Comorbidities in COPD: Current and Future Treatment Challenges
Abstract
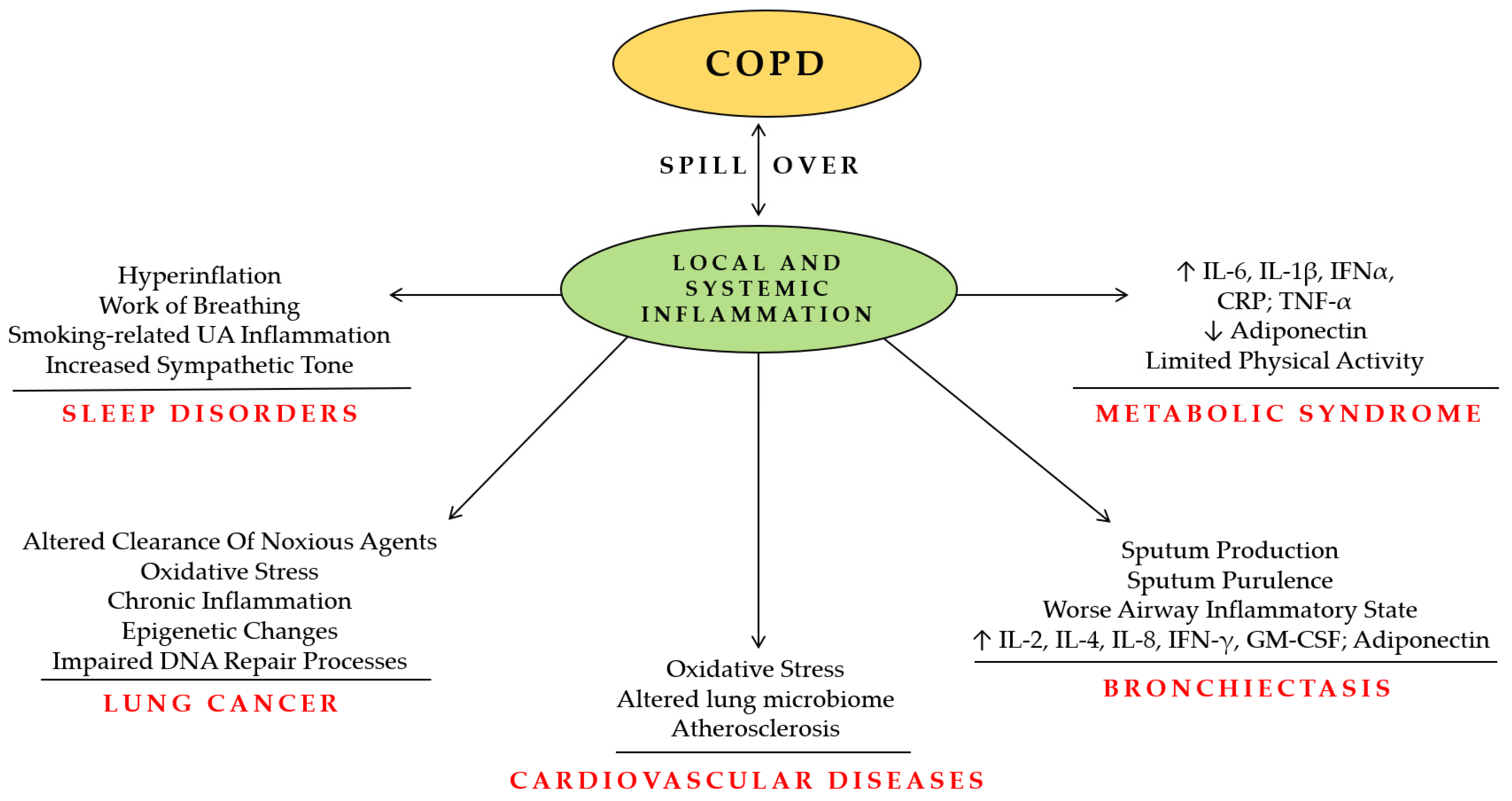
:1. Introduction
2. COPD and Cardiovascular Diseases
2.1. Pathogenesis and Mechanisms of Cardiopulmonary Interaction
2.2. Cardiovascular Mortality in COPD Pharmacological Trials
2.3. Efficacy and Safety of Cardiovascular Agents in Patients with COPD
2.4. Summary of Evidence and Future Perspective
3. COPD and Lung Cancer
Summary of Evidence and Future Perspective
4. COPD and Bronchiectasis
Summary of Evidence and Future Perspective
5. COPD and OSA
Summary of Evidence and Perspective
6. COPD and Metabolic Syndrome
Summary of Evidence and Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, P.J.; Celli, B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Investig. 2008, 118, 3546–3556. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Robinson, S.C.; Mills, G.D.; Sullivan, G.D.; Karalus, N.C.; McLachlan, J.D.; Hancox, R.J. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax 2011, 66, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Decramer, M.; Janssens, W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir. Med. 2013, 1, 73–83. [Google Scholar] [CrossRef]
- Sin, D.D.; Man, S.F.P. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc. Am. Thorac. Soc. 2005, 2, 8–11. [Google Scholar] [CrossRef]
- André, S.; Conde, B.; Fragoso, E.; Boléo-Tomé, J.P.; Areias, V.; Cardoso, J. COPD and Cardiovascular Disease. Pulmonology 2019, 25, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Howe, M.; Leidal, A.; Montgomery, D.; Jackson, E. Role of Cigarette Smoking and Gender in Acute Coronary Syndrome Events. Am. J. Cardiol. 2011, 108, 1382–1386. [Google Scholar] [CrossRef]
- Cheyne, W.S.; Gelinas, J.C.; Eves, N.D. Hemodynamic effects of incremental lung hyperinflation. Am. J. Physiol. Circ. Physiol. 2018, 315, H474–H481. [Google Scholar] [CrossRef]
- Cosio, M.G.; Saetta, M.; Agusti, A. Immunologic Aspects of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2009, 360, 2445–2454. [Google Scholar] [CrossRef]
- Rabe, K.F.; Hurst, J.R.; Suissa, S. Cardiovascular disease and COPD: Dangerous liaisons? Eur. Respir. Rev. 2018, 27, 180057. [Google Scholar] [CrossRef]
- Donaldson, G.C.; Hurst, J.R.; Smith, C.J.; Hubbard, R.B.; Wedzicha, J.A. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest 2010, 137, 1091–1097. [Google Scholar] [CrossRef]
- Adrish, M.; Nannaka, V.B.; Cano, E.J.; Bajantri, B.; Diaz-Fuentes, G. Significance of NT-pro-BNP in acute exacerbation of COPD patients without underlying left ventricular dysfunction. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1183–1189. [Google Scholar] [CrossRef]
- Baillard, C.; Boussarsar, M.; Fosse, J.-P.; Girou, E.; Le Toumelin, P.; Cracco, C.; Jaber, S.; Cohen, Y.; Brochard, L. Cardiac troponin I in patients with severe exacerbation of chronic obstructive pulmonary disease. Intensiv. Care Med. 2003, 29, 584–589. [Google Scholar] [CrossRef]
- Brekke, P.H.; Omland, T.; Holmedal, S.H.; Smith, P.; Soyseth, V. Troponin T elevation and long-term mortality after chronic obstructive pulmonary disease exacerbation. Eur. Respir. J. 2008, 31, 563–570. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, L.P.; Magder, S.; Burkhart, D.; Kesten, S.; Liu, D.; Manuel, R.C.; Niewoehner, D.E. Cause-specific mortality adjudication in the UPLIFT® COPD trial: Findings and recommendations. Respir. Med. 2012, 106, 515–521. [Google Scholar] [CrossRef]
- Vestbo, J.; Calverley, P.; Celli, B.; Ferguson, G.; Jenkins, C.; Jones, P.; Pauwels, R.; Pride, N.; Anderson, J.; Devoy, M.; et al. The TORCH (TOwards a Revolution in COPD Health) survival study protocol. Eur. Respir. J. 2004, 24, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Vestbo, J.; Anderson, J.A.; Brook, R.D.; Calverley, P.M.A.; Celli, B.R.; Crim, C.; Martinez, F.; Yates, J.; Newby, D.E. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): A double-blind randomised controlled trial. Lancet 2016, 387, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, G.J.; Neffen, H. A Systematic Review of the Efficacy and Safety of a Fixed-Dose Combination of Umeclidinium and Vilanterol for the Treatment of COPD. Chest 2015, 148, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Farne, H.A.; Cates, C.J. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2015, 2015, CD008989. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Matera, M.G.; Ora, J.; Cazzola, M.; Calzetta, L. The impact of dual bronchodilation on cardiovascular serious adverse events and mortality in COPD: A quantitative synthesis. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 3469–3485. [Google Scholar] [CrossRef]
