Embolization of De Novo Pulmonary Arteriovenous Malformations Using High-Volume Detachable Non-Fibered Coils: Propensity-Matched Comparison to Traditional Coils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Treatment Groups
2.2. Additional Variables
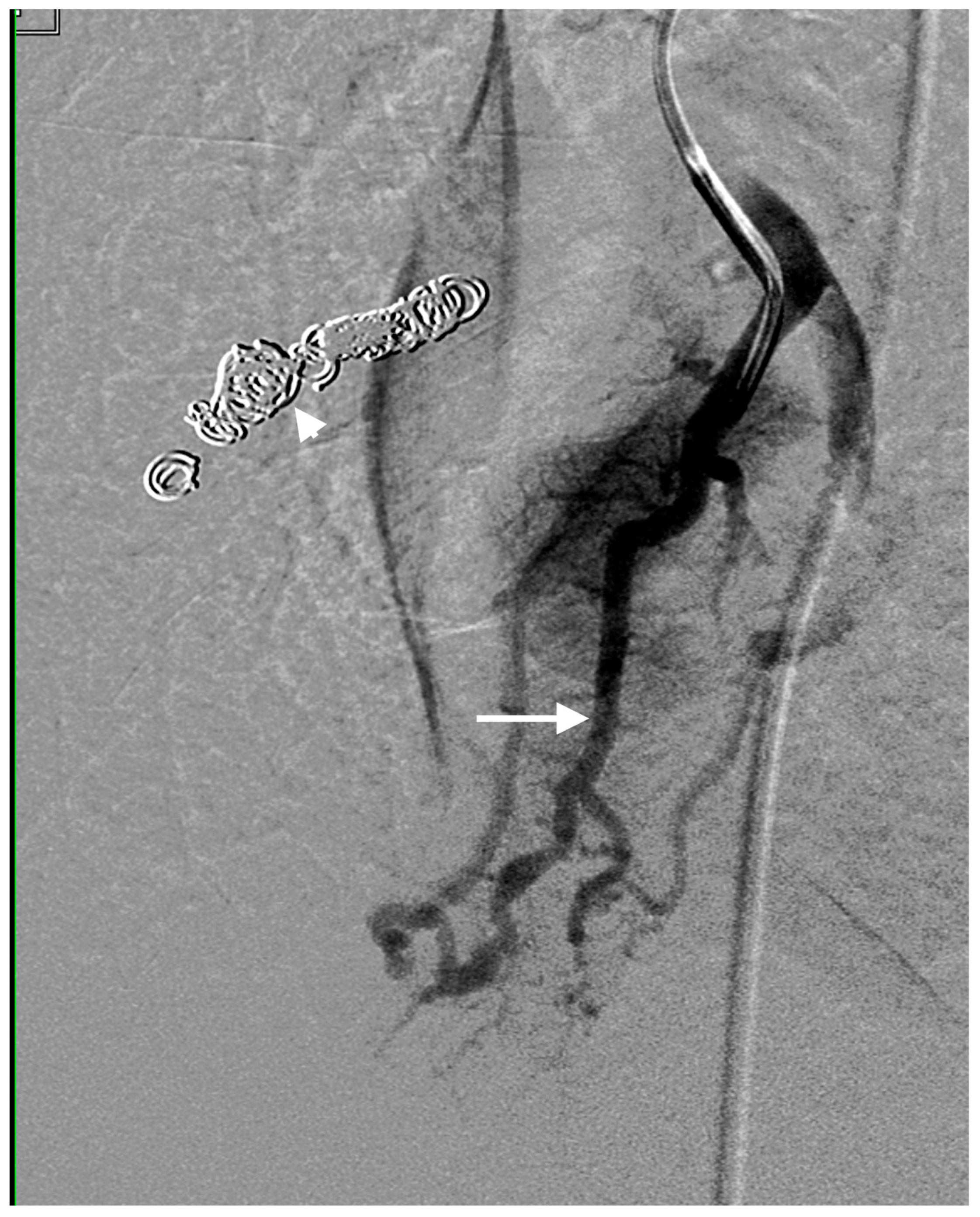
2.3. Technique
2.4. Statistical Analysis
3. Results
3.1. Persistent-Occlusion Rates
3.2. Embolic Quantity and Cost Analysis
3.3. Propensity Matching
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gossage, J.R.; Kanj, G. Pulmonary Arteriovenous Malformations: A State of the Art Review. Am. J. Respir. Crit. Care Med. 1998, 158, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Shovlin, C.L. Pulmonary Arteriovenous Malformations. Am. J. Respir. Crit. Care Med. 2014, 190, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; McWilliams, J.P. Approach to Pulmonary Arteriovenous Malformations: A Comprehensive Update. J. Clin. Med. 2020, 9, 1927. [Google Scholar] [CrossRef] [PubMed]
- Faughnan, M.E.; Palda, V.A.; Garcia-Tsao, G.; Geisthoff, U.W.; McDonald, J.; Proctor, D.D.; Spears, J.; Brown, D.H.; Buscarini, E.; Chesnutt, M.S.; et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J. Med. Genet. 2011, 48, 73–87. [Google Scholar] [CrossRef]
- Cottin, V.; Plauchu, H.; Bayle, J.Y.; Barthelet, M.; Revel, D.; Cordier, J.F. Pulmonary Arteriovenous Malformations in Patients with Hereditary Hemorrhagic Telangiectasia. Am. J. Respir. Crit. Care Med. 2004, 169, 994–1000. [Google Scholar] [CrossRef]
- Haitjema, T.; Disch, F.; Overtoom, T.T.C.; Westermann, C.J.J.; Lammers, J.W.J. Screening family members of patients with hereditary hemorrhagic telangiectasia. Am. J. Med. 1995, 99, 519–524. [Google Scholar] [CrossRef]
- Mager, J.J.; Overtoom, T.T.C.; Blauw, H.; Lammers, J.W.J.; Westermann, C.J.J. Embolotherapy of Pulmonary Arteriovenous Malformations: Long-term Results in 112 Patients. J. Vasc. Interv. Radiol. 2004, 15, 451–456. [Google Scholar] [CrossRef]
- Remy-Jardin, M.; Dumont, P.; Brillet, P.Y.; Dupuis, P.; Duhamel, A.; Remy, J. Pulmonary Arteriovenous Malformations Treated with Embolotherapy: Helical CT Evaluation of Long-term Effectiveness after 2–21-Year Follow-up. Radiology 2006, 239, 576–585. [Google Scholar] [CrossRef]
- Narsinh, K.H.; Ramaswamy, R.; Kinney, T.B. Management of Pulmonary Arteriovenous Malformations in Hereditary Hemorrhagic Telangiectasia Patients. Semin. Interv. Radiol. 2013, 30, 408–412. [Google Scholar] [CrossRef]
