Differences in the Incidence of Hypotension and Hypertension between Sexes during Non-Cardiac Surgery: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility
2.3. Outcome
2.4. Data Extraction
2.5. Statistical Analysis
2.6. Sensitivity Analyses
- Using the least severe threshold of IOH when multiple thresholds for IOH were reported in one study, as relying exclusively on the most severe threshold of IOH could lead to the omission of numerous cases of milder hypotension;
- Using only studies that were classified as generalizable based on the NOS scale (question S1, representativeness of the exposed cohort) to assess the possible influence of the generalizability of the study population on the overall meta-analysis.
2.7. Subgroup Analyses
- Analyzing the studies with a mean or median age of ≥65 years and those with a mean or median age of <65 years, since BP differences between the sexes vary through age [3] and an increase in BP is observed in post-menopausal women [23]. Even though substantial overlap in age may be present between the relatively older and younger subgroups, we accepted this limitation due to biological plausibility of a potential difference in BP combined with the exploratory nature of the present study;
- Analyzing the studies only reporting absolute thresholds (e.g., MAP < 65 mmHg for IOH), only reporting relative thresholds (e.g., >30% reduction in MAP for IOH), and studies reporting a combination of absolute and relative thresholds, with the rationale that the baseline difference in blood pressure between sexes is accounted for in relative thresholds, thus obscuring potential sex-differences.
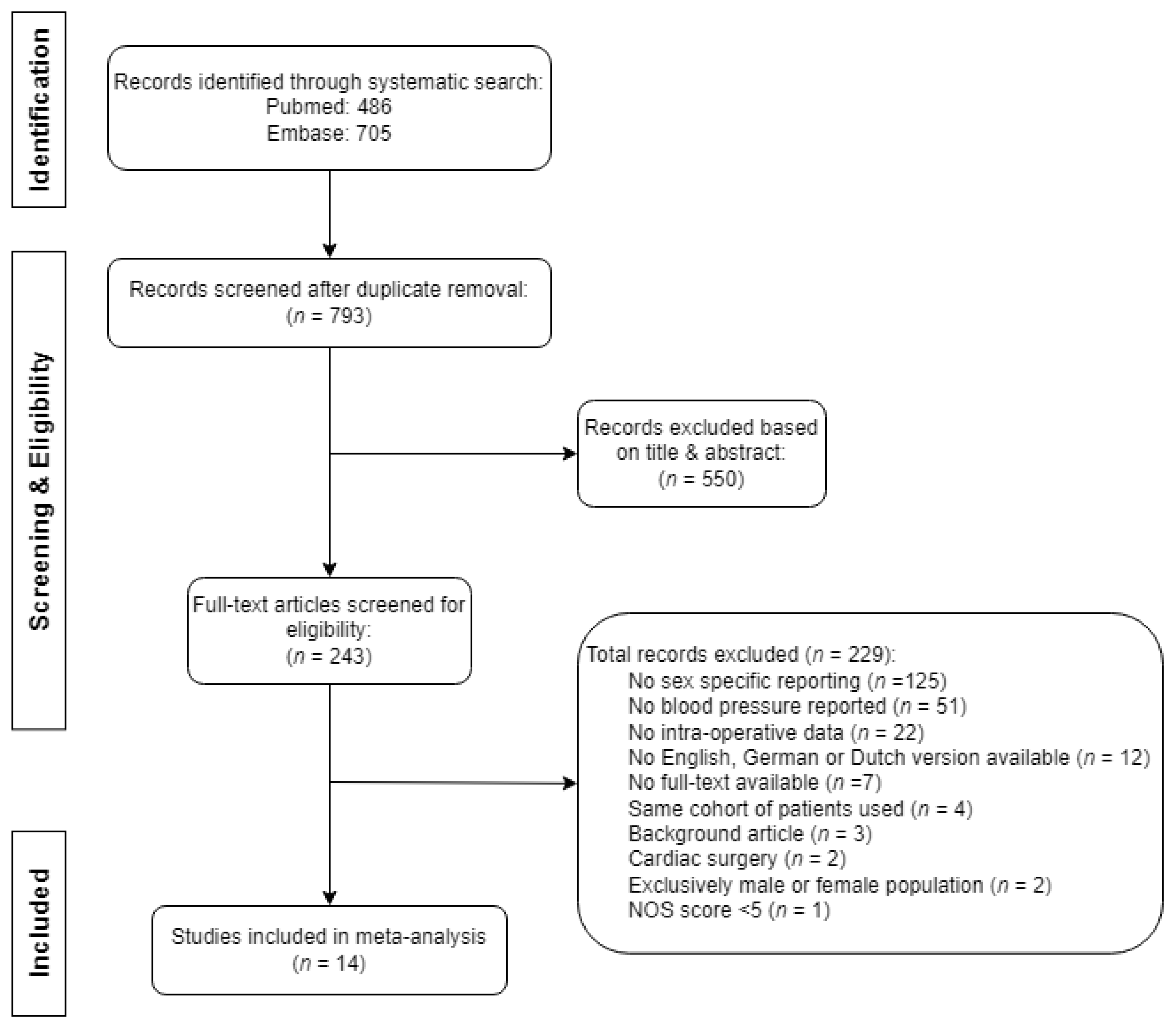
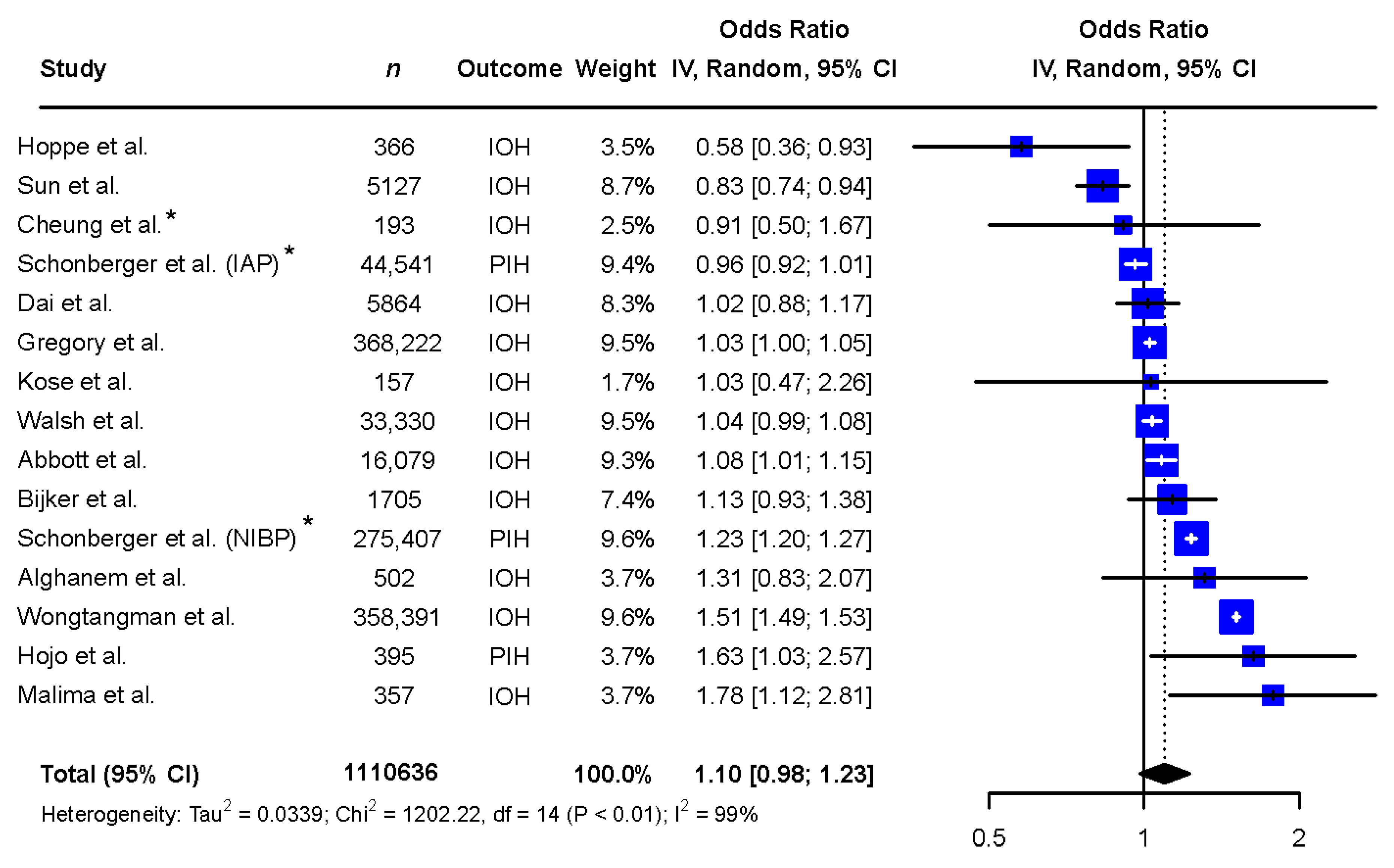
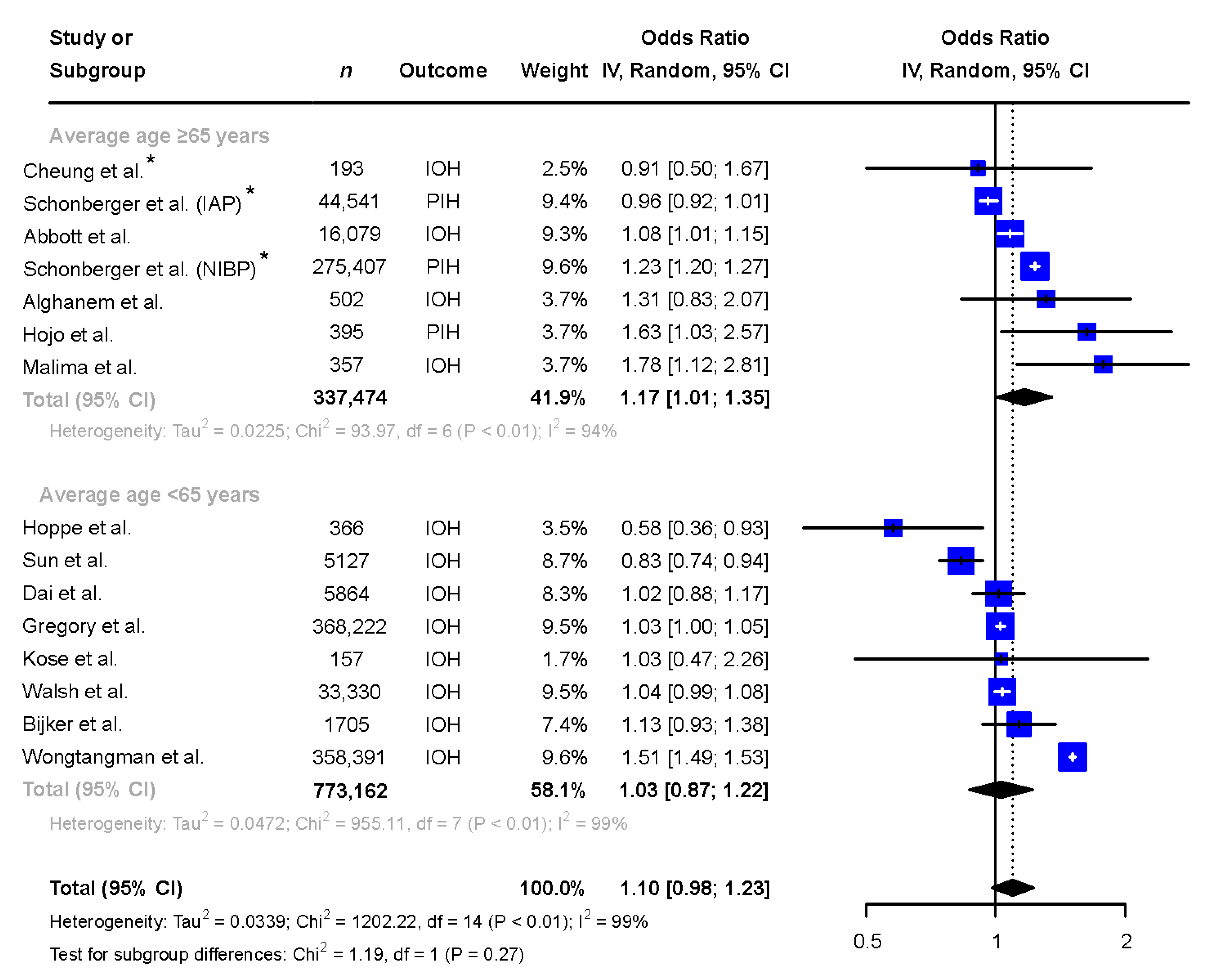
3. Results
3.1. Meta-Analysis
3.2. Sensitivity Analyses
3.3. Subgroup Analyses
3.4. Secondary Outcomes
4. Discussion
4.1. Limitations
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| aOR | Adjusted odds ratio |
| BP | Blood pressure |
| DBP | Diastolic blood pressure |
| IOH | Intraoperative hypotension |
| MAP | Mean arterial pressure |
| NOS | Newcastle–Ottawa scale |
| OR | Odds ratio |
| PIH | Post induction hypotension |
| SBP | Systolic blood pressure |
References
- Garcia, M.; Mulvagh, S.L.; Bairey Merz, C.N.; Buring, J.E.; Manson, J.E. Cardiovascular disease in women: Clinical perspectives. Circ. Res. 2016, 118, 1273–1293. [Google Scholar] [CrossRef] [PubMed]