- Kato, M.; Tomii, K.; Hashimoto, K.; Nezu, Y.; Ishii, T.; Jones, C.E.; Kilbride, S.; Gross, A.S.; Clifton, C.S.; Lipson, D.A. The IMPACT Study–Single Inhaler Triple Therapy (FF/UMEC/VI) versus FF/VI and UMEC/VI in Patients with COPD: Efficacy and Safety in a Japanese Population. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 2849–2861. [Google Scholar] [CrossRef]
- Rabe, K.F.; Martinez, F.J.; Ferguson, G.T.; Wang, C.; Singh, D.; Wedzicha, J.A.; Trivedi, R.; Rose, E.S.; Ballal, S.; McLaren, J.; et al. Triple Inhaled Therapy at Two Glucocorticoid Doses in Moderate-to-Very-Severe COPD. N. Engl. J. Med. 2020, 383, 35–48. [Google Scholar] [CrossRef]
- Suissa, S. Triple therapy in COPD: Understanding the data. ERJ Open Res. 2023, 9, 00615-2022. [Google Scholar] [CrossRef] [PubMed]
- Egred, M.; Shaw, S.; Mohammad, B.; Waitt, P.; Rodrigues, E. Under-use of beta-blockers in patients with ischaemic heart disease and concomitant chronic obstructive pulmonary disease. Qjm Int. J. Med. 2005, 98, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Rutten, F.H.; Zuithoff, N.P.; Hak, E.; Grobbee, D.E.; Hoes, A.W. Beta-blockers may reduce mortality and risk of exacerbations in patients with chronic obstructive pulmonary disease. Arch. Intern. Med. 2010, 170, 880–887. [Google Scholar] [CrossRef]
- Ekström, M.P.; Hermansson, A.B.; Ström, K.E. Effects of Cardiovascular Drugs on Mortality in Severe Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 715–720. [Google Scholar] [CrossRef]
- Kunadian, V.; Chan, D.; Ali, H.; Wilkinson, N.; Howe, N.; McColl, E.; Thornton, J.; von Wilamowitz-Moellendorff, A.; Holstein, E.-M.; Burns, G.; et al. Antiplatelet therapy in the primary prevention of cardiovascular disease in patients with chronic obstructive pulmonary disease: Protocol of a randomised controlled proof-of-concept trial (APPLE COPD-ICON 2). BMJ Open 2018, 8, e020713. [Google Scholar] [CrossRef] [PubMed]
- Pavasini, R.; Biscaglia, S.; D’Ascenzo, F.; Del Franco, A.; Contoli, M.; Zaraket, F.; Guerra, F.; Ferrari, R.; Campo, G. Antiplatelet Treatment Reduces All-Cause Mortality in COPD Patients: A Systematic Review and Meta-Analysis. COPD J. Chronic Obstr. Pulm. Dis. 2015, 13, 509–514. [Google Scholar] [CrossRef]
- Quist-Paulsen, P. Statins and inflammation: An update. Curr. Opin. Cardiol. 2010, 25, 399–405. [Google Scholar] [CrossRef]
- Cao, C.; Wu, Y.; Xu, Z.; Lv, D.; Zhang, C.; Lai, T.; Li, W.; Shen, H. The effect of statins on chronic obstructive pulmonary disease exacerbation and mortality: A systematic review and meta-analysis of observational research. Sci. Rep. 2015, 5, 16461. [Google Scholar] [CrossRef]
- Ingebrigtsen, T.S.; Marott, J.L.; Nordestgaard, B.G.; Lange, P.; Hallas, J.; Vestbo, J. Statin use and exacerbations in individuals with chronic obstructive pulmonary disease. Thorax 2014, 70, 33–40. [Google Scholar] [CrossRef]
- Walsh, A.; Perrem, L.M.; Elshafi, M.; Khashan, A.S.; Henry, M.; Ni Chroinin, M. Statins versus placebo for people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst. Rev. 2019, 7, CD011959. [Google Scholar] [CrossRef]
