A Contemporary Review of the Use of Extracorporeal CytoSorb® Hemoadsorption Therapy in Patients with Infective Endocarditis
Abstract
1. Introduction
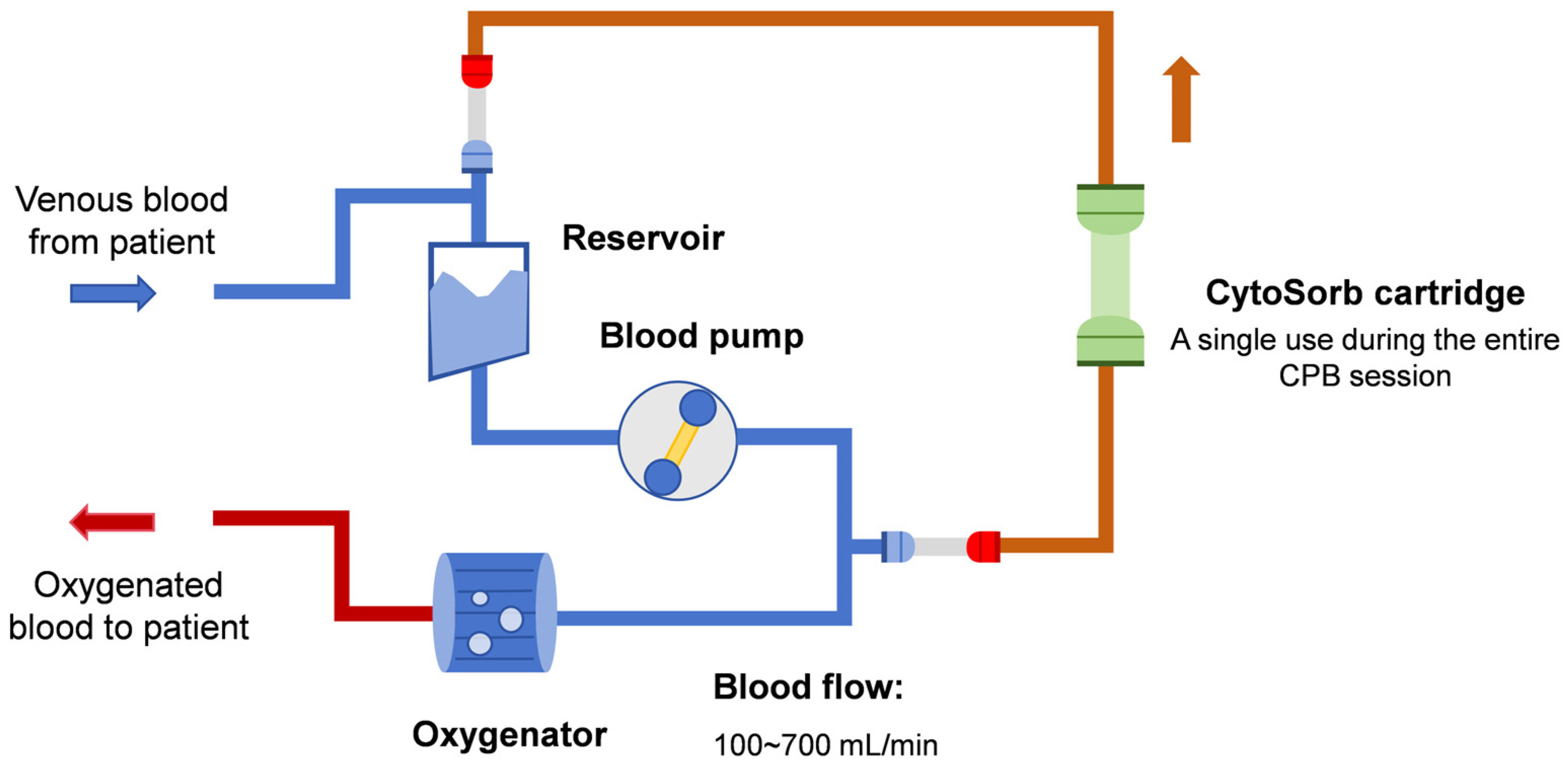
2. Rationale for Hemoadsorption Therapy in IE Patients Undergoing Cardiac Surgery
3. Clinical Evidence for Hemoadsorption Therapy in IE Patients Undergoing Cardiac Surgery
| Author, Publication Year | Study Location | Study Design | Study Period | Sample Size | Mean or Median EuroScore II | Hemoadsorption Prescription | Main Findings |
|---|---|---|---|---|---|---|---|
| Silke Asch, 2021 [14] | Göttingen, Germany | RCT | November 2018 to March 2020 | 20 | Cytosorb: 8.5 Control: 3.6 | Cytosorb® hemoadsorption was initiated intraoperatively and continued for 24 h postoperatively. | Cytosorb® hemoadsorption neither resulted in a reduction of inflammatory parameters nor an improvement of hemodynamics in IE patients. |
| Mahmoud Diab, 2022 [9] | Multicenter, Germany | RCT | 17 January 2018 to 31 January 2020 | 282 | Cytosorb: 19.1 ± 17.3 Control: 20.2 ± 17.8 | Hemadsorption during CPB. | Although Cytosorb® hemoadsorption reduced plasma cytokines, there was no difference in clinically relevant outcome measures and no reduction in postoperative organ dysfunction. |
| Ingo Garau, 2019 [37] | Hamburg, Germany | RCT | September 2013 to June 2015 | 40 | Cytosorb: 6.1 Control: 6.3 | Hemadsorption during CPB with a blood flow of 300 mL/min. | In elective on-pump cardiac surgery patients, Cytosorb® hemoadsorption was associated with a short-term reduction in pro-inflammatory cytokine levels of IL-8 and TNFα. |
| Elettra C Poli, 2019 [38] | Lausanne, Switzerland | RCT | May 2016 to January 2018 | 30 | Cytosorb: 3.0 Control: 5.1 | Hemadsorption during CPB. | CytoSorb® hemoadsorption during CPB was not associated with a decrease in pro- or anti-inflammatory cytokines nor with an improvement in relevant clinical outcomes. |
| Anna Holmen, 2022 [13] | Gothenburg, Sweden | RCT | April 2019 to September 2020 | 19 | NA | Hemadsorption during CPB. | Cytosorb® hemoadsorption contributed to an insignificantly decreased vasopressor use after surgery in IE patients. |
| Zaki Haidari, 2023 [41] | Essen and Nuremberg, Germany | Retrospective study | January 2015 to March 2022 | 130 | Cytosorb: 11.9 Control: 12.0 | Hemadsorption during CPB with a blood flow ranging from 100 to 700 mL/min. | Intraoperative Cytosorb® hemoadsorption significantly contributed to reduced sepsis-associated mortality, 30- and 90-day mortality, and improved hemodynamics in high-risk IE patients. |