- Gerdts, E.; Sudano, I.; Brouwers, S.; Borghi, C.; Bruno, R.M.; Ceconi, C.; Cornelissen, V.; Diévart, F.; Ferrini, M.; Kahan, T.; et al. Sex differences in arterial hypertension: A scientific statement from the ESC Council on Hypertension, the European Association of Preventive Cardiology, Association of Cardiovascular Nursing and Allied Professions, the ESC Council for Cardiology Practice, and the ESC Working Group on Cardiovascular Pharmacotherapy. Eur. Heart J. 2022, 43, 4777–4788. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Kim, A.; Ebinger, J.E.; Niiranen, T.J.; Claggett, B.L.; Bairey Merz, C.N.; Cheng, S. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol. 2020, 5, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Wijnberge, M.; Schenk, J.; Bulle, E.; Vlaar, A.P.; Maheshwari, K.; Hollmann, M.W.; Binnekade, J.M.; Geerts, B.F.; Veelo, D.P. Association of intraoperative hypotension with postoperative morbidity and mortality: Systematic review and meta-analysis. BJS Open 2021, 5, zraa018. [Google Scholar] [CrossRef] [PubMed]
- Bijker, J.B.; Persoon, S.; Peelen, L.M.; Moons, K.G.; Kalkman, C.J.; Kappelle, L.J.; Van Klei, W.A. Intraoperative hypotension and perioperative ischemic stroke after general surgery: A nested case-control study. J. Am. Soc. Anesthesiol. 2012, 116, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Bijker, J.B.; Van Klei, W.A.; Vergouwe, Y.; Eleveld, D.J.; Van Wolfswinkel, L.; Moons, K.G.; Kalkman, C.J. Intraoperative hypotension and 1-year mortality after noncardiac surgery. J. Am. Soc. Anesthesiol. 2009, 111, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Sessler, D.I.; Khanna, A.K. Perioperative myocardial injury and the contribution of hypotension. Intensive Care Med. 2018, 44, 811–822. [Google Scholar] [CrossRef]
- Maheshwari, K.; Turan, A.; Mao, G.; Yang, D.; Niazi, A.K.; Agarwal, D.; Sessler, D.I.; Kurz, A. The association of hypotension during non-cardiac surgery, before and after skin incision, with postoperative acute kidney injury: A retrospective cohort analysis. Anaesthesia 2018, 73, 1223–1228. [Google Scholar] [CrossRef]
- Gregory, A.; Stapelfeldt, W.H.; Khanna, A.K.; Smischney, N.J.; Boero, I.J.; Chen, Q.; Stevens, M.; Shaw, A.D. Intraoperative hypotension is associated with adverse clinical outcomes after noncardiac surgery. Anesth. Analg. 2021, 132, 1654–1665. [Google Scholar] [CrossRef]
- Malima, Z.; Torborg, A.; Cronjé, L.; Biccard, B. Predictors of post-spinal hypotension in elderly patients; a prospective observational study in the Durban Metropole. South. Afr. J. Anaesth. Analg. 2019, 25, 13–17. [Google Scholar] [CrossRef]
- Hojo, T.; Kimura, Y.; Shibuya, M.; Fujisawa, T. Predictors of hypotension during anesthesia induction in patients with hypertension on medication: A retrospective observational study. BMC Anesthesiol. 2022, 22, 343. [Google Scholar] [CrossRef] [PubMed]