- Lai, C.-C.; Wang, Y.-H.; Wang, C.-Y.; Wang, H.-C.; Yu, C.-J.; Chen, L. Comparative effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on the risk of pneumonia and severe exacerbations in patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.A.; Aaron, C.P.; Hoffman, E.A.; Schwartz, J.E.; Madrigano, J.; Austin, J.H.; Kalhan, R.; Lovasi, G.; Watson, K.; Stukovsky, K.H.; et al. Angiotensin-Converting Inhibitors and Angiotensin II Receptor Blockers and Longitudinal Change in Percent Emphysema on Computed Tomography. The Multi-Ethnic Study of Atherosclerosis Lung Study. Ann. Am. Thorac. Soc. 2017, 14, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, F.; D’agnano, V.; Scialò, F.; Komici, K.; Allocca, V.; Nucera, F.; Salvi, R.; Stella, G.M.; Bianco, A. Evolving concepts in COPD and lung cancer: A narrative review. Minerva Medica 2022, 113, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Young, R.; Hopkins, R.J.; Gamble, G.D.; Etzel, C.; El-Zein, R.; Crapo, J.D. Genetic evidence linking lung cancer and COPD: A new perspective. Appl. Clin. Genet. 2011, 4, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Eisner, M.D.; Anthonisen, N.; Coultas, D.; Kuenzli, N.; Perez-Padilla, R.; Postma, D.; Romieu, I.; Silverman, E.K.; Balmes, J.R. An Official American Thoracic Society Public Policy Statement: Novel Risk Factors and the Global Burden of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2010, 182, 693–718. [Google Scholar] [CrossRef]
- Moayedi-Nia, S.; Pasquet, R.; Siemiatycki, J.; Koushik, A.; Ho, V. Occupational Exposures and Lung Cancer Risk-An Analysis of the CARTaGENE Study. J. Occup. Environ. Med. 2022, 64, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, D.; Liu, Y.; Piao, H.; Zhang, T.; Li, X.; Zhao, E.; Zhang, D.; Zheng, Y.; Tang, X. The effect of ambient PM2.5 exposure on survival of lung cancer patients after lobectomy. Environ. Health 2023, 22, 23. [Google Scholar] [CrossRef]
- Mazzarella, G.; Ferraraccio, F.; Prati, M.; Annunziata, S.; Bianco, A.; Mezzogiorno, A.; Liguori, G.; Angelillo, I.; Cazzola, M. Effects of diesel exhaust particles on human lung epithelial cells: An in vitro study. Respir. Med. 2007, 101, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska-Suchanek, I.; Mosor, M.; Gabryel, P.; Grabicki, M.; Żurawek, M.; Fichna, M.; Strauss, E.; Batura-Gabryel, H.; Dyszkiewicz, W.; Nowak, J. Susceptibility loci in lung cancer and COPD: Association of IREB2 and FAM13A with pulmonary diseases. Sci. Rep. 2015, 5, srep13502. [Google Scholar] [CrossRef] [PubMed]
- Young, R.P.; Whittington, C.F.; Hopkins, R.J.; Hay, B.A.; Epton, M.J.; Gamble, G.D. FAM13A locus in COPD is independently associated with lung cancer–evidence of a molecular genetic link between COPD and lung cancer. Appl. Clin. Genet. 2010, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shang, Y.; Cai, F.; Zhang, S.; Xiao, Z.; Wang, H.; Fan, Y.; Li, T.; Sheng, S.; Fu, Y.; et al. Correlation between lung cancer and the HHIP polymorphisms of chronic obstructive pulmonary disease (COPD) in the Chinese Han population. Genes Immun. 2018, 20, 273–280. [Google Scholar] [CrossRef]
- Young, R.P.; Whittington, C.F.; Hopkins, R.J.; Hay, B.A.; Epton, M.J.; Black, P.N.; Gamble, G.D. Chromosome 4q31 locus in COPD is also associated with lung cancer. Eur. Respir. J. 2010, 36, 1375–1382. [Google Scholar] [CrossRef]
- Tessema, M.; Yingling, C.M.; Picchi, M.A.; Wu, G.; Liu, Y.; Weissfeld, J.L.; Siegfried, J.M.; Tesfaigzi, Y.; Belinsky, S.A. Epigenetic Repression of CCDC37 and MAP1B Links Chronic Obstructive Pulmonary Disease to Lung Cancer. J. Thorac. Oncol. 2015, 10, 1181–1188. [Google Scholar] [CrossRef]
- Mahmood, M.Q.; Ward, C.; Muller, H.K.; Sohal, S.S.; Walters, E.H. Epithelial mesenchymal transition (EMT) and non-small cell lung cancer (NSCLC): A mutual association with airway disease. Med. Oncol. 2017, 34, 45. [Google Scholar] [CrossRef]
- Qi, C.; Sun, S.-W.; Xiong, X.-Z. From COPD to Lung Cancer: Mechanisms Linking, Diagnosis, Treatment, and Prognosis. Int. J. Chron. Obstruct. Pulmon. Dis. 2022, 17, 2603–2621. [Google Scholar] [CrossRef]
- Clere, N.; Renault, S.; Corre, I. Endothelial-to-Mesenchymal Transition in Cancer. Front. Cell Dev. Biol. 2020, 8, 747. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Nam, J.-K.; Kim, J.-H.; Choi, S.-H.; Lee, Y.-J. Endothelial-to-mesenchymal transition in anticancer therapy and normal tissue damage. Exp. Mol. Med. 2020, 52, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.M. Mechanistic links between COPD and lung cancer. Nat. Rev. Cancer 2013, 13, 233–245. [Google Scholar] [CrossRef]