- Makimoto, S.; Hiraki, T.; Gobara, H.; Fujiwara, H.; Iguchi, T.; Matsui, Y.; Mimura, H.; Kanazawa, S. Association between reperfusion and shrinkage percentage of the aneurysmal sac after embolization of pulmonary arteriovenous malformation: Evaluation based on contrast-enhanced thin-section CT images. Jpn. J. Radiol. 2014, 32, 266–273. [Google Scholar] [CrossRef]
- Pollak, J.S.; Saluja, S.; Thabet, A.; Henderson, K.J.; Denbow, N.; White, R.I. Clinical and Anatomic Outcomes after Embolotherapy of Pulmonary Arteriovenous Malformations. J. Vasc. Interv. Radiol. 2006, 17, 35–45. [Google Scholar] [CrossRef]
- Haitjema, T.J.; Overtoom, T.T.; Westermann, C.J.; Lammers, J.W. Embolisation of pulmonary arteriovenous malformations: Results and follow up in 32 patients. Thorax 1995, 50, 719–723. [Google Scholar] [CrossRef]
- Lee, D.W.; White, R.I.; Egglin, T.K.; Pollak, J.S.; Fayad, P.B.; Wirth, J.A.; Rosenblatt, M.M.; Dickey, K.W.; Burdge, C.M. Embolotherapy of Large Pulmonary Arteriovenous Malformations: Long-Term Results. Ann. Thorac. Surg. 1997, 64, 930–940. [Google Scholar] [CrossRef]
- Prasad, V.; Chan, R.P.; Faughnan, M.E. Embolotherapy of Pulmonary Arteriovenous Malformations: Efficacy of Platinum versus Stainless Steel Coils. J. Vasc. Interv. Radiol. 2004, 15, 153–160. [Google Scholar] [CrossRef]
- Milic, A.; Chan, R.P.; Cohen, J.H.; Faughnan, M.E. Reperfusion of Pulmonary Arteriovenous Malformations after Embolotherapy. J. Vasc. Interv. Radiol. 2005, 16, 1675–1683. [Google Scholar] [CrossRef]
- Shimohira, M.; Kawai, T.; Hashizume, T.; Ohta, K.; Nakagawa, M.; Ozawa, Y.; Sakurai, K.; Shibamoto, Y. Reperfusion Rates of Pulmonary Arteriovenous Malformations after Coil Embolization: Evaluation with Time-Resolved MR Angiography or Pulmonary Angiography. J. Vasc. Interv. Radiol. 2015, 26, 856–864.e1. [Google Scholar] [CrossRef]
- Shimohira, M.; Kawai, T.; Hashizume, T.; Muto, M.; Kitase, M.; Shibamoto, Y. Usefulness of Hydrogel-Coated Coils in Embolization of Pulmonary Arteriovenous Malformations. Cardiovasc. Interv. Radiol. 2018, 41, 848–855. [Google Scholar] [CrossRef]
- Stein, E.J.; Chittams, J.L.; Miller, M.; Trerotola, S.O. Persistence in Coil-Embolized Pulmonary Arteriovenous Malformations with Feeding Artery Diameters of 3 mm or Less: A Retrospective Single-Center Observational Study. J. Vasc. Interv. Radiol. 2017, 28, 442–449. [Google Scholar] [CrossRef]
