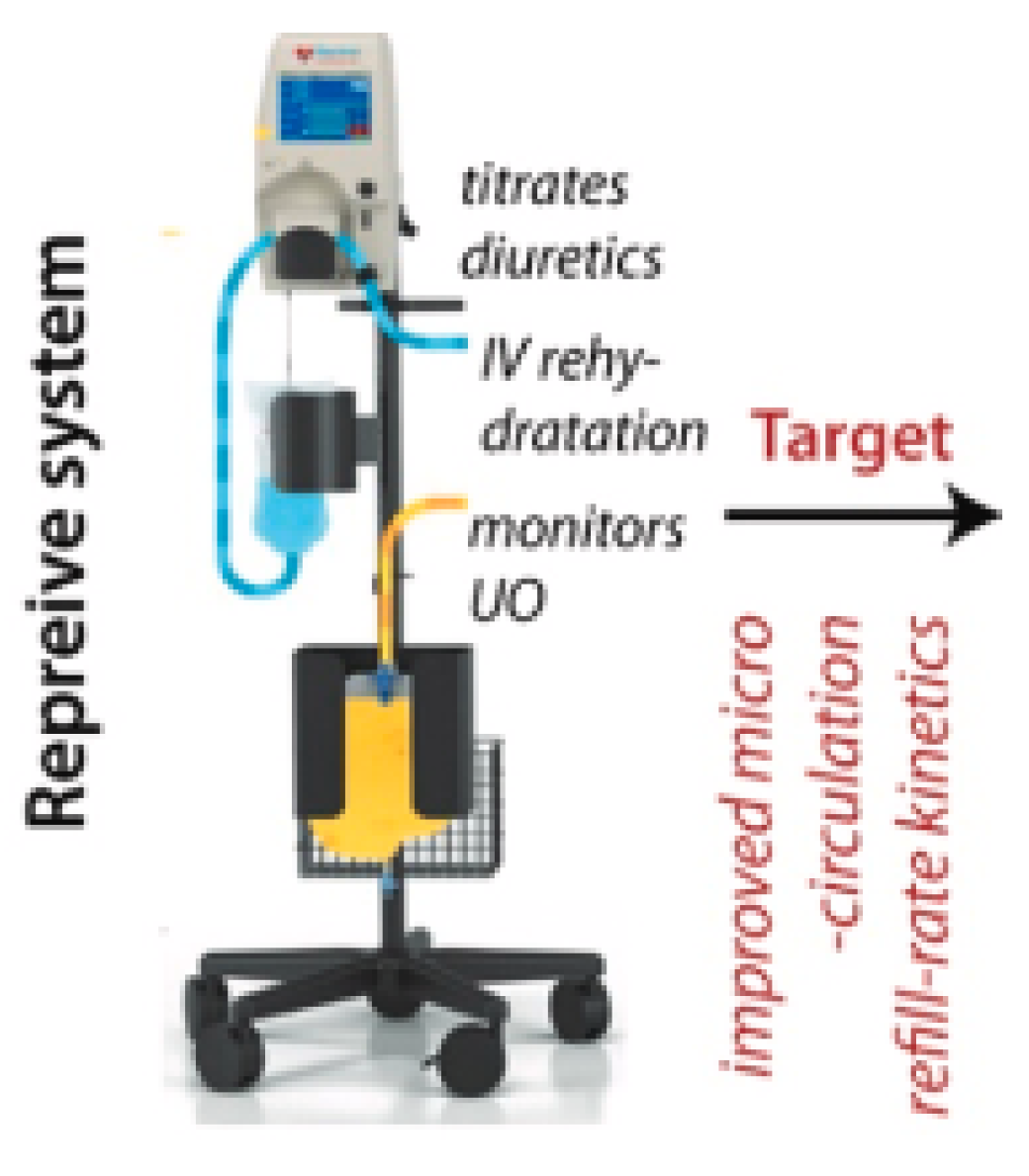
- R—Renal Replacement (CRRT-continuous renal replacement therapy, peritoneal dialysis)—AlfaPump, Reprieve System.
- I(1)—Inotropes (cardiac plexus nerve stimulation)—Cardionomic, NeuroTronik.
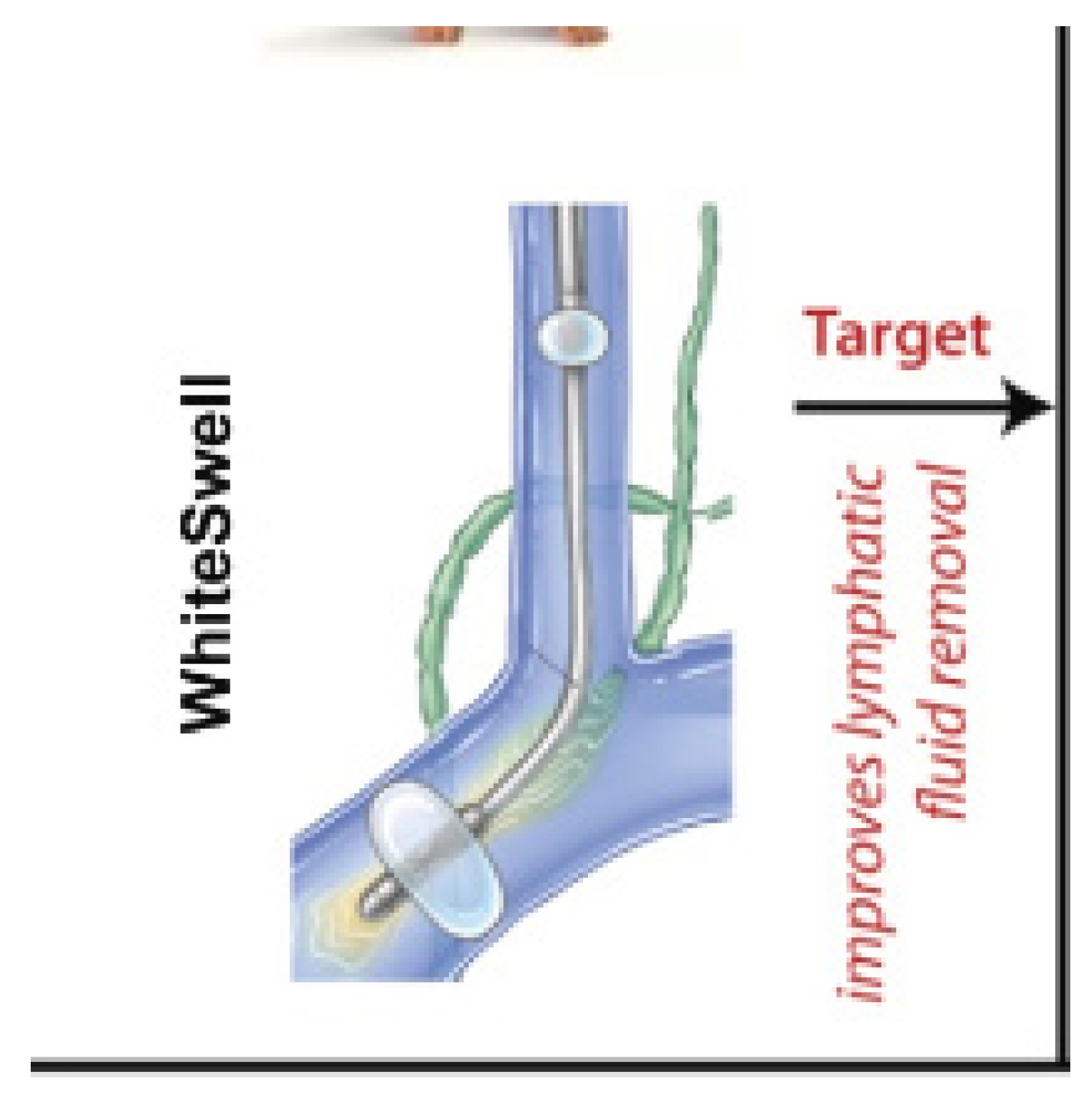
- I(2)—Interstitial (fluid management: lymphatic duct compression)—WhiteSwell.