- Eapen, M.S.; Hansbro, P.M.; McAlinden, K.; Kim, R.Y.; Ward, C.; Hackett, T.L.; Walters, E.H.; Sohal, S.S. Abnormal M1/M2 macrophage phenotype profiles in the small airway wall and lumen in smokers and chronic obstructive pulmonary disease (COPD). Sci. Rep. 2017, 7, 13392. [Google Scholar] [CrossRef]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef]
- Suzuki, H.; Yamada, Y.; Koh, E.; Yoshino, I.; Sekine, Y. Severity of Chronic Obstructive Pulmonary Disease and Its Relationship to Lung Cancer Prognosis after Surgical Resection. Thorac. Cardiovasc. Surg. 2012, 61, 124–130. [Google Scholar] [CrossRef]
- Roy, E.; Rheault, J.; Pigeon, M.-A.; Ugalde, P.A.; Racine, C.; Simard, S.; Chouinard, G.; Lippens, A.; Lacasse, Y.; Maltais, F. Lung cancer resection and postoperative outcomes in COPD: A single-center experience. Chronic Respir. Dis. 2020, 17, 1479973120925430. [Google Scholar] [CrossRef] [PubMed]
- Bugge, A.; Lund, M.B.; Brunborg, C.; Solberg, S.; Kongerud, J. Survival After Surgical Resection for Lung Cancer in Patients With Chronic Obstructive Pulmonary Disease. Ann. Thorac. Surg. 2016, 101, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Malapelle, U.; Rocco, D.; Perrotta, F.; Mazzarella, G. Targeting immune checkpoints in non small cell lung cancer. Curr. Opin. Pharmacol. 2018, 40, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Della Gravara, L.; Battiloro, C.; Letizia, A.; Cantile, R.; D’Agnano, V.; Sica, G.; Rocco, D. Emerging Biomarkers for the Selection of Advanced NSCLC-Affected Immunotherapy Patients. J. Mol. Pathol. 2021, 2, 197–206. [Google Scholar] [CrossRef]
- Bianco, A.; D’agnano, V.; Matera, M.G.; Della Gravara, L.; Perrotta, F.; Rocco, D. Immune checkpoint inhibitors: A new landscape for extensive stage small cell lung cancer treatment. Expert Rev. Respir. Med. 2021, 15, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, F.; Nankivell, M.; Adizie, J.B.; Maqsood, U.; Elshafi, M.; Jafri, S.; Lerner, A.D.; Woolhouse, I.; Munavvar, M.; Evison, M.; et al. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for PD-L1 Testing in Non-small Cell Lung Cancer. Chest 2020, 158, 1230–1239. [Google Scholar] [CrossRef]
- Polverino, F.; Mirra, D.; Yang, C.X.; Esposito, R.; Spaziano, G.; Rojas-Quintero, J.; Sgambato, M.; Piegari, E.; Cozzolino, A.; Cione, E.; et al. Similar programmed death ligand 1 (PD-L1) expression profile in patients with mild COPD and lung cancer. Sci. Rep. 2022, 12, 22402. [Google Scholar] [CrossRef]
- Shin, S.H.; Park, H.Y.; Im, Y.; Jung, H.A.; Sun, J.; Ahn, J.S.; Ahn, M.; Park, K.; Lee, H.Y.; Lee, S. Improved treatment outcome of pembrolizumab in patients with nonsmall cell lung cancer and chronic obstructive pulmonary disease. Int. J. Cancer 2019, 145, 2433–2439. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, C.; Gao, J.; Yu, P.; Lin, X.; Xie, X.; Liu, M.; Zhang, J.; Xie, Z.; Cui, F.; et al. Treatment response and safety of immunotherapy for advanced non-small cell lung cancer with comorbid chronic obstructive pulmonary disease: A retrospective cohort study. Transl. Lung Cancer Res. 2022, 11, 2306–2317. [Google Scholar] [CrossRef]
- Zhou, J.; Chao, Y.; Yao, D.; Ding, N.; Li, J.; Gao, L.; Zhang, Y.; Xu, X.; Zhou, J.; Halmos, B.; et al. Impact of chronic obstructive pulmonary disease on immune checkpoint inhibitor efficacy in advanced lung cancer and the potential prognostic factors. Transl. Lung Cancer Res. 2021, 10, 2148–2162. [Google Scholar] [CrossRef]
- Mark, N.M.; Kargl, J.; Busch, S.E.; Yang, G.H.Y.; Metz, H.E.; Zhang, H.; Hubbard, J.J.; Pipavath, S.N.J.; Madtes, D.K.; Houghton, A.M. Chronic Obstructive Pulmonary Disease Alters Immune Cell Composition and Immune Checkpoint Inhibitor Efficacy in Non–Small Cell Lung Cancer. Am. J. Respir. Crit. Care Med. 2018, 197, 325–336. [Google Scholar] [CrossRef]