- Andersen, P.E.; Duvnjak, S.; Gerke, O.; Kjeldsen, A.D. Long-Term Single-Center Retrospective Follow-Up After Embolization of Pulmonary Arteriovenous Malformations Treated Over a 20-year Period: Frequency of Re-canalization with Various Embolization Materials and Clinical Outcome. Cardiovasc. Interv. Radiol. 2019, 42, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.A.; Faughnan, M.E.; Vozoris, N.T.; Prabhudesai, V. Reperfusion of Pulmonary Arteriovenous Malformations Following Embolotherapy: A Randomized Controlled Trial of Detachable Versus Pushable Coils. Cardiovasc. Interv. Radiol. 2020, 43, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, L.R.; Duckwiler, G.R.; Roberts, D.G.; McWilliams, J.P. Treatment of Recurrent Pulmonary Arteriovenous Malformations: Comparison of Proximal Versus Distal Embolization Technique. Cardiovasc. Interv. Radiol. 2020, 43, 29–36. [Google Scholar] [CrossRef]
- Trerotola, S.O.; Pyeritz, R.E. Does use of coils in addition to amplatzer vascular plugs prevent recanalization? AJR Am. J. Roentgenol. 2010, 195, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Tau, N.; Atar, E.; Mei-Zahav, M.; Bachar, G.N.; Dagan, T.; Birk, E.; Bruckheimer, E. Amplatzer Vascular Plugs Versus Coils for Embolization of Pulmonary Arteriovenous Malformations in Patients with Hereditary Hemorrhagic Telangiectasia. Cardiovasc. Interv. Radiol. 2016, 39, 1110–1114. [Google Scholar] [CrossRef]
- Adachi, A.; Ohta, K.; Jahangiri, Y.; Matsui, Y.; Horikawa, M.; Geeratikun, Y.; Chansanti, O.; Yata, S.; Fujii, S.; Steinberger, J.; et al. Treatment of pulmonary arteriovenous malformations: Clinical experience using different embolization strategies. Jpn. J. Radiol. 2020, 38, 382–386. [Google Scholar] [CrossRef]
- Iv, J.V.; Gemender, M.; Samoilov, D. Packing density and long-term occlusion after transcatheter vessel embolization with soft, bare-platinum detachable coils. Am. J. Interv. Radiol. 2020, 4, 2. [Google Scholar] [CrossRef]
- Yasumoto, T.; Osuga, K.; Yamamoto, H.; Ono, Y.; Masada, M.; Mikami, K.; Kanamori, D.; Nakamura, M.; Tanaka, K.; Nakazawa, T.; et al. Long-term outcomes of coil packing for visceral aneurysms: Correlation between packing density and incidence of coil compaction or recanalization. J. Vasc. Interv. Radiol. JVIR 2013, 24, 1798–1807. [Google Scholar] [CrossRef]
- Sluzewski, M.; van Rooij, W.J.; Slob, M.J.; Bescós, J.O.; Slump, C.H.; Wijnalda, D. Relation between Aneurysm Volume, Packing, and Compaction in 145 Cerebral Aneurysms Treated with Coils. Radiology 2004, 231, 653–658. [Google Scholar] [CrossRef]
- White, R.; Mitchell, S.; Barth, K.; Kaufman, S.; Kadir, S.; Chang, R.; Terry, P.; White, J.R.; Trerotola, S.O.; Pyeritz, R.E.; et al. Angioarchitecture of pulmonary arteriovenous malformations: An important consideration before embolotherapy. Am. J. Roentgenol. 1983, 140, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Baba, Y.; Senokuchi, T.; Nakajo, M. Efficacy of venous sac embolization for pulmonary arteriovenous malformations: Comparison with feeding artery embolization. J. Vasc. Interv. Radiol. JVIR 2012, 23, 1566–1577, quiz p. 1581. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Carter, R.E. Behind the Numbers: Propensity Score Analysis—A Primer for the Diagnostic Radiologist. Radiology 2013, 269, 640–645. [Google Scholar] [CrossRef]