- Schonberger, R.B.; Dai, F.; Michel, G.; Vaughn, M.T.; Burg, M.M.; Mathis, M.; Kheterpal, S.; Akhtar, S.; Shah, N.; Bardia, A. Association of propofol induction dose and severe pre-incision hypotension among surgical patients over age 65. J. Clin. Anesth. 2022, 80, 110846. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, P.; Burfeindt, C.; Reese, P.C.; Briesenick, L.; Flick, M.; Kouz, K.; Pinnschmidt, H.; Hapfelmeier, A.; Sessler, D.I.; Saugel, B. Chronic arterial hypertension and nocturnal non-dipping predict postinduction and intraoperative hypotension: A secondary analysis of a prospective study. J. Clin. Anesth. 2022, 79, 110715. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Wijeysundera, D.N.; Tait, G.A.; Beattie, W.S. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology 2015, 123, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Li, X.; Yang, Y.; Cao, Y.; Wang, E.; Dong, Z. A retrospective cohort analysis for the risk factors of intraoperative hypotension. Int. J. Clin. Pract. 2020, 74, e13521. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses; University of Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2000; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 15 September 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wesselink, E.M.; Kappen, T.H.; Torn, H.M.; Slooter, A.J.C.; van Klei, W.A. Intraoperative hypotension and the risk of postoperative adverse outcomes: A systematic review. Br. J. Anaesth. 2018, 121, 706–721. [Google Scholar] [CrossRef]
- Harbord, R.M.; Egger, M.; Sterne, J.A. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006, 25, 3443–3457. [Google Scholar] [CrossRef]
- Samargandy, S.; Matthews, K.A.; Brooks, M.M.; Barinas-Mitchell, E.; Magnani, J.W.; Thurston, R.C.; Khoudary, S.R.E. Trajectories of Blood Pressure in Midlife Women: Does Menopause Matter? Circ. Res. 2022, 130, 312–322. [Google Scholar] [CrossRef]
- RuDusky, B.M. Perioperative Sublingual Isosorbide Dinitrate for the Prevention of Cardiac Complications in Cardiac Patients Undergoing Noncardiac Surgery. Angiology 2005, 56, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.; Martyn, A.; Campbell, N.; Frost, S.; Gilbert, K.; Michota, F.; Seal, D.; Ghali, W.; Khan, N.A. Predictors of intraoperative hypotension and bradycardia. Am. J. Med. 2015, 128, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Abbott, T.E.; Pearse, R.M.; Archbold, R.A.; Ahmad, T.; Niebrzegowska, E.; Wragg, A.; Rodseth, R.N.; Devereaux, P.J.; Ackland, G.L. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: Results of the VISION study. Anesth. Analg. 2018, 126, 1936. [Google Scholar] [CrossRef] [PubMed]
- Alghanem, S.M.; Massad, I.M.; Almustafa, M.M.; Al-Shwiat, L.H.; El-Masri, M.K.; Samarah, O.Q.; Khalil, O.A.; Ahmad, M. Relationship between intra-operative hypotension and post-operative complications in traumatic hip surgery. Indian J. Anaesth. 2020, 64, 18. [Google Scholar] [PubMed]
- Kose, E.A.; Kabul, H.K.; Yildirim, V.; Tulmac, M. Preoperative exercise heart rate recovery predicts intraoperative hypotension in patients undergoing noncardiac surgery. J. Clin. Anesth. 2012, 24, 471–476. [Google Scholar] [CrossRef]