- Biton, J.; Ouakrim, H.; Dechartres, A.; Alifano, M.; Mansuet-Lupo, A.; Si, H.; Halpin, R.; Creasy, T.; Bantsimba-Malanda, C.; Arrondeau, J.; et al. Impaired Tumor-Infiltrating T Cells in Patients with Chronic Obstructive Pulmonary Disease Impact Lung Cancer Response to PD-1 Blockade. Am. J. Respir. Crit. Care Med. 2018, 198, 928–940. [Google Scholar] [CrossRef]
- Galvano, A.; Gristina, V.; Malapelle, U.; Pisapia, P.; Pepe, F.; Barraco, N.; Castiglia, M.; Perez, A.; Rolfo, C.; Troncone, G.; et al. The prognostic impact of tumor mutational burden (TMB) in the first-line management of advanced non-oncogene addicted non-small-cell lung cancer (NSCLC): A systematic review and meta-analysis of randomized controlled trials. ESMO Open 2021, 6, 100124. [Google Scholar] [CrossRef]
- Hill, A.T.; Sullivan, A.L.; Chalmers, J.D.; De Soyza, A.; Elborn, J.S.; Floto, R.A.; Grillo, L.; Gruffydd-Jones, K.; Harvey, A.; Haworth, C.S.; et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 2019, 74 (Suppl. S1), 1–69. [Google Scholar] [CrossRef]
- Huang, J.T.J.; Cant, E.; Keir, H.R.; Barton, A.K.; Kuzmanova, E.; Shuttleworth, M.; Pollock, J.; Finch, S.; Polverino, E.; Bottier, M.; et al. Endotyping Chronic Obstructive Pulmonary Disease, Bronchiectasis, and the “Chronic Obstructive Pulmonary Disease-Bronchiectasis Association”. Am. J. Respir. Crit. Care Med. 2022, 206, 417–426. [Google Scholar] [CrossRef]
- Sethi, S.; Murphy, T.F. Infection in the Pathogenesis and Course of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef]
- O’Brien, C.; Guest, P.J.; Hill, S.L.; Stockley, R.A. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax 2000, 55, 635–642. [Google Scholar] [CrossRef]
- Martínez-García, M.Á.; Soler-Cataluña, J.J.; Sanz, Y.D.; Serra, P.C.; Lerma, M.A.; Vicente, J.B.; Perpiñá-Tordera, M. Factors associated with bronchiectasis in patients with COPD. Chest 2011, 140, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.A.; Young, T.P.; Maselli, D.J.; Martinez, C.H.; Gill, R.; Nardelli, P.; Wang, W.; Kinney, G.L.; Hokanson, J.E.; Washko, G.R.; et al. Quantitative CT Measures of Bronchiectasis in Smokers. Chest 2017, 151, 1255–1262. [Google Scholar] [CrossRef]
- Martínez-García, M.-A.; Carrillo, D.d.l.R.; Soler-Cataluña, J.-J.; Donat-Sanz, Y.; Serra, P.C.; Lerma, M.A.; Ballestín, J.; Sánchez, I.V.; Ferrer, M.J.S.; Dalfo, A.R.; et al. Prognostic Value of Bronchiectasis in Patients with Moderate-to-Severe Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 823–831. [Google Scholar] [CrossRef]
- Marsland, I.; Sobala, R.; De Soyza, A.; Witham, M. Multimorbidity in bronchiectasis: A systematic scoping review. ERJ Open Res. 2023, 9, 00296–2022. [Google Scholar] [CrossRef]
- Du, Q.; Jin, J.; Liu, X.; Sun, Y. Bronchiectasis as a Comorbidity of Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0150532. [Google Scholar] [CrossRef]
- De Soyza, A.; McDonnell, M.J.; Goeminne, P.C.; Aliberti, S.; Lonni, S.; Davison, J.; Dupont, L.J.; Fardon, T.C.; Rutherford, R.M.; Hill, A.T.; et al. Bronchiectasis Rheumatoid Overlap Syndrome Is an Independent Risk Factor for Mortality in Patients With Bronchiectasis: A Multicenter Cohort Study. Chest 2017, 151, 1247–1254. [Google Scholar] [CrossRef]
- Dou, S.; Zheng, C.; Cui, L.; Xie, M.; Wang, W.; Tian, H.; Li, K.; Liu, K.; Tian, X.; Wang, X.; et al. High prevalence of bronchiectasis in emphysema-predominant COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2041–2047. [Google Scholar] [CrossRef]
- Sever, Z.K.; Bircan, H.A.; Sirin, F.B.; Evrimler, S.; Celik, S.; Merd, N. Serum biomarkers in patients with stable and exacerbated COPD-bronchiectasis overlap syndrome. Clin. Respir. J. 2020, 14, 1032–1039. [Google Scholar] [CrossRef]
- Bianco, A.; Mazzarella, G.; Turchiarelli, V.; Nigro, E.; Corbi, G.; Scudiero, O.; Sofia, M.; Daniele, A. Adiponectin: An Attractive Marker for Metabolic Disorders in Chronic Obstructive Pulmonary Disease (COPD). Nutrients 2013, 5, 4115–4125. [Google Scholar] [CrossRef]