- P—Pullers (suprarenal IVC blood pump, intrarenal vein pump, infrarenal partial obstruction, intermittent SVC occlusion)—preCardia, Doraya catheter, transcatheter renal decongestion system.
- P—Pushers (suprarenal descending aortic pumps)—Reitan catheter pump, Aortix, Second Heart Assist.
- S—Selective (selective intrarenal artery vasodilator drug delivery)—Benephit catheter.
2. Decongestive (D)
- Loop Diuretics (e.g., furosemide, torasemide) are often first-line therapies to promote diuresis and decrease fluid overload. Trials like ADVOR [11] (acetazolamide) or CLOROTIC [12] (combination of loop diuretics with thiazidic) have proven to relieve congestion to a somewhat greater extent, but without significant impact on mortality and readmission for heart failure.
- Aquapheresis is a form of ultrafiltration therapy that removes excess fluid directly from the bloodstream, offering precise control of fluid removal in cases of diuretic resistance. As the AVOID-HF [20] trial, in which aquapheresis was compared with IV diuretics and hospitalization, showed, the aquapheresis group trended toward a longer time to first HF event within 90 days and fewer HF and cardiovascular events. However, more patients in the aquapheresis group experienced adverse events of special interest or serious product-related adverse events, with similar renal function changes in both arms.
3. Renal Replacement (R)
- Continuous Renal Replacement Therapy (CRRT) is often used in critically ill CRS patients to provide continuous fluid and solute removal without destabilizing blood pressure. This method is preferable for patients who are hemodynamically unstable [30].
- Intermittent Hemodialysis can be used for patients with less severe CRS who can tolerate the more rapid fluid and solute shifts [31].
4. Inotropes (I1)