- Roberts, D.G.; Sparks, H.D.; Cusumano, L.R.; Mathevosian, S.; Duckwiler, G.R.; McWilliams, J.P. Comparison of Feeding-Artery-Only versus Nidus-Plus-Feeding-Artery Embolization of Pulmonary Arteriovenous Malformations. J. Vasc. Interv. Radiol. JVIR 2021, 32, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Imai, K.; King, G.; Stuart, E.A. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Softw. 2011, 42, 1–28. [Google Scholar] [CrossRef]
- Kurth, T.; Walker, A.M.; Glynn, R.J.; Chan, K.A.; Gaziano, J.M.; Berger, K.; Robins, J.M. Results of Multivariable Logistic Regression, Propensity Matching, Propensity Adjustment, and Propensity-based Weighting under Conditions of Nonuniform Effect. Am. J. Epidemiol. 2006, 163, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, I.A.; van der Molen, A.J. Propensity Score Matching as a Substitute for Randomized Controlled Trials on Acute Kidney Injury After Contrast Media Administration: A Systematic Review. Am. J. Roentgenol. 2018, 211, 822–826. [Google Scholar] [CrossRef]
- Imbens, G.W. Nonparametric Estimation of Average Treatment Effects Under Exogeneity: A Review. Rev. Econ. Stat. 2004, 86, 4–29. [Google Scholar] [CrossRef]
- Austin, P.C.; Thomas, N.; Rubin, D.B. Covariate-adjusted survival analyses in propensity-score matched samples: Imputing potential time-to-event outcomes. Stat. Methods Med. Res. 2020, 29, 728–751. [Google Scholar] [CrossRef]
- Morgan, C.J. Reducing bias using propensity score matching. J. Nucl. Cardiol. 2018, 25, 404–406. [Google Scholar] [CrossRef]
- Bailey, C.R.; Arun, A.; Towsley, M.; Choi, W.K.; Betz, J.F.; MacKenzie, S.; Areda, M.A.; Duvvuri, M.; Mitchell, S.; Weiss, C.R. MVPTM Micro Vascular Plug Systems for the Treatment of Pulmonary Arteriovenous Malformations. Cardiovasc. Interv. Radiol. 2019, 42, 389–395. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, J.; Kim, Y.H.; Kang, U.R.; Cha, J.G.; Lee, J.; Cha, S.-I.; Kim, C.-H. Efficacy and Safety of AMPLATZER Vascular Plug Type IV for Embolization of Pulmonary Arteriovenous Malformations. J. Vasc. Interv. Radiol. 2019, 30, 1082–1088. [Google Scholar] [CrossRef]