| Jurij Matija Kalisnik, 2022 [12] | Nuremberg, Germany | Retrospective study | January 2015 to April 2021 | 202 | Cytosorb: 9.89 Control: 8.95 | Hemadsorption during CPB. | After propensity score match, intraoperative Cytosorb® hemoadsorption significantly reduced sepsis and sepsis-associated mortality after cardiac surgery in high-risk patients with active left-sided native and prosthetic valve IE. |
| David Santer, 2021 [15] | Basel, Switzerland | Retrospective study | 2009 to 2019 | 241 | Cytosorb: 7.8 Control: 8.6 | Hemadsorption during CPB with a blood flow of 500 mL/min. | Intraoperative Cytosorb® hemoadsorption did not reduce in-hospital mortality, incidence of delirium, myocardial ischemia, stroke, and postoperative renal failure, but was significantly associated with increased in-hospital stay and incidence of reoperation for bleeding in IE patients. |
| Lars-Uwe Kühne, 2019 [43] | Hamburg, Germany | Case series | July 2017 to April 2018 | 20 | Group 1: 26.8 Group 2: 33.8 | Group 1: intraoperative hemoadsorption with a blood flow rate between 300 and 600 mL/min. Group 2: intraoperative plus postoperative hemoadsorption with a blood flow rate between 300 and 600 mL/min. | IE patients undergoing intraoperative plus postoperative Cytosorb® hemoadsorption showed a similar ICU and 90-day survival compared to those undergoing intraoperative Cytosorb® hemoadsorption only. However, postoperative continuation of hemoadsorption treatment was associated with a higher rate of postoperative complications and a longer intensive care unit stay despite a significant difference in baseline disease severity between the two groups. |
| Karl Träger, 2017 [40] | Ulm, Germany | Case series with a historical group | September 2013 to August 2016 | 67 | Cytosorb: 11 Historical control: 9 for ICU survivors | Hemadsorption during CPB with a blood flow ranging from 200 to 400 mL/min. | Intraoperative Cytosorb® hemoadsorption contributed to reduced plasma IL-6 and IL-8 levels and improved hemodynamics in IE patients. |
4. Safety Concerns
5. Health Economics
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hubers, S.A.; DeSimone, D.C.; Gersh, B.J.; Anavekar, N.S. Infective Endocarditis: A Contemporary Review. Mayo Clin. Proc. 2020, 95, 982–997. [Google Scholar] [CrossRef]
- Iung, B.; Duval, X. Infective endocarditis: Innovations in the management of an old disease. Nat. Rev. Cardiol. 2019, 16, 623–635. [Google Scholar] [CrossRef]