- Nigro, E.; Mosella, M.; Daniele, A.; Mallardo, M.; Accardo, M.; Bianco, A.; Perrotta, F.; Scialò, F. Adiponectin Increase in Patients Affected by Chronic Obstructive Pulmonary Disease with Overlap of Bronchiectasis. Life 2023, 13, 444. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.O.; Polverino, E.; Hoefsloot, W.; Codecasa, L.R.; Diel, R.; Jenkins, S.G.; Loebinger, M.R. Pulmonary disease by non-tuberculous mycobacteria-clinical management, unmet needs and future perspectives. Expert Rev. Respir. Med. 2017, 11, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Polverino, E.; Dimakou, K.; Hurst, J.; Martinez-Garcia, M.-A.; Miravitlles, M.; Paggiaro, P.; Shteinberg, M.; Aliberti, S.; Chalmers, J.D. The overlap between bronchiectasis and chronic airway diseases: State of the art and future directions. Eur. Respir. J. 2018, 52, 1800328. [Google Scholar] [CrossRef]
- Martínez-García, M.; Oscullo, G.; García-Ortega, A.; Matera, M.G.; Rogliani, P.; Cazzola, M. Inhaled Corticosteroids in Adults with Non-cystic Fibrosis Bronchiectasis: From Bench to Bedside. A Narrative Review. Drugs 2022, 82, 1453–1468. [Google Scholar] [CrossRef] [PubMed]
- Oscullo, G.; Gómez-Olivas, J.D.; Ingles, M.; Mompean, S.; Martinez-Perez, R.; Suarez-Cuartin, G.; la Rosa-Carrillo, D.; Martinez-Garcia, M.A. Bronchiectasis-COPD Overlap Syndrome: Role of Peripheral Eosinophil Count and Inhaled Corticosteroid Treatment. J. Clin. Med. 2023, 12, 6417. [Google Scholar] [CrossRef] [PubMed]
- Veasey, S.C.; Rosen, I.M. Obstructive Sleep Apnea in Adults. N. Engl. J. Med. 2019, 380, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Schwartz, A.R.; Schneider, H.; Owens, R.L.; DeYoung, P.; Han, M.K.; Wedzicha, J.A.; Hansel, N.N.; Zeidler, M.R.; Wilson, K.C.; et al. Research Priorities in Pathophysiology for Sleep-disordered Breathing in Patients with Chronic Obstructive Pulmonary Disease. An Official American Thoracic Society Research Statement. Am. J. Respir. Crit. Care Med. 2018, 197, 289–299. [Google Scholar] [CrossRef]
- Flenley, D. Sleep in Chronic Obstructive Lung Disease. Clin. Chest Med. 1985, 6, 651–661. [Google Scholar] [CrossRef]
- Shawon, S.R.; Perret, J.L.; Senaratna, C.V.; Lodge, C.; Hamilton, G.S.; Dharmage, S.C. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: A systematic review. Sleep Med. Rev. 2016, 32, 58–68. [Google Scholar] [CrossRef]
- Marin, J.M.; Soriano, J.B.; Carrizo, S.J.; Boldova, A.; Celli, B.R. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: The overlap syndrome. Am. J. Respir. Crit. Care Med. 2010, 182, 325–331. [Google Scholar] [CrossRef]
- McNicholas, W.T.; Hansson, D.; Schiza, S.; Grote, L. Sleep in chronic respiratory disease: COPD and hypoventilation disorders. Eur. Respir. Rev. 2019, 28, 190064. [Google Scholar] [CrossRef]
- Naranjo, M.; Willes, L.; Prillaman, B.A.; Quan, S.F.; Sharma, S. Undiagnosed OSA May Significantly Affect Outcomes in Adults Admitted for COPD in an Inner-City Hospital. Chest 2020, 158, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Biselli, P.; Grossman, P.R.; Kirkness, J.P.; Patil, S.P.; Smith, P.L.; Schwartz, A.R.; Schneider, H. The effect of increased lung volume in chronic obstructive pulmonary disease on upper airway obstruction during sleep. J. Appl. Physiol. 2015, 119, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Badr, C.; Elkins, M.R.; Ellis, E.R. The effect of body position on maximal expiratory pressure and flow. Aust. J. Physiother. 2002, 48, 95–102. [Google Scholar] [CrossRef]
- White, L.H.; Bradley, T.D. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J. Physiol. 2013, 591, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Renner, B.; Mueller, C.A.; Shephard, A. Environmental and non-infectious factors in the aetiology of pharyngitis (sore throat). Inflamm. Res. 2012, 61, 1041–1052. [Google Scholar] [CrossRef]
- Bouloukaki, I.; Fanaridis, M.; Testelmans, D.; Pataka, A.; Schiza, S. Overlaps between obstructive sleep apnoea and other respiratory diseases, including COPD, asthma and interstitial lung disease. Breathe 2022, 18, 220073. [Google Scholar] [CrossRef] [PubMed]
- Chaouat, A.; Weitzenblum, E.; Krieger, J.; Ifoundza, T.; Oswald, M.; Kessler, R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 1995, 151, 82–86. [Google Scholar] [CrossRef]