- Walsh, M.; Devereaux, P.J.; Garg, A.X.; Kurz, A.; Turan, A.; Rodseth, R.N.; Cywinski, J.; Thabane, L.; Sessler, D.I. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: Toward an empirical definition of hypotension. Anesthesiology 2013, 119, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Wongtangman, K.; Wachtendorf, L.J.; Blank, M.; Grabitz, S.D.; Linhardt, F.C.; Azimaraghi, O.; Raub, D.; Pham, S.; Kendale, S.M.; Low, Y.H. Effect of intraoperative arterial hypotension on the risk of perioperative stroke after noncardiac surgery: A retrospective multicenter cohort study. Anesth. Analg. 2021, 133, 1000–1008. [Google Scholar] [CrossRef]
- Mansukhani, N.A.; Yoon, D.Y.; Teter, K.A.; Stubbs, V.C.; Helenowski, I.B.; Woodruff, T.K.; Kibbe, M.R. Determining If Sex Bias Exists in Human Surgical Clinical Research. JAMA Surg. 2016, 151, 1022–1030. [Google Scholar] [CrossRef]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Merz, C.N.B.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.; Maas, A.H. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
- Gaudino, M.; Di Franco, A.; Alexander, J.H.; Bakaeen, F.; Egorova, N.; Kurlansky, P.; Boening, A.; Chikwe, J.; Demetres, M.; Devereaux, P.J.; et al. Sex differences in outcomes after coronary artery bypass grafting: A pooled analysis of individual patient data. Eur. Heart J. 2021, 43, 18–28. [Google Scholar] [CrossRef]
- Dixon, L.K.; Di Tommaso, E.; Dimagli, A.; Sinha, S.; Sandhu, M.; Benedetto, U.; Angelini, G.D. Impact of sex on outcomes after cardiac surgery: A systematic review and meta-analysis. Int. J. Cardiol. 2021, 343, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Oreel, T.H.; Nieuwkerk, P.T.; Hartog, I.D.; Netjes, J.E.; Vonk, A.B.A.; Lemkes, J.; van Laarhoven, H.W.M.; Scherer-Rath, M.; Sprangers, M.A.G.; Henriques, J.P.S. Gender differences in quality of life in coronary artery disease patients with comorbidities undergoing coronary revascularization. PLoS ONE 2020, 15, e0234543. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, H.; Jessup, J.A.; Lindsey, S.H.; Chappell, M.C.; Groban, L. Role of estrogen in diastolic dysfunction. Am. J. Physiol.-Heart Circ. Physiol. 2014, 306, H628–H640. [Google Scholar] [CrossRef]
- Kimura, M.; Sekiguchi, H.; Shimamoto, K.; Kawana, M.; Takemura, Y.; Hagiwara, N.; Yamaguchi, J. High-normal diastolic blood pressure as a risk factor for left ventricular diastolic dysfunction in healthy postmenopausal women. Hypertens. Res. 2022, 45, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Osude, N.; Durazo-Arvizu, R.; Markossian, T.; Liu, K.; Michos, E.D.; Rakotz, M.; Wozniak, G.; Egan, B.; Kramer, H. Age and sex disparities in hypertension control: The multi-ethnic study of atherosclerosis (MESA). Am. J. Prev. Cardiol. 2021, 8, 100230. [Google Scholar] [CrossRef] [PubMed]