- Dobutamine is a common inotropic agent used to enhance cardiac output by stimulating beta-adrenergic receptors. However, it is generally reserved for acute decompensations, due to its arrhythmogenic potential [32].
- Milrinone is a phosphodiesterase-3 inhibitor that has both inotropic and vasodilatory effects, making it useful for patients with low cardiac output and high systemic vascular resistance [33].
- Cardiac plexus nerve stimulation has recently been proven useful in treating patients with type 2 cardio-renal syndrome [34].
5. Interstitial Fluid Management (I2)
6. Pullers (P1)
7. Pushers (P2)
8. Sympatholytics (S)
- Beta-blockers (e.g., carvedilol) are used to inhibit sympathetic activity, reducing heart rate and decreasing myocardial oxygen demand. This allows the heart to pump more efficiently, reducing stress on both the heart and kidneys [43].
8.1. “Pullers” in Cardio-Renal Syndrome
8.2. Venous Circulation in CRS
8.3. Device-Based Therapies for Venous Congestion in CRS
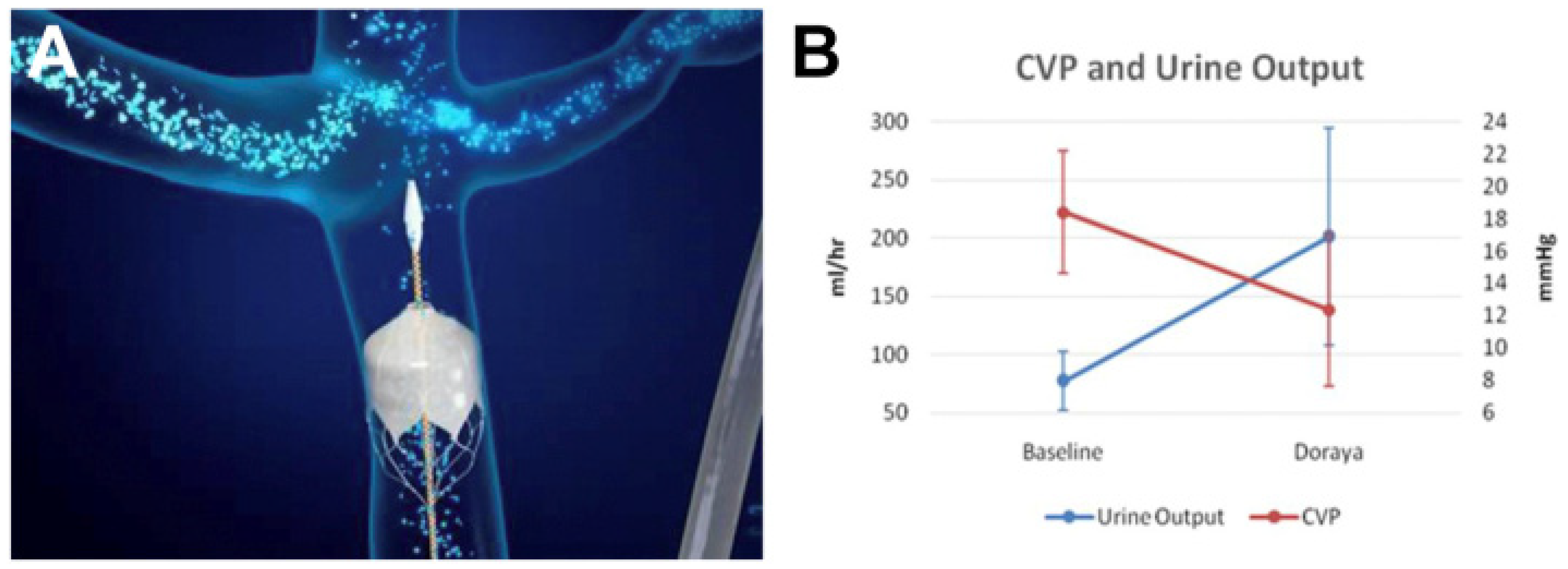
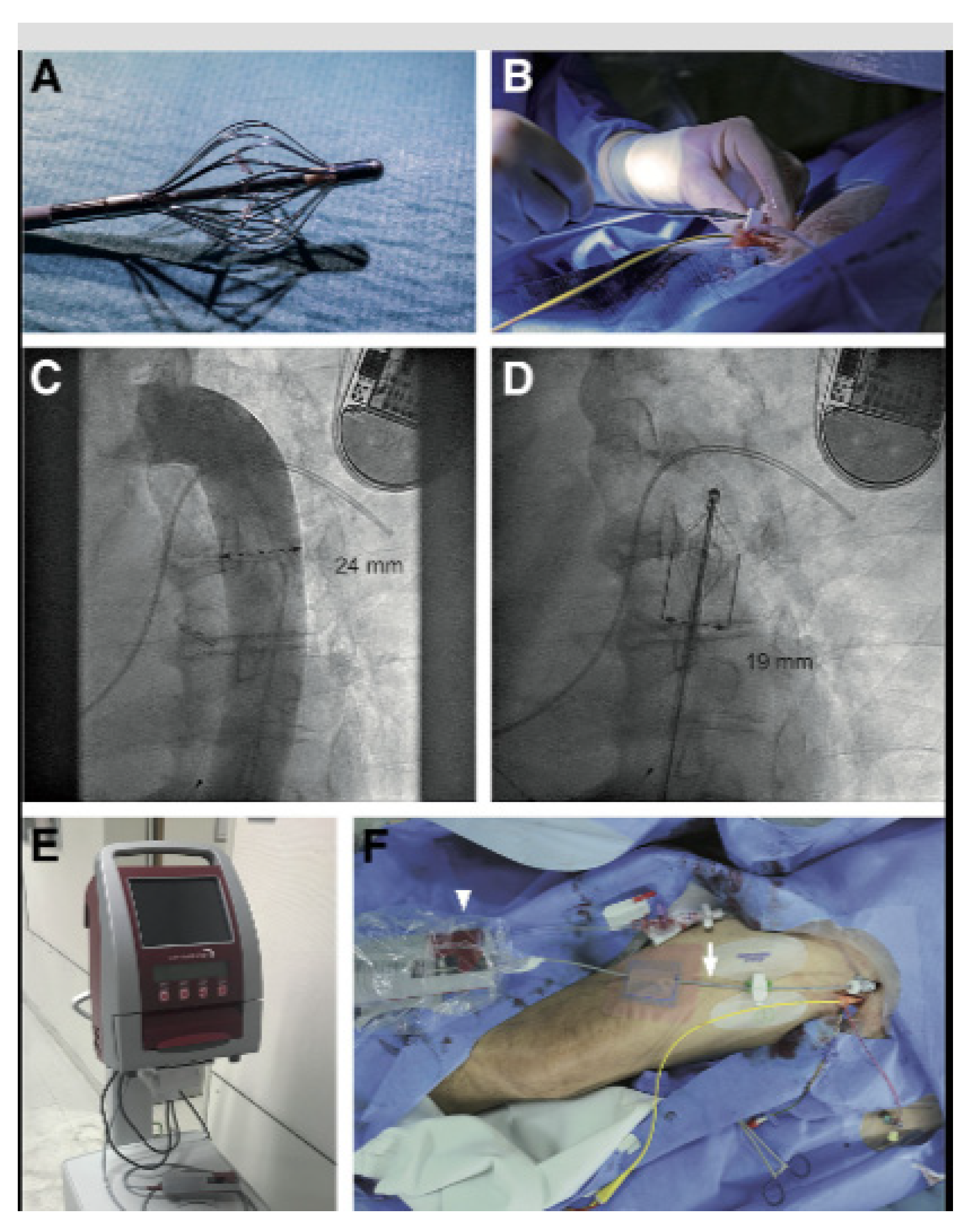
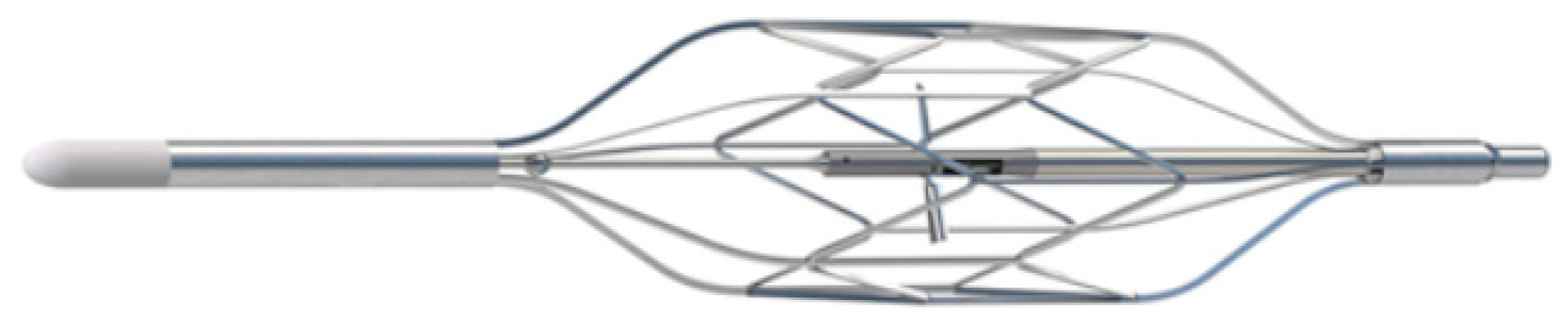
8.3.1. The Doraya Catheter
8.3.2. The preCARDIA System
8.3.3. The Perfusor Device
8.4. “Pushers” in Cardio-Renal Syndrome
8.5. Arterial Circulation in CRS
8.6. Device-Based Therapies for Arterial Circulation in CRS
8.6.1. The Reitan Catheter Pump
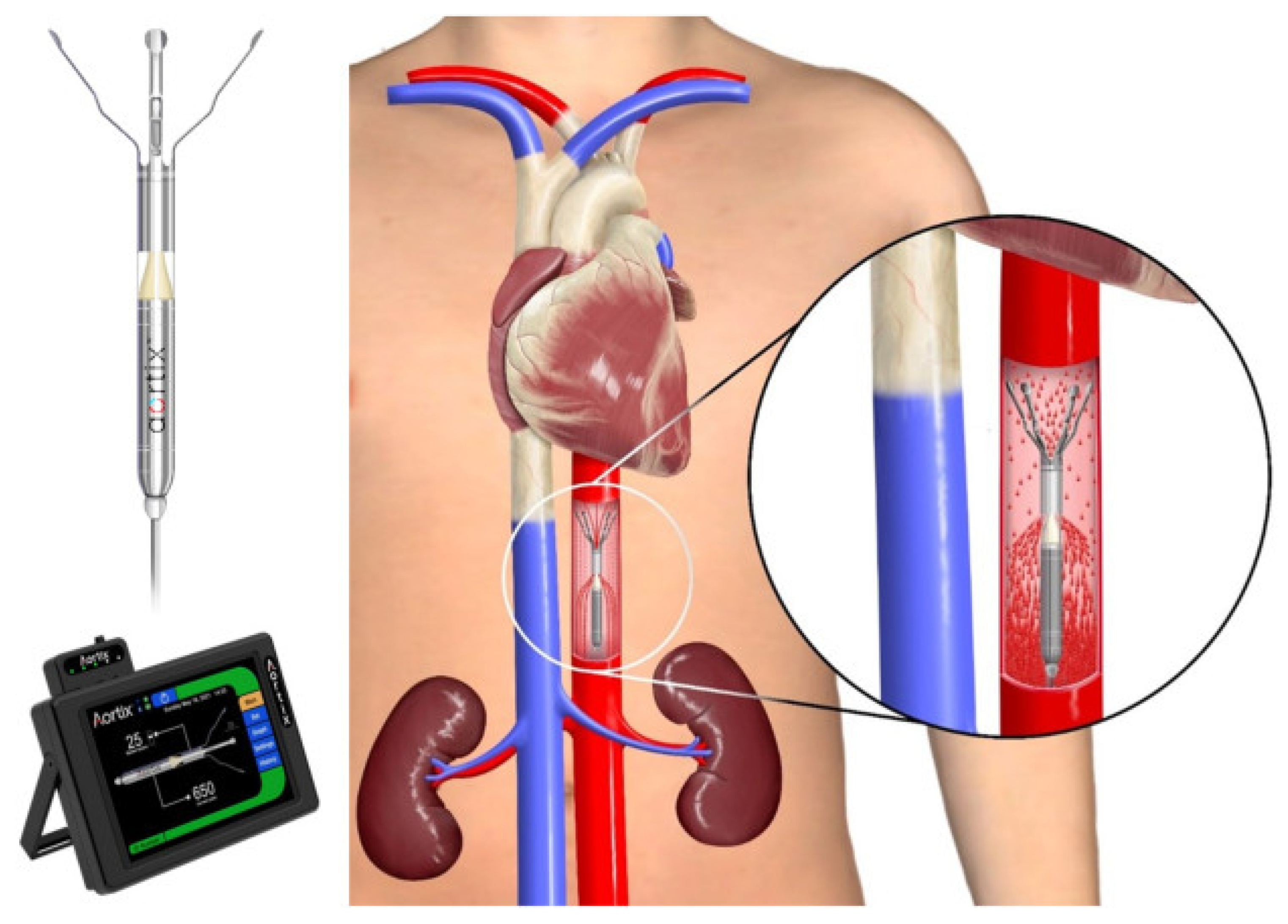
8.6.2. Aortix
8.6.3. ModulHeart
8.6.4. Second Heart Assist
8.7. “Fluid Shifters” in Cardio-Renal Syndrome
8.8. The Microcirculation–Interstitium–Lymphatic (MIL) Axis in Cardio-Renal Syndrome
- Capillary Leakage: Elevated capillary pressure in heart failure increases fluid transudation into the interstitial space. This heightened capillary pressure disrupts the balance, contributing to the development of edema.