- Hundt, W.; Kalinowski, M.; Kiessling, A.; Heverhagen, J.T.; Eivazi, B.; Werner, J.; Steinbach, S.; Klose, K.J.; Burbelko, M. Novel approach to complex pulmonary arteriovenous malformation embolization using detachable coils and Amplatzer vascular plugs. Eur. J. Radiol. 2012, 81, e732–e738. [Google Scholar] [CrossRef]
- Kucukay, F.; Özdemir, M.; Şenol, E.; Okten, S.; Ereren, M.; Karan, A. Large Pulmonary Arteriovenous Malformations: Long-Term Results of Embolization with AMPLATZER Vascular Plugs. J. Vasc. Interv. Radiol. 2014, 25, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Mahdjoub, E.; Tavolaro, S.; Parrot, A.; Cornelis, F.; Khalil, A.; Carette, M.F. Pulmonary Arteriovenous Malformations: Safety and Efficacy of Microvascular Plugs. Am. J. Roentgenol. 2018, 211, 1135–1143. [Google Scholar] [CrossRef] [PubMed]




| Embolic Brand | Coil Category | Manufacturer | Total | |
|---|---|---|---|---|
| Interlock | Detachable Fibered | Boston Scientific, Marlborough, Massachusetts, USA | 650 | |
| Interlock 018 | 529 | |||
| Interlock 018 soft | 50 | |||
| Interlock 035 | 71 | |||
| Axium | Detachable Non-Fibered | Medtronic, Minneapolis, MN, USA | 58 | |
| Tornado | Pushable Fibered | Cook Medical, Bloomington, IN, USA | 50 | |
| Ruby | High-Volume Detachable Non-Fibered (HVDNF) | Penumbra, Alameda, CA, USA | 209 | |
| Ruby Standard Coil | 61 | |||
| Mandrel diameter, range (3–14 mm) | ||||
| Coil length, range (12–60 cm) | ||||
| Ruby Soft Coil | 84 | |||
| Mandrel diameter, range (2–8 mm) | ||||
| Coil length, range (4–60 cm) | ||||
| Packing Coil | 62 | |||
| Coil length, range (15–60 cm) | ||||
| POD Coil (POD4, POD8) | 2 | |||
| Nester | Pushable Fibered | Cook Medical, Bloomington, IN, USA | 210 | |
| microNester | Pushable Fibered | Cook Medical, Bloomington, IN, USA | 132 | |
| Azur | Detachable Non-Fibered Hydrogel | Terumo Medical, Somerset, NJ, USA | 31 | |
| Concerto | Detachable Fibered | Medtronic, Minneapolis, MN, USA | 64 | |
| 1404 | ||||
| Embolic Group | Age * (Years) | Female * | HHT * | Follow-Up (Months) | Angioarchitecture | Fluoroscopy Time (Minutes) | Feeding-Artery Diameter (mm) | Embolic Quantity | Cost Analysis (Dollars) | Success | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Simple | Complex | Number of Coils | |||||||||
| Group 1: Non-HVDNF Coils (n = 192) | 43.3 ± 17.1 (10–87) | 73.2% | 85.4% | 30.7 ± 31.9 (0.3–122.4) | 67.7% (130/192) | 32.3% (62/192) | 48.6 | 3.2 ± 1.2 (2–11) | 5.7 ± 4.5 (1–36) | 3977 ± 3725 (164–27,937) | 81.8% (157/192) |
| Group 2: HVDNF Coils (n = 80) | 45.4 ± 15.8 (16–69) | 68.4% | 73.6% | 14.7 ± 13.4 (1.2–56.8) | 58.8% (47/80) | 41.3% (33/80) | 49.7 | 3.4 ± 1.3 (2–10) | 3.9 ± 3.6 (1–23) | 4775 ± 3553 (1540–20,152) | 96.3% (77/80) |