- Wenger, N.K.; Arnold, A.; Bairey Merz, C.N.; Cooper-DeHoff, R.M.; Ferdinand, K.C.; Fleg, J.L.; Gulati, M.; Isiadinso, I.; Itchhaporia, D.; Light-McGroary, K. Hypertension across a woman’s life cycle. J. Am. Coll. Cardiol. 2018, 71, 1797–1813. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Niiranen, T.J.; Rader, F.; Henglin, M.; Kim, A.; Ebinger, J.E.; Claggett, B.; Merz, C.N.B.; Cheng, S. Sex differences in blood pressure associations with cardiovascular outcomes. Circulation 2021, 143, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Kringeland, E.; Tell, G.S.; Midtbø, H.; Igland, J.; Haugsgjerd, T.R.; Gerdts, E. Stage 1 hypertension, sex, and acute coronary syndromes during midlife: The Hordaland Health Study. Eur. J. Prev. Cardiol. 2022, 29, 147–154. [Google Scholar] [CrossRef]
- Crowther, M.; van der Spuy, K.; Roodt, F.; Nejthardt, M.B.; Davids, J.G.; Roos, J.; Cloete, E.; Pretorius, T.; Davies, G.L.; van der Walt, J.G.; et al. The relationship between pre-operative hypertension and intra-operative haemodynamic changes known to be associated with postoperative morbidity. Anaesthesia 2018, 73, 812–818. [Google Scholar] [CrossRef]
- Rook, W.; Yeung, J. Pre-operative hypertension and intra-operative hypotension. Anaesthesia 2018, 73, 1437–1438. [Google Scholar] [CrossRef]
- Naqvi, S.; Godfrey, A.K.; Hughes, J.F.; Goodheart, M.L.; Mitchell, R.N.; Page, D.C. Conservation, acquisition, and functional impact of sex-biased gene expression in mammals. Science 2019, 365, eaaw7317. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Wassertheurer, S.; O’Rourke, M.F.; Haiden, A.; Zweiker, R.; Rammer, M.; Hametner, B.; Eber, B. Pulsatile hemodynamics in patients with exertional dyspnea: Potentially of value in the diagnostic evaluation of suspected heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2013, 61, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Gelzinis, T.A. New insights into diastolic dysfunction and heart failure with preserved ejection fraction. Semin. Cardiothorac. Vasc. Anesth. 2014, 18, 208–217. [Google Scholar] [CrossRef]
- Sessler, D.I.; Bloomstone, J.A.; Aronson, S.; Berry, C.; Gan, T.J.; Kellum, J.A.; Plumb, J.; Mythen, M.G.; Grocott, M.P.W.; Edwards, M.R.; et al. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br. J. Anaesth. 2019, 122, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, E.; Tartler, T.M.; Suleiman, A.; Wachtendorf, L.J.; Ma, H.; Chen, G.; Kendale, S.M.; Kienbaum, P.; Subramaniam, B.; Wagner, S.; et al. Dose-dependent relationship between intra-procedural hypoxaemia or hypocapnia and postoperative delirium in older patients. Br. J. Anaesth. 2023, 130, e298–e306. [Google Scholar] [CrossRef]