- Interstitial Fluid Accumulation: As fluid accumulates in the interstitial space, it exerts backpressure on capillaries, further impeding fluid reabsorption [69].
- Lymphatic Insufficiency: In CRS, the lymphatic system often becomes overwhelmed, failing to adequately clear excess interstitial fluid, which exacerbates congestion and worsens symptoms.
8.9. Device-Based Therapies for Fluid Shifting in CRS
8.9.1. WhiteSwell eLym
- The device was deployed and activated in nine patients, with a mean treatment time of 24 h.
- No patient experienced a serious procedure-, device-, or therapy-related adverse event.
- Patients who underwent therapy with the eLym system plus loop diuretic lost a mean of 6.0 ± 4.6 kg from baseline to hospital discharge while maintaining kidney function, as measured by a stable or improved creatinine (mean Δ −0.10 ± 0.12 mg/dL).
- The loop diuretic-only group lost a mean of 3.3 ± 3.7 kg.
- One of the nine treated patients (11%) was hospitalized within 30 days of discharge.
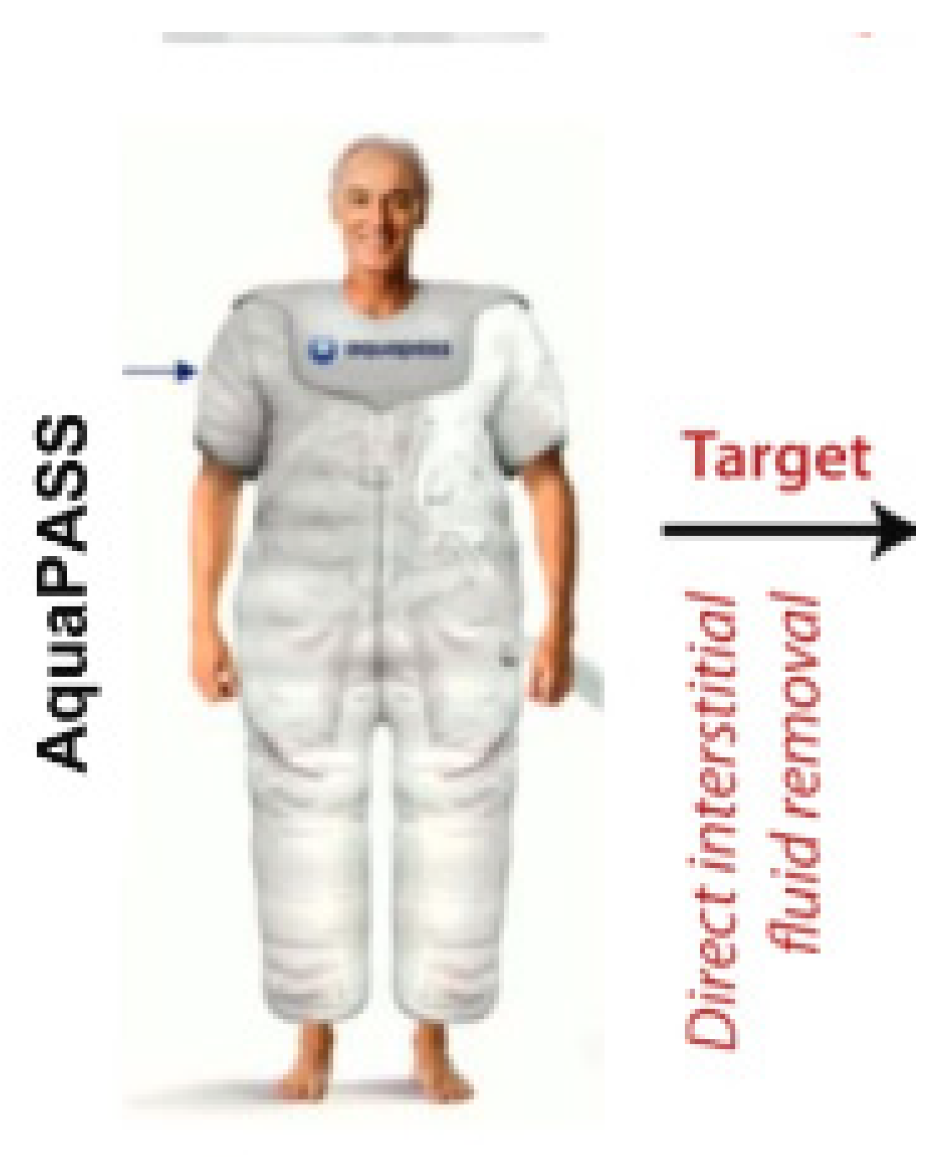
8.9.2. AquaPASS
8.9.3. Reprieve
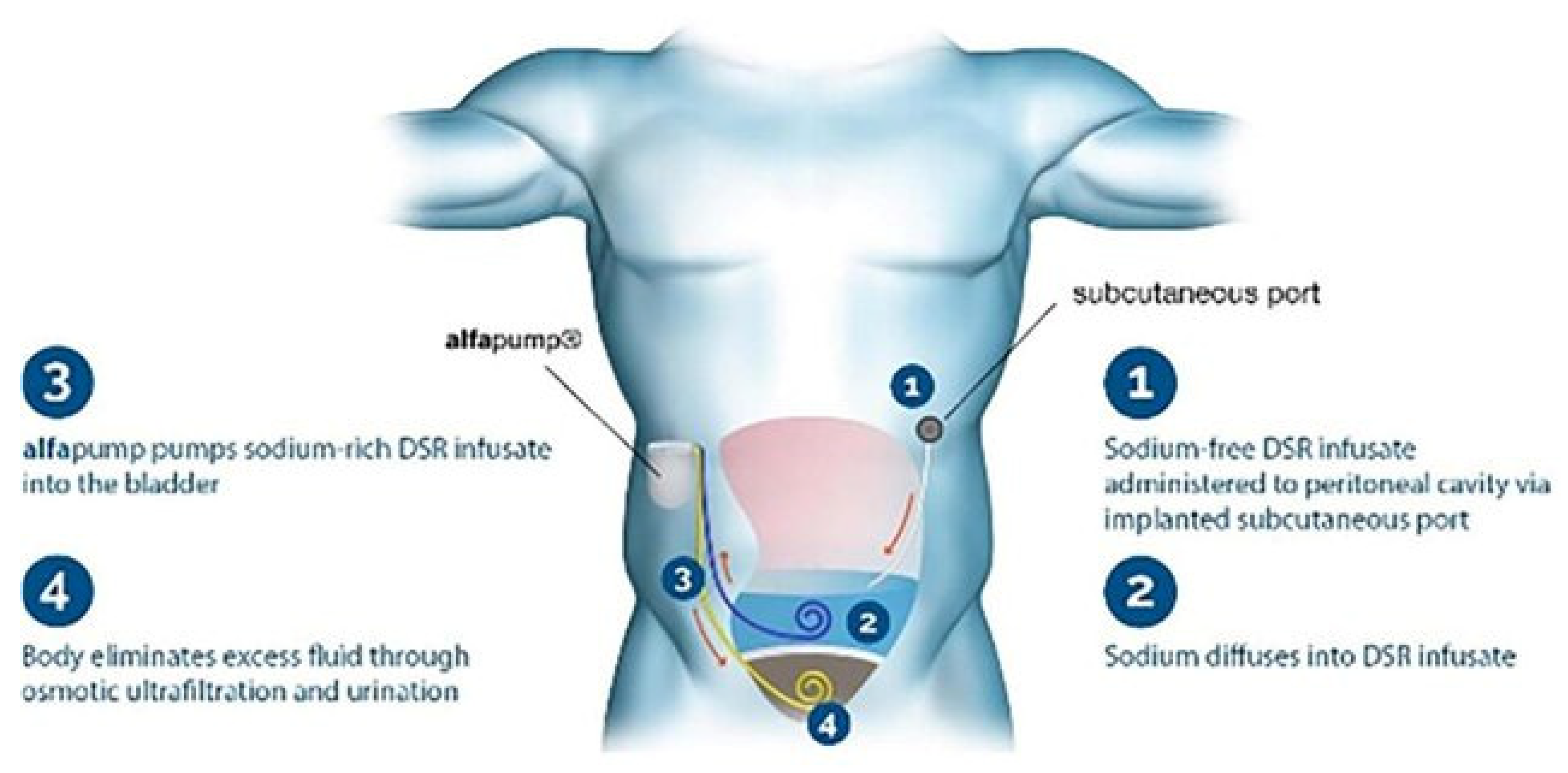
8.9.4. DSR 2.0 (Direct Sodium Removal)
8.10. Are These Studies Really Useful? How? When? Can We Make Them Better?
8.11. Limitations of Current Trial Endpoints in CRS
- Changes in Creatinine Levels: While serum creatinine is an indicator of kidney function, it is affected by many factors beyond actual renal health, including body mass, hydration status, and medications. Moreover, short-term fluctuations in creatinine do not necessarily reflect long-term kidney health.
- Glomerular Filtration Rate (GFR): GFR is another widely used measure, yet it can remain stable or even improve transiently without signifying sustained renal recovery.
- Hospitalization Rates: While a reduction in hospitalizations can indicate improved outcomes, it is also influenced by factors such as local healthcare policies and patient adherence, making it an indirect measure of device effectiveness.
- Mortality: Although mortality is a critical endpoint, it is a relatively coarse measure that fails to capture the nuanced benefits of improved quality of life or symptom relief that many device therapies offer in CRS.
8.12. Suggested Novel Endpoints for CRS Trials
- Central Venous Pressure (CVP): CVP directly reflects venous congestion, a core pathophysiological component in CRS, particularly in “backward failure” scenarios. Monitoring CVP changes could provide direct insight into the effectiveness of “puller” devices such as the Doraya catheter.
- Cardiac Output and Renal Perfusion Pressure: Measuring renal perfusion directly can provide a clearer picture of how “pushers” like Aortix and ModulHeart are improving renal function by enhancing forward flow, which is often inadequately represented by traditional endpoints.
- Quality of Life Metrics: Patient-reported outcomes that measure symptom relief, exercise tolerance, and daily functioning should be prioritized, as these reflect real-world benefits and may correlate better with the overall impact of therapy.
8.13. Challenges and Uncertainties to Overcome
- Complexity of CRS Pathophysiology: The mechanisms driving CRS are multifaceted, involving interactions among cardiac output, venous congestion, neurohormonal activation, and more. Determining the optimal device for each patient based on individual pathophysiology requires sophisticated diagnostic tools and experience.
- Patient Selection: The heterogeneity of CRS poses a challenge for patient selection. Identifying which patients will benefit most from “pullers”, “pushers”, or “fluid shifters” requires precise criteria and biomarkers, which are still under development.
- Safety and Long-Term Data: Most device trials have focused on short-term efficacy. The long-term safety of devices like the Doraya catheter, Aortix, and WhiteSwell eLym needs to be assessed, particularly regarding risks of thrombosis, infection, and device durability. Ongoing studies like MOJAVE [36] are gathering data but may take years to yield comprehensive results.
- Integration with Existing Care Models: Incorporating device-based therapies into current heart failure and nephrology care pathways requires collaboration across specialties, which can be logistically challenging. Additionally, many healthcare systems are not yet equipped to handle the operational demands of these devices.
- Cost-Effectiveness: Device-based therapies are generally more expensive than pharmacologic treatments. Demonstrating cost-effectiveness through reductions in hospitalizations, emergency visits, and long-term health improvements will be crucial for their adoption.