| TOTAL | 44.3 ± 16.5 (10–87) | 68.5% | 76.8% | 26.0 ± 28.7 (0.3–122.4) | 65.0% (177/272) | 35.0% (95/272) | 48.9 | 3.3 ± 1.3 (2–11) | 5.2 ± 4.4 (1–36) | 4212 ± 3687 (164–27,937) | 86.0% (234/272) |
| p-Value | p < 0.0001 | p > 0.05 | p = 0.17 | p < 0.0001 | p = 0.0012 | p = 0.0017 | |||||
| All Data | Matched Data | Treatment | Control | ||
|---|---|---|---|---|---|
| Std. Mean Difference (n = 265) | Std. Mean Difference (n = 160) | HVDNF (n = 80) | Non-HVDNF (n = 80) | p-Value | |
| Age (Years) | 0.075 | 0.030 | 45.8 (15.4) | 46.2 (16.9) | 0.73 |
| HHT | |||||
| Present | 0.24 | 0.035 | 68 (85.0%) | 69 (86.2%) | 0.82 |
| Absent | “ | “ | 12 (15.0%) | 11 (13.8%) | |
| FA Diameter (mm) | 0.17 | 0.14 | 3.4 (1.5) | 3.1 (1.1) | 0.63 |
| Angioarchitecture | |||||
| Complex | 0.18 | 0.0 | 33 (41.2%) | 33 (41.2%) | 1.0 |
| Simple | “ | “ | 47 (58.8%) | 47 (58.8%) | |
| Embolization Technique | |||||
| DFA | 0.34 | 0.0 | 33 (41.2%) | 33 (41.2%) | 1.0 |
| NFA | “ | “ | 47 (58.8%) | 47 (58.8%) | |
| Follow-Up (Months) | 1.25 | 0.06 | 14.0 (15.6) | 14.9 (13.5) | 0.13 |
| Predictors | Risk-Ratio Estimates | CI | p-Value |
|---|---|---|---|
| Age (Years) | 0.99 | (0.96–1.03) | 0.61 |
| HHT | |||
| Absent | ref | ||
| Present | 0.70 | (0.13–3.80) | 0.68 |
| FA Diameter (mm) | 1.4 | (0.79–2.45) | 0.25 |
| Angioarchitecture | |||
| Simple | ref | ||
| Complex | 3.95 | (1.19–13.17) | 0.025 * |
| Embolization Technique | |||
| DFA | ref | ||
| NFA | 0.4 | (0.12–1.37) | 0.14 |
| Coil Type | |||
| Non-HVDNF | ref | ||
| HVDNF | 0.19 | (0.05–0.78) | 0.021 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathevosian, S.; Sparks, H.D.; Cusumano, L.R.; Roberts, D.G.; Majumdar, S.; McWilliams, J.P. Embolization of De Novo Pulmonary Arteriovenous Malformations Using High-Volume Detachable Non-Fibered Coils: Propensity-Matched Comparison to Traditional Coils. J. Clin. Med. 2024, 13, 648. https://doi.org/10.3390/jcm13030648
Mathevosian S, Sparks HD, Cusumano LR, Roberts DG, Majumdar S, McWilliams JP. Embolization of De Novo Pulmonary Arteriovenous Malformations Using High-Volume Detachable Non-Fibered Coils: Propensity-Matched Comparison to Traditional Coils. Journal of Clinical Medicine. 2024; 13(3):648. https://doi.org/10.3390/jcm13030648
Chicago/Turabian StyleMathevosian, Sipan, Hiro D. Sparks, Lucas R. Cusumano, Dustin G. Roberts, Shamaita Majumdar, and Justin P. McWilliams. 2024. "Embolization of De Novo Pulmonary Arteriovenous Malformations Using High-Volume Detachable Non-Fibered Coils: Propensity-Matched Comparison to Traditional Coils" Journal of Clinical Medicine 13, no. 3: 648. https://doi.org/10.3390/jcm13030648