- Salmasi, V.; Maheshwari, K.; Yang, D.; Mascha, E.J.; Singh, A.; Sessler, D.I.; Kurz, A. Relationship between Intraoperative Hypotension, Defined by Either Reduction from Baseline or Absolute Thresholds, and Acute Kidney and Myocardial Injury after Noncardiac Surgery: A Retrospective Cohort Analysis. Anesthesiology 2017, 126, 47–65. [Google Scholar] [CrossRef]
- Halvorsen, S.; Mehilli, J.; Cassese, S.; Hall, T.S.; Abdelhamid, M.; Barbato, E.; De Hert, S.; de Laval, I.; Geisler, T.; Hinterbuchner, L. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery: Developed by the task force for cardiovascular assessment and management of patients undergoing non-cardiac surgery of the European Society of Cardiology (ESC) Endorsed by the European Society of Anaesthesiology and Intensive Care (ESAIC). Eur. Heart J. 2022, 43, 3826–3924. [Google Scholar]

| Author | n | Female (%) | Age | Surgery type | Surgery Duration (in Min) | Anesthesia Type (%) | Other Selection Criteria | History of Arterial Hypertension | Method BP Measurement | Outcome | Hypo/Hypertension Threshold | Minimum Duration | Incidence Men | Incidence Women | Incidence Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbott, 2018 [26] | 16,079 | 8316 (51.7%) | 65 (11.9) | Noncardiac | NS | Regional and/or GA | ≥1 day hospital LOS | 8171 (50.8%) | NIBP | IOH | SBP < 100 mmHg | NS | 4702 (61%) | 5189 (62%) | 9891 (62%) |
| IOHT | SBP > 160 mmHg | NS | 2197 (28%) | 2557 (31%) | 4754 (30%) | ||||||||||
| Alghanem, 2020 [27] | 502 | 237 (47.2%) | 71.7 (14.2) | Unilateral femur fracture | NS | Spinal (74.3%) or GA (25.7%) | None | 291 (58.0%) | NIBP or IAP | IOH | ≥30% reduction in SBP | ≥10 min | 43 (16%) | 48 (20%) | 91 (18%) |
| Bijker, 2009 [6] | 1705 | 825 (48.4%) | 52 (15.8) | General or vascular surgery | 112 (IQR 73–163) | Neuraxial (11.8%) GA (71.9%) Combined (16.3%) | None | 380 (22.3%) | NIBP or IAP | IOH | SBP < 80 mmHg | ≥1 min | 324 (37%) | 328 (40%) | 652 (38%) |
| Cheung, 2015 [25] | 193 | 128 (66.3%) | 67.6 (11.3) | Noncardiac | NS | Neuraxial (23.3%) GA (69.4%) Combined (7.3%) | On loop diuretic on regular basis before surgery. Loop diuretic or placebo on day of surgery. | NS | NIBP or IAP | IOH | SBP < 90 mmHg OR > 35% decrease in MAP OR administration of vasopressors | ≥5 min * | NS | NS | 107 (55%) |
| Dai, 2020 [15] | 5864 | 3103 (52.9%) | 47.4 * | Noncardiac | NS | Neuraxial (6.9%) GA (92.2%) IA (4.7%) GA + NB (2.9%) GA + IA (0.2%) | None | 855 (14.5%) | NIBP or IAP | IOH | SBP < 90 mmHg OR > 20% decrease in MAP | ≥10 min | 435 (16%) | 496 (16%) | 931 (16%) |
| Gregory, 2021 [9] | 368,222 | 226,694 (62%) | 60.1 * | Noncardiac, non-cesarian | 30 (IQR 16–60) | Not specified | ≥1 day hospital LOS | 230,482 (63%) | NIBP or IAP | IOH | MAP < 75 mmHg | ≥1 measurement | 51,725 (37%) | 94,018 (41%) | 14,5743 (40%) |