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Haapio, M.; Mankad, S.; Zamperetti, N.; Massie, B.; Bellomo, R.; Berl, T.; Anker, S.D.; Anand, I.; Aspromonte, N.; et al. Prevention of cardio-renal syndromes: Workgroup statements from the 7th ADQI Consensus Conference. Nephrol. Dial. Transplant. 2010, 25, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Mullens, W. Cardiorenal syndrome in decompensated heart failure. Heart 2010, 96, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C. Cardiorenal syndromes: Definition and classification. Contrib. Nephrol. 2010, 164, 33–38. [Google Scholar] [PubMed]
- Hostetter, T.H. Chronic kidney disease predicts cardiovascular disease. N. Engl. J. Med. 2004, 351, 1344–1346. [Google Scholar] [CrossRef]
- de Oliveira Cardoso, C.; Elgalad, A.; Li, K.; Perin, E.C. Device-based therapy for decompensated heart failure: An updated review of devices in development based on the DRI(2)P(2)S classification. Front. Cardiovasc. Med. 2022, 9, 962839. [Google Scholar] [CrossRef]
- Kapur, N.K.; Kiernan, M.S.; Gorgoshvili, I.; Yousefzai, R.; Vorovich, E.E.; Tedford, R.J.; Sauer, A.J.; Abraham, J.; Resor, C.D.; Kimmelstiel, C.D.; et al. Intermittent Occlusion of the Superior Vena Cava to Improve Hemodynamics in Patients With Acutely Decompensated Heart Failure: The VENUS-HF Early Feasibility Study. Circ. Heart Fail. 2022, 15, e008934. [Google Scholar] [CrossRef]
- Cunningham, J.W.; Sun, J.L.; Mc Causland, F.R.; Ly, S.; Anstrom, K.J.; Lindenfeld, J.; Givertz, M.M.; Stevenson, L.W.; Lakdawala, N.K. Lower urine sodium predicts longer length of stay in acute heart failure patients: Insights from the ROSE AHF trial. Clin. Cardiol. 2020, 43, 43–49. [Google Scholar] [CrossRef]
- Hoen, M.; Hofman, D.E.; Hompes, B.H.A.; Peeters, L.E.E.; Langenveld, B.; van Kimmenade, R.R.J.; Frenken, L.A.M.; Lenderink, T.; Brunner-La Rocca, H.P.; Sanders-Van Wijk, S. The role of urine sodium in acutely decompensated heart failure. Int. J. Cardiol. Heart Vasc. 2024, 55, 101509. [Google Scholar] [CrossRef]
- Ganes, A.; Davis, J.A.; Virtanen, J.K.; Voutilainen, A.; Tuomainen, T.P.; Atherton, J.J.; Amerena, J.; Driscoll, A.; Hare, D.L.; Wittert, G.; et al. Urinary sodium concentration predicts time to major adverse coronary events and all-cause mortality in men with heart failure over a 28-33-year period: A prospective cohort study. BMC Cardiovasc. Disord. 2022, 22, 391. [Google Scholar] [CrossRef]
- Mullens, W.; Dauw, J.; Martens, P.; Meekers, E.; Nijst, P.; Verbrugge, F.H.; Chenot, F.; Moubayed, S.; Dierckx, R.; Blouard, P.; et al. Acetazolamide in Decompensated Heart Failure with Volume Overload trial (ADVOR): Baseline characteristics. Eur. J. Heart Fail. 2022, 24, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Trullàs, J.C.; Morales-Rull, J.L.; Casado, J.; Carrera-Izquierdo, M.; Sánchez-Marteles, M.; Conde-Martel, A.; Dávila-Ramos, M.F.; Llácer, P.; Salamanca-Bautista, P.; Pérez-Silvestre, J.; et al. Combining loop with thiazide diuretics for decompensated heart failure: The CLOROTIC trial. Eur. Heart J. 2023, 44, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Claggett, B.L.; Lam, C.S.P.; Pitt, B.; Senni, M.; Shah, S.J.; Voors, A.A.; Zannad, F.; Desai, A.S.; Jhund, P.S.; et al. Finerenone in patients with heart failure with mildly reduced or preserved ejection fraction: Rationale and design of the FINEARTS-HF trial. Eur. J. Heart Fail. 2024, 26, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Mody, R.; Nee Sheth, A.B.; Dash, D.; Mody, B.; Agrawal, A.; Monga, I.S.; Rastogi, L.; Munjal, A. Device therapies for heart failure with reduced ejection fraction: A new era. Front. Cardiovasc. Med. 2024, 11, 1388232. [Google Scholar] [CrossRef] [PubMed]
- Bart, B.A.; Boyle, A.; Bank, A.J.; Anand, I.; Olivari, M.T.; Kraemer, M.; Mackedanz, S.; Sobotka, P.A.; Schollmeyer, M.; Goldsmith, S.R. Ultrafiltration versus usual care for hospitalized patients with heart failure: The Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) trial. J. Am. Coll. Cardiol. 2005, 46, 2043–2046. [Google Scholar] [CrossRef]
- Costanzo, M.R.; Saltzberg, M.T.; Jessup, M.; Teerlink, J.R.; Sobotka, P.A. Ultrafiltration is associated with fewer rehospitalizations than continuous diuretic infusion in patients with decompensated heart failure: Results from UNLOAD. J. Card. Fail. 2010, 16, 277–284. [Google Scholar] [CrossRef]
- Schrier, R.W.; Abraham, W.T. Hormones and hemodynamics in heart failure. N. Engl. J. Med. 1999, 341, 577–585. [Google Scholar] [CrossRef]