- McNicholas, W.T. Comorbid obstructive sleep apnoea and chronic obstructive pulmonary disease and the risk of cardiovascular disease. J. Thorac. Dis. 2018, 10 (Suppl. S34), S4253–S4261. [Google Scholar] [CrossRef]
- Jean-Louis, G.; Zizi, F.; Brown, D.B.; Ogedegbe, G.; Borer, J.S.; McFarlane, S.I. Obstructive sleep apnea and cardiovascular disease: Evidence and underlying mechanisms. Minerva Pneumol. 2009, 48, 277–293. [Google Scholar]
- Sharma, B.; Neilan, T.G.; Kwong, R.Y.; Mandry, D.; Owens, R.L.; McSharry, D.; Bakker, J.P.; Malhotra, A. Evaluation of Right Ventricular Remodeling Using Cardiac Magnetic Resonance Imaging in Co-Existent Chronic Obstructive Pulmonary Disease and Obstructive Sleep Apnea. COPD J. Chronic Obstr. Pulm. Dis. 2012, 10, 4–10. [Google Scholar] [CrossRef]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2019, 15, 335–343. [Google Scholar] [CrossRef]
- Suri, T.M.; Suri, J.C. A review of therapies for the overlap syndrome of obstructive sleep apnea and chronic obstructive pulmonary disease. FASEB BioAdvances 2021, 3, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.C.; Venekamp, L.N.; Peeters, G.A.; Pijpers, A.; Pevernagie, D.A. Management of insomnia in sleep disordered breathing. Eur. Respir. Rev. 2019, 28, 190080. [Google Scholar] [CrossRef]
- Javaheri, S.; Redline, S. Insomnia and Risk of Cardiovascular Disease. Chest 2017, 152, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Budhiraja, R.; Parthasarathy, S.; Budhiraja, P.; Habib, M.P.; Wendel, C.; Quan, S.F. Insomnia in Patients with COPD. Sleep 2012, 35, 369–375. [Google Scholar] [CrossRef]
- Luyster, F.S.; Boudreaux-Kelly, M.Y.; Bon, J.M. Insomnia in chronic obstructive pulmonary disease and associations with healthcare utilization and costs. Respir. Res. 2023, 24, 93. [Google Scholar] [CrossRef]
- Sarber, K.M.; Patil, R.D. Comorbid Insomnia and Sleep Apnea: Challenges and Treatments. Otolaryngol. Clin. N. Am. 2023, in press. [Google Scholar] [CrossRef]
- Anderson, G.; Cheng, J.; Moline, M.; Lorch, D.G.; Hall, N.; Shah, D. Respiratory safety of lemborexant in adult and elderly patients with moderate to severe copd. Chest 2022, 162, A2617. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Lorch, D.; Lowe, A.D.; Uchimura, N.; Hall, N.; Shah, D.; Moline, M. A randomized, double-blind, placebo-controlled, crossover study of respiratory safety of lemborexant in moderate to severe obstructive sleep apnea. J. Clin. Sleep Med. 2024, 20, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Cebron Lipovec, N.; Beijers, R.J.; van den Borst, B.; Doehner, W.; Lainscak, M.; Schols, A.M. The Prevalence of Metabolic Syndrome in Chronic Obstructive Pulmonary Disease: A Systematic Review. COPD J. Chronic Obstr. Pulm. Dis. 2016, 13, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Díez-Manglano, J.; Barquero-Romero, J.; Almagro, P.; Cabrera, F.J.; García, F.L.; Montero, L.; Soriano, J.B. COPD patients with and without metabolic syndrome: Clinical and functional differences. Intern. Emerg. Med. 2013, 9, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Watz, H.; Waschki, B.; Kirsten, A.; Müller, K.C.; Kretschmar, G.; Meyer, T.; Holz, O.; Magnussen, H. The metabolic syndrome in patients with chronic bronchitis and COPD: Frequency and associated consequences for systemic inflammation and physical inactivity. Chest 2009, 136, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, T.; Wang, Z.; Yu, F.; Xu, Q.; Guo, W.; Wu, C.; He, J. Body mass index and mortality in chronic obstructive pulmonary disease: A dose-response meta-analysis. Medicine 2016, 95, e4225. [Google Scholar] [CrossRef] [PubMed]
- Clini, E.; Crisafulli, E.; Radaeli, A.; Malerba, M. COPD and the metabolic syndrome: An intriguing association. Intern. Emerg. Med. 2011, 8, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Röpcke, S.; Holz, O.; Lauer, G.; Müller, M.; Rittinghausen, S.; Ernst, P.; Lahu, G.; Elmlinger, M.; Krug, N.; Hohlfeld, J.M. Repeatability of and relationship between potential COPD biomarkers in bronchoalveolar lavage, bronchial biopsies, serum, and induced sputum. PLoS ONE 2012, 7, e46207. [Google Scholar] [CrossRef]