| IOH | MAP < 65 mmHg | ≥1 measurement | 25,874 (18%) | 45,064 (20%) | 70,938 (19%) | ||||||||||
| IOH | MAP < 55 mmHg | ≥1 measurement | 10,402 (7%) | 17,071 (8%) | 27,473 (7%) | ||||||||||
| Hojo, 2022 [11] | 395 | 184 (47%) | 70 [61–78] | Oral/maxillofacial | NA | GA | Hypertensive patients, on medication | 395 (100%) | NIBP or IAP | PIH | MAP < 55 mmHg | ≥1 min | 87 (41%) | 101 (55%) | 188 (48%) |
| Hoppe, 2022 [13] | 366 | 195 (53%) | 52 [47–57] | Elective noncardiac | 51 (IQR 30–84) | GA | ASA class I or II, no DM, no CHF, no CKD | 87 (24.0%) | NIBP or IAP | PIH | ≥30% reduction in MAP compared to preoperative nighttime MAP | NS | 84 (49%) | 76 (39%) | 160 (44%) |
| IOH | ≥30% reduction in MAP compared to preoperative nighttime MAP | NS | 71 (42%) | 54 (28%) | 125 (34%) | ||||||||||
| Kose, 2012 [28] | 157 | 75 (47.8%) | 45.3 (10.8) | Elective noncardiac | 127.8 (SD 35.7) | GA | ASA class I or II, no betablockers/calcium channel blockers | 29 (18.5%) | Continuous non invasive BP | IOH | >30% reduction in MAP | NS | 16 (20%) | 15 (20%) | 31 (20%) |
| Malima, 2019 [10] | 357 | 212 (59.4%) | 67 (8.3) | Lower limb surgery | NS | Spinal | None | NS | NIBP | IOH | ≥25% reduction in SBP or SBP < 100 mmHg | NS | 68 (47%) | 132 (62%) | 200 (56%) |
| Schonberger, 2022 [12] | 275,470 | 142,672 (51.8%) | 72.8 (6.3) | Non-minor surgery | 192.1 (SD 110.3) | GA | GA using propofol | NS | NIBP | PIH | MAP < 55 mmHg | NS | NS | NS | 57,009 (20.7%) |
| 44,541 | 19,477 (43.7%) | 73.7 (6.6) | Non-minor surgery | 302.3 (SD 139.3) | GA | GA using propofol | NS | IAP | PIH | MAP < 55 mmHg | NS | NS | NS | 15,589 (35.0%) | |
| Sun, 2015 [14] | 5127 | 2708 (53%) | 61.3 (14.2) | Noncardiac, non-urologic | <2 h 509 (31.9%) 2–5 h 874 (54.7%) >5 h 214 (13.4%) | GA | ≥1 day hospital LOS, invasive BP monitoring and etCO2 available | 2437 (47.5%) | IAP | IOH | MAP < 65 mmHg | Various durations reported | 2281 (94%) | 2530 (93%) | 4811 (94%) |
| IOH | MAP < 60 mmHg | Various durations reported | 2097 (87%) | 2276 (84%) | 4373 (85%) | ||||||||||
| IOH | MAP < 55 mmHg | Various durations reported | 1716 (71%) | 1814 (67%) | 3530 (69%) | ||||||||||
| Walsh, 2013 [29] | 33,330 | 16,836 (50.5%) | 55.8 (15.5) | Noncardiac, non-urologic | NS | GA | ≥1 day hospital LOS, pre- and post-operative creatinine | NS | NIBP or IAP | IOH | MAP < 55 mmHg | ≥1 measurement | 7024 (43%) | 7317 (43%) | 14,341 (43%) |
| Wongtangman, 2021 [30] | 358,391 | 198,915 (55.5%) | 54 (16.6) | Noncardiac | NS | GA | None | 144,880 (40.4%) | NIBP or IAP | IOH | MAP < 55 mmHg | NS | 62,292 (39%) | 97,817 (49%) | 160,109 (45%) |
| Author | Year | Study Design | S1 | S2 | S3 | S4 | C | O1 | O2 | O3 | Total NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbott [26] | 2018 | Multicenter prospective cohort study | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 |