- Damman, K.; Navis, G.; Voors, A.A.; Asselbergs, F.W.; Smilde, T.D.; Cleland, J.G.; van Veldhuisen, D.J.; Hillege, H.L. Worsening renal function and prognosis in heart failure: Systematic review and meta-analysis. J. Card. Fail. 2007, 13, 599–608. [Google Scholar] [CrossRef]
- Wilson Tang, W.H.; Wilfried Mullens, F.H.V. Cardiorenal Syndrome in Heart Failure; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Costanzo, M.R.; Negoianu, D.; Jaski, B.E.; Bart, B.A.; Heywood, J.T.; Anand, I.S.; Smelser, J.M.; Kaneshige, A.M.; Chomsky, D.B.; Adler, E.D.; et al. Aquapheresis Versus Intravenous Diuretics and Hospitalizations for Heart Failure. JACC Heart Fail. 2016, 4, 95–105. [Google Scholar] [CrossRef]
- Kapur, N.K.; Karas, R.H.; Newman, S.; Jorde, L.; Chabrashvili, T.; Annamalai, S.; Esposito, M.; Kimmelstiel, C.D.; Lenihan, T.; Burkhoff, D. First-in-human experience with occlusion of the superior vena cava to reduce cardiac filling pressures in congestive heart failure. Catheter. Cardiovasc. Interv. 2019, 93, 1205–1210. [Google Scholar] [CrossRef]
- Chen, H.H.; Anstrom, K.J.; Givertz, M.M.; Stevenson, L.W.; Semigran, M.J.; Goldsmith, S.R.; Bart, B.A.; Bull, D.A.; Stehlik, J.; LeWinter, M.M.; et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The ROSE acute heart failure randomized trial. JAMA 2013, 310, 2533–2543. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; Jones, W.S.; Boortz-Marx, R.L.; Ganesh, A.; Green, C.L.; Hernandez, A.F.; Patel, M.R. Splanchnic Nerve Block for Acute Heart Failure. Circulation 2018, 138, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; Ponikowski, P.P.; Burkhoff, D.; Dunlap, M.E.; Sobotka, P.A.; Molinger, J.; Patel, M.R.; Felker, G.M.; Hernandez, A.F.; Litwin, S.E.; et al. Splanchnic nerve modulation in heart failure: Mechanistic overview, initial clinical experience, and safety considerations. Eur. J. Heart Fail. 2021, 23, 1076–1084. [Google Scholar] [CrossRef]
- Fudim, M.; Khan, M.S.; Paracha, A.A.; Sunagawa, K.; Burkhoff, D. Targeting Preload in Heart Failure: Splanchnic Nerve Blockade and Beyond. Circ. Heart Fail. 2022, 15, e009340. [Google Scholar] [CrossRef]
- Fudim, M.; Boortz-Marx, R.L.; Ganesh, A.; DeVore, A.D.; Patel, C.B.; Rogers, J.G.; Coburn, A.; Johnson, I.; Paul, A.; Coyne, B.J.; et al. Splanchnic Nerve Block for Chronic Heart Failure. JACC Heart Fail. 2020, 8, 742–752. [Google Scholar] [CrossRef]
- Fudim, M.; Engelman, Z.J.; Reddy, V.Y.; Shah, S.J. Splanchnic Nerve Ablation for Volume Management in Heart Failure. JACC Basic Transl. Sci. 2022, 7, 319–321. [Google Scholar] [CrossRef]
- Neri, M.; Villa, G.; Garzotto, F.; Bagshaw, S.; Bellomo, R.; Cerda, J.; Ferrari, F.; Guggia, S.; Joannidis, M.; Kellum, J.; et al. Nomenclature for renal replacement therapy in acute kidney injury: Basic principles. Crit. Care 2016, 20, 318. [Google Scholar] [CrossRef]
- Costanzo, M.R.; Ronco, C. Isolated ultrafiltration in heart failure patients. Curr. Cardiol. Rep. 2012, 14, 254–264. [Google Scholar] [CrossRef]
- Saunders, H.; Rehan, A.; Hashmi, M.F.; Sanghavi, D.K. Continuous Renal Replacement Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Gaudry, S.; Grolleau, F.; Barbar, S.; Martin-Lefevre, L.; Pons, B.; Boulet, É.; Boyer, A.; Chevrel, G.; Montini, F.; Bohe, J.; et al. Continuous renal replacement therapy versus intermittent hemodialysis as first modality for renal replacement therapy in severe acute kidney injury: A secondary analysis of AKIKI and IDEAL-ICU studies. Crit. Care 2022, 26, 93. [Google Scholar] [CrossRef]
- Al-Hesayen, A.; Parker, J.D. The effects of dobutamine on renal sympathetic activity in human heart failure. J. Cardiovasc. Pharmacol. 2008, 51, 434–436. [Google Scholar] [CrossRef]
- Kumar, N.; Reeves, J.; Hussain, N.; Essandoh, M. Dispelling Dogma: Milrinone Therapy Is Safe and Effective in Renally-Impaired Patients, and We Knew This! J. Cardiothorac. Vasc. Anesth. 2023, 37, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Zucker, I.H.; Xia, Z.; Wang, H.J. Potential Neuromodulation of the Cardio-Renal Syndrome. J. Clin. Med. 2023, 12, 803. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Lin, M.H.; Chen, C.L.; Lai, Y.C.; Chen, C.Y.; Lin, Y.C.; Hung, C.C. Comprehensive Comparison of the Effect of Inotropes on Cardiorenal Syndrome in Patients with Advanced Heart Failure: A Network Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 4120. [Google Scholar] [CrossRef]
- Available online: https://www.nutritioncare.org/Guidelines_and_Clinical_Resources/Enteral_Nutrition_Resources/ (accessed on 1 November 2024).