- Cyphert, T.J.; Morris, R.T.; House, L.M.; Barnes, T.M.; Otero, Y.F.; Barham, W.J.; Hunt, R.P.; Zaynagetdinov, R.; Yull, F.E.; Blackwell, T.S.; et al. NF-κB-dependent airway inflammation triggers systemic insulin resistance. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 309, R1144–R1152. [Google Scholar] [CrossRef]
- Franssen, F.M.E.; O’Donnell, D.E.; Goossens, G.H.; Blaak, E.E.; Schols, A.M.W.J. Obesity and the lung: 5. Obesity and COPD. Thorax 2008, 63, 1110–1117. [Google Scholar] [CrossRef]
- Monteiro, F.; Camillo, C.A.; Vitorasso, R.; Sant’anna, T.; Hernandes, N.A.; Probst, V.S.; Pitta, F. Obesity and Physical Activity in the Daily Life of Patients with COPD. Lung 2012, 190, 403–410. [Google Scholar] [CrossRef]
- Tkacova, R. Systemic Inflammation in Chronic Obstructive Pulmonary Disease: May Adipose Tissue Play a Role? Review of the Literature and Future Perspectives. Mediat. Inflamm. 2010, 2010, 585989. [Google Scholar] [CrossRef]
- Perrotta, F.; Nigro, E.; Mollica, M.; Costigliola, A.; D’agnano, V.; Daniele, A.; Bianco, A.; Guerra, G. Pulmonary Hypertension and Obesity: Focus on Adiponectin. Int. J. Mol. Sci. 2019, 20, 912. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, F.; Nigro, E.; Pafundi, P.C.; Polito, R.; Nucera, F.; Scialò, F.; Caramori, G.; Bianco, A.; Daniele, A. Adiponectin is Associated with Neutrophils to Lymphocyte Ratio in Patients with Chronic Obstructive Pulmonary Disease. COPD J. Chronic Obstr. Pulm. Dis. 2020, 18, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Krommidas, G.; Kostikas, K.; Papatheodorou, G.; Koutsokera, A.; Gourgoulianis, K.I.; Roussos, C.; Koulouris, N.G.; Loukides, S. Plasma leptin and adiponectin in COPD exacerbations: Associations with inflammatory biomarkers. Respir. Med. 2010, 104, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Sexton, P.; Metcalf, P.; Kolbe, J. Respiratory Effects of Insulin Sensitisation with Metformin: A Prospective Observational Study. COPD J. Chronic Obstr. Pulm. Dis. 2013, 11, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Schick, M.A.; Schlegel, N. Clinical Implication of Phosphodiesterase-4-Inhibition. Int J Mol Sci. 2022, 23, 1209. [Google Scholar] [CrossRef]
- Andrews, C.S.; Matsuyama, S.; Lee, B.-C.; Li, J.-D. Resveratrol suppresses NTHi-induced inflammation via up-regulation of the negative regulator MyD88 short. Sci. Rep. 2016, 6, srep34445. [Google Scholar] [CrossRef]
- Altintas Dogan, A.D.; Hilberg, O.; Hess, S.; Jensen, T.T.; Bladbjerg, E.M.; Juhl, C.B. Respiratory Effects of Treatment with a Glucagon-Like Peptide-1 Receptor Agonist in Patients Suffering from Obesity and Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 405–414. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariniello, D.F.; D’Agnano, V.; Cennamo, D.; Conte, S.; Quarcio, G.; Notizia, L.; Pagliaro, R.; Schiattarella, A.; Salvi, R.; Bianco, A.; et al. Comorbidities in COPD: Current and Future Treatment Challenges. J. Clin. Med. 2024, 13, 743. https://doi.org/10.3390/jcm13030743
Mariniello DF, D’Agnano V, Cennamo D, Conte S, Quarcio G, Notizia L, Pagliaro R, Schiattarella A, Salvi R, Bianco A, et al. Comorbidities in COPD: Current and Future Treatment Challenges. Journal of Clinical Medicine. 2024; 13(3):743. https://doi.org/10.3390/jcm13030743
Chicago/Turabian StyleMariniello, Domenica Francesca, Vito D’Agnano, Donatella Cennamo, Stefano Conte, Gianluca Quarcio, Luca Notizia, Raffaella Pagliaro, Angela Schiattarella, Rosario Salvi, Andrea Bianco, and et al. 2024. "Comorbidities in COPD: Current and Future Treatment Challenges" Journal of Clinical Medicine 13, no. 3: 743. https://doi.org/10.3390/jcm13030743
APA StyleMariniello, D. F., D’Agnano, V., Cennamo, D., Conte, S., Quarcio, G., Notizia, L., Pagliaro, R., Schiattarella, A., Salvi, R., Bianco, A., & Perrotta, F. (2024). Comorbidities in COPD: Current and Future Treatment Challenges. Journal of Clinical Medicine, 13(3), 743. https://doi.org/10.3390/jcm13030743