| Alghanem [27] | 2020 | Single center retrospective cohort study | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Bijker [6] | 2009 | Single center retrospective cohort study | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Cheung [25] | 2015 | Single center prospective cohort study | 0 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 6 |
| Dai [15] | 2020 | Cohort study using data of multicenter RCT | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Gregory [9] | 2021 | Single center retrospective cohort study | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Hojo [11] | 2022 | Multicenter retrospective cohort study | 0 | 1 | 1 | 0 | 2 | 1 | 0 | 1 | 6 |
| Hoppe [13] | 2022 | Prospective observational stugy | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Kose [28] | 2012 | Prospective observational study | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 5 |
| Malima [10] | 2019 | Single center retrospective cohort study | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Schonberger | 2022 | Multicenter retrospective cohort study | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Sun [14] | 2015 | Single center prospective cohort study | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Walsh [29] | 2013 | Single center retrospective cohort study | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 5 |
| Wongtangman [30] | 2021 | Single center prospective cohort study | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bos, E.M.E.; Tol, J.T.M.; de Boer, F.C.; Schenk, J.; Hermanns, H.; Eberl, S.; Veelo, D.P. Differences in the Incidence of Hypotension and Hypertension between Sexes during Non-Cardiac Surgery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 666. https://doi.org/10.3390/jcm13030666
Bos EME, Tol JTM, de Boer FC, Schenk J, Hermanns H, Eberl S, Veelo DP. Differences in the Incidence of Hypotension and Hypertension between Sexes during Non-Cardiac Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(3):666. https://doi.org/10.3390/jcm13030666
Chicago/Turabian StyleBos, Elke M. E., Johan T. M. Tol, Fabienne C. de Boer, Jimmy Schenk, Henning Hermanns, Susanne Eberl, and Denise P. Veelo. 2024. "Differences in the Incidence of Hypotension and Hypertension between Sexes during Non-Cardiac Surgery: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 3: 666. https://doi.org/10.3390/jcm13030666
APA StyleBos, E. M. E., Tol, J. T. M., de Boer, F. C., Schenk, J., Hermanns, H., Eberl, S., & Veelo, D. P. (2024). Differences in the Incidence of Hypotension and Hypertension between Sexes during Non-Cardiac Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(3), 666. https://doi.org/10.3390/jcm13030666