- Available online: https://trial2cure.com/trialsindex.php?study=reducing-fluid-overload-in-heart-failure-patients-using-a-non-invasive-renal-independent-system (accessed on 1 November 2024).
- Gnanaraj, J.F.; von Haehling, S.; Anker, S.D.; Raj, D.S.; Radhakrishnan, J. The relevance of congestion in the cardio-renal syndrome. Kidney Int. 2013, 83, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Zymliński, R.; Biegus, J.; Vanderheyden, M.; Gajewski, P.; Dierckx, R.; Bartunek, J.; Ponikowski, P. Safety, Feasibility of Controllable Decrease of Vena Cava Pressure by Doraya Catheter in Heart Failure. JACC Basic Transl. Sci. 2023, 8, 394–402. [Google Scholar] [CrossRef]
- Grafton, G.; Tita, C.; Heuring, J.J.; Fain, E.S.; Shah, P.; Cowger, J.A.; Alqarqaz, M.; Basir, M.B. Continuous-Flow Intra-Aortic Percutaneous Mechanical Circulatory Support in Heart Failure With Worsening Renal Function. Circ. Heart Fail. 2023, 16, e009842. [Google Scholar] [CrossRef]
- Cowger, J.A.; Basir, M.B.; Baran, D.A.; Hayward, C.S.; Rangaswami, J.; Walton, A.; Tita, C.; Minear, S.; Hakemi, E.; Klein, L.; et al. Safety and Performance of the Aortix Device in Acute Decompensated Heart Failure and Cardiorenal Syndrome. JACC Heart Fail. 2023, 11, 1565–1575. [Google Scholar] [CrossRef]
- Georges, G.; Trudeau, F.; Doucet-Martineau, J.; Rochon, M.; Potvin, J.; Ebner, A.; Généreux, P. First-in-Human Experience With the ModulHeart Device for Mechanical Circulatory Support and Renal Perfusion. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100449. [Google Scholar] [CrossRef]
- Kim, C.S. Pharmacologic Management of the Cardio-renal Syndrome. Electrolyte Blood Press 2013, 11, 17–23. [Google Scholar] [CrossRef]
- Nathan, S.; Basir, M.B. Emerging Device Therapies for Cardiorenal Syndrome. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2 Pt B, 101210. [Google Scholar] [CrossRef]
- Ross, E.A. Congestive Renal Failure: The Pathophysiology and Treatment of Renal Venous Hypertension. J. Card. Fail. 2012, 18, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Ter Maaten, J.M.; Damman, K.; Verhaar, M.C.; Paulus, W.J.; Duncker, D.J.; Cheng, C.; van Heerebeek, L.; Hillege, H.L.; Lam, C.S.; Navis, G.; et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: The role of endothelial dysfunction and inflammation. Eur. J. Heart Fail. 2016, 18, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; Salah, H.; Sathananthan, J.; Bernier, M.; Pabon-Ramos, W.; Schwartz, R.; Rodés-Cabau, J.; Côté, F.; Khalifa, A.; Virani, S.; et al. Lymphatic Dysregulation in Patients With Heart Failure. J. Am. Coll. Cardiol. 2021, 78, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Maisel, A. Volume overload and cardiorenal syndromes. Congest. Heart Fail. 2010, 16 (Suppl. 1), Si–Siv; quiz Svi. [Google Scholar] [CrossRef]
- Kawada, T.; Matsushita, H.; Yokota, S.; Yoshida, Y.; Fukumitsu, M.; Alexander, J.; Saku, K. Short-term dynamic characteristics of diuresis during exogenous pressure perturbations with and without arterial baroreflex control. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2024, 326, R230–R241. [Google Scholar]
- Testani, J.M.; Coca, S.G.; McCauley, B.D.; Shannon, R.P.; Kimmel, S.E. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur. J. Heart Fail. 2011, 13, 877–884. [Google Scholar]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Sokos, G.; Taylor, D.O.; Starling, R.C.; Young, J.B.; Tang, W.H.W. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 2009, 53, 589–596. [Google Scholar]
- Rafouli-Stergiou, P.; Parissis, J.T.; Anastasiou-Nana, M. Inotropes for the management of acute heart failure patients with renal dysfunction. Still an option? Expert Opin. Pharmacother. 2012, 13, 2637–2647. [Google Scholar] [CrossRef]
- Testani, J.M.; Damman, K. Venous congestion and renal function in heart failure… it’s complicated. Eur. J. Heart Fail. 2013, 15, 599–601. [Google Scholar] [CrossRef]
- Boorsma, E.M.; Ter Maaten, J.M.; Damman, K.; Dinh, W.; Gustafsson, F.; Goldsmith, S.; Burkhoff, D.; Zannad, F.; Udelson, J.E.; Voors, A.A. Congestion in heart failure: A contemporary look at physiology, diagnosis and treatment. Nat. Rev. Cardiol. 2020, 17, 641–655. [Google Scholar] [CrossRef]
- Uthoff, H.; Breidthardt, T.; Klima, T.; Aschwanden, M.; Arenja, N.; Socrates, T.; Heinisch, C.; Noveanu, M.; Frischknecht, B.; Baumann, U.; et al. Central venous pressure and impaired renal function in patients with acute heart failure. Eur. J. Heart Fail. 2011, 13, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Tamayo-Gutierrez, A.; Ibrahim, H.N. The Kidney in Heart Failure: The Role of Venous Congestion. Methodist Debakey Cardiovasc. J. 2022, 18, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, S.M. Hemodynamic monitoring. Chest 2013, 143, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Zymliński, R.; Dierckx, R.; Biegus, J.; Vanderheyden, M.; Bartunek, J.; Ponikowski, P. Novel IVC Doraya Catheter Provides Congestion Relief in Patients With Acute Heart Failure. JACC Basic Transl. Sci. 2022, 7, 326–327. [Google Scholar] [CrossRef]
- Krum, H.; Iyngkaran, P.; Lekawanvijit, S. Pharmacologic management of the cardiorenal syndrome in heart failure. Curr. Heart Fail. Rep. 2009, 6, 105–111. [Google Scholar] [CrossRef]
- Rangaswami, J.; Mathew, R.O. Pathophysiological Mechanisms in Cardiorenal Syndrome. Adv. Chronic Kidney Dis. 2018, 25, 400–407. [Google Scholar] [CrossRef]
- Zannad, F.; Rossignol, P. Cardiorenal Syndrome Revisited. Circulation 2018, 138, 929–944. [Google Scholar] [CrossRef]
- Kosiorek, A.; Biegus, J.; Rozentryt, P.; Hurkacz, M.; Zymliński, R. Cardiorenal syndrome: Decongestion in heart failure across wide spectrum of kidney pathophysiology. Adv. Clin. Exp. Med. 2022, 31, 445–455. [Google Scholar] [CrossRef]
- Chandramohan, D.; Simhadri, P.K.; Jena, N.; Palleti, S.K. Strategies for the Management of Cardiorenal Syndrome in the Acute Hospital Setting. Hearts 2024, 5, 329–348. [Google Scholar] [CrossRef]
- Keeble, T.R.; Karamasis, G.V.; Rothman, M.T.; Ricksten, S.E.; Ferrari, M.; Hullin, R.; Scherstén, F.; Reitan, O.; Kirking, S.T.; Cleland, J.G.F.; et al. Percutaneous haemodynamic and renal support in patients presenting with decompensated heart failure: A multi-centre efficacy study using the Reitan Catheter Pump (RCP). Int. J. Cardiol. 2019, 275, 53–58. [Google Scholar] [CrossRef]
- Napp, L.C.; Mariani, S.; Ruhparwar, A.; Schmack, B.; Keeble, T.R.; Reitan, O.; Hanke, J.S.; Dogan, G.; Hiss, M.; Bauersachs, J.; et al. First-in-Man Use of the Percutaneous 10F Reitan Catheter Pump for Cardiorenal Syndrome. Asaio. J. 2022, 68, e99–e101. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Cunningham, M.; Richardson, A.; Burton, B. Wireless Powered and Water-Proof Mechanical Circulatory Support Device for Ambulatory Class III Heart Failure. JACC Basic Transl. Sci. 2022, 7, 328–329. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.W.M.; Song, L.; Tam, K.; Roth Flach, R.J. Targeting lymphatic function in cardiovascular-kidney-metabolic syndrome: Preclinical methods to analyze lymphatic function and therapeutic opportunities. Front. Cardiovasc. Med. 2024, 11, 1412857. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Shimizu, Y.; Hayashi, T.; Che, Y.; Suzuki, J.; Tsuzuki, K.; Narita, S.; Shibata, R.; Calvert, J.W.; Murohara, T. Cardiac Lymphatic Insufficiency Leads to Diastolic Dysfunction Via Myocardial Morphologic Change. JACC Basic Transl. Sci. 2023, 8, 958–972. [Google Scholar] [CrossRef]
- Abraham, W.T.; Jonas, M.; Dongaonkar, R.M.; Geist, B.; Ueyama, Y.; Render, K.; Youngblood, B.; Muir, W.; Hamlin, R.; Del Rio, C.L. Direct Interstitial Decongestion in an Animal Model of Acute-on-Chronic Ischemic Heart Failure. JACC Basic Transl. Sci. 2021, 6, 872–881. [Google Scholar] [CrossRef]
- Zhong, J.; Kirabo, A.; Yang, H.C.; Fogo, A.B.; Shelton, E.L.; Kon, V. Intestinal Lymphatic Dysfunction in Kidney Disease. Circ. Res. 2023, 132, 1226–1245. [Google Scholar] [CrossRef]
- Martens, P.; Burkhoff, D.; Cowger, J.A.; Jorde, U.P.; Kapur, N.K.; Tang, W.H.W. Emerging Individualized Approaches in the Management of Acute Cardiorenal Syndrome With Renal Assist Devices. JACC Heart Fail. 2023, 11, 1289–1303. [Google Scholar] [CrossRef]
- Available online: https://whiteswell.com/whiteswell-announces-six-month-outcomes-after-elym-system-treatment-for-acute-decompensated-heart-failure-patients-in-its-delta-hf-study/ (accessed on 15 November 2014).
- Aronson, D.; Nitzan, Y.; Petcherski, S.; Bravo, E.; Habib, M.; Burkhoff, D.; Abraham, W.T. Enhancing Sweat Rate Using a Novel Device for the Treatment of Congestion in Heart Failure. Circ. Heart Fail. 2023, 16, e009787. [Google Scholar] [CrossRef]
- Biegus, J.; Zymlinski, R.; Siwolowski, P.; Testani, J.; Szachniewicz, J.; Tycińska, A.; Banasiak, W.; Halpert, A.; Levin, H.; Ponikowski, P. Controlled decongestion by Reprieve therapy in acute heart failure: Results of the TARGET-1 and TARGET-2 studies. Eur. J. Heart Fail. 2019, 21, 1079–1087. [Google Scholar] [CrossRef]
- Rao, V.S.; Ivey-Miranda, J.B.; Cox, Z.L.; Moreno-Villagomez, J.; Ramos-Mastache, D.; Neville, D.; Balkcom, N.; Asher, J.L.; Bellumkonda, L.; Bigvava, T.; et al. Serial direct sodium removal in patients with heart failure and diuretic resistance. Eur. J. Heart Fail. 2024, 26, 1215–1230. [Google Scholar] [CrossRef]
- Assavapokee, T.; Rola, P.; Assavapokee, N.; Koratala, A. Decoding VExUS: A practical guide for excelling in point-of-care ultrasound assessment of venous congestion. Ultrasound J. 2024, 16, 48. [Google Scholar] [CrossRef]
- Gupta, B.; Ahluwalia, P.; Gupta, A.; Ranjan, N.; Kakkar, K.; Aneja, P. Utility of VExUS score in the peri-operative care unit, intensive care unit, and emergency setting—A systematic review. Indian J. Anaesth. 2023, 67 (Suppl. 4), S218–S226. [Google Scholar]


| Category | Mechanism | Examples |
|---|---|---|
| Decongestive (D) or Dilators (D) | Reduce fluid overload and congestion Improve blood flow, reduce vascular resistance | Loop diuretics (furosemide), aquapheresis Splanchnic nerve blockade |
| Renal Replacement (R) | Provides artificial renal function | CRRT, intermittent hemodialysis |
| Inotropes (I) | Enhance cardiac contractility | Dobutamine, Levosimendan |
| Interstitial (Fluid Management) (I) | Manages interstitial fluid | WhiteSwell eLym, AquaPASS |
| Pullers (P) | Reduce venous congestion | Doraya catheter, preCARDIA system |
| Pushers (P) | Enhance arterial flow and renal perfusion | Aortix, ModulHeart |
| Sympatholytics (S) | Inhibit SNS activity to lower vascular resistance | Beta-blockers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meche, V.; Kundnani, N.R.; Sharma, A.; Căpăstraru, F.-M.; Nistor, D.; Sarau, C.A.; Gaita, L. Cardio-Renal Syndrome: Latest Developments in Device-Based Therapy. J. Clin. Med. 2024, 13, 7814. https://doi.org/10.3390/jcm13247814
Meche V, Kundnani NR, Sharma A, Căpăstraru F-M, Nistor D, Sarau CA, Gaita L. Cardio-Renal Syndrome: Latest Developments in Device-Based Therapy. Journal of Clinical Medicine. 2024; 13(24):7814. https://doi.org/10.3390/jcm13247814
Chicago/Turabian StyleMeche, Vlad, Nilima Rajpal Kundnani, Abhinav Sharma, Flavia-Maria Căpăstraru, Daciana Nistor, Cristian Andrei Sarau, and Laura Gaita. 2024. "Cardio-Renal Syndrome: Latest Developments in Device-Based Therapy" Journal of Clinical Medicine 13, no. 24: 7814. https://doi.org/10.3390/jcm13247814
APA StyleMeche, V., Kundnani, N. R., Sharma, A., Căpăstraru, F.-M., Nistor, D., Sarau, C. A., & Gaita, L. (2024). Cardio-Renal Syndrome: Latest Developments in Device-Based Therapy. Journal of Clinical Medicine, 13(24), 7814. https://doi.org/10.3390/jcm13247814