- Adema, J.L.; Ahiskali, A.; Fida, M.; Mediwala Hornback, K.; Stevens, R.W.; Rivera, C.G. Heartbreaking Decisions: The Dogma and Uncertainties of Antimicrobial Therapy in Infective Endocarditis. Pathogens 2023, 12, 703. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals from the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef]
- Pettersson, G.B.; Coselli, J.S.; Pettersson, G.B.; Coselli, J.S.; Hussain, S.T.; Griffin, B.; Blackstone, E.H.; Gordon, S.M.; LeMaire, S.A.; Woc-Colburn, L.E. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: Surgical treatment of infective endocarditis: Executive summary. J. Thorac. Cardiovasc. Surg. 2017, 153, 1241–1258.e1229. [Google Scholar] [CrossRef]
- Wan, S.; LeClerc, J.L.; Vincent, J.L. Inflammatory response to cardiopulmonary bypass—Mechanisms involved and possible therapeutic strategies. Chest 1997, 112, 676–692. [Google Scholar] [CrossRef]
- Giacinto, O.; Satriano, U.; Nenna, A.; Spadaccio, C.; Lusini, M.; Mastroianni, C.; Nappi, F.; Chello, M. Inflammatory Response and Endothelial Dysfunction Following Cardiopulmonary Bypass: Pathophysiology and Pharmacological Targets. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Diab, M.; Lehmann, T.; Bothe, W.; Akhyari, P.; Platzer, S.; Wendt, D.; Deppe, A.-C.; Strauch, J.; Hagel, S.; Guenther, A.; et al. Cytokine Hemoadsorption during Cardiac Surgery Versus Standard Surgical Care for Infective Endocarditis (REMOVE): Results from a Multicenter Randomized Controlled Trial. Circulation 2022, 145, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Diab, M.; Tasar, R.; Sponholz, C.; Lehmann, T.; Pletz, M.W.; Bauer, M.; Brunkhorst, F.M.; Doenst, T. Changes in inflammatory and vasoactive mediator profiles during valvular surgery with or without infective endocarditis: A case control pilot study. PLoS ONE 2020, 15, e0228286. [Google Scholar] [CrossRef] [PubMed]
- Haidari, Z.; Demircioglu, E.; Boss, K.; Tyczynski, B.; Thielmann, M.; Schmack, B.; Kribben, A.; Weymann, A.; El Gabry, M.; Ruhparwar, A.; et al. Intraoperative hemoadsorption in high-risk patients with infective endocarditis. PLoS ONE 2022, 17, e0266820. [Google Scholar] [CrossRef]
- Kalisnik, J.M.; Leiler, S.; Mamdooh, H.; Zibert, J.; Bertsch, T.; Vogt, F.A.; Bagaev, E.; Fittkau, M.; Fischlein, T. Single-Centre Retrospective Evaluation of Intraoperative Hemoadsorption in Left-Sided Acute Infective Endocarditis. J. Clin. Med. 2022, 11, 3954. [Google Scholar] [CrossRef]
- Holmen, A.; Corderfeldt, A.; Lannemyr, L.; Dellgren, G.; Hansson, E.C. Whole Blood Adsorber during CPB and Need for Vasoactive Treatment after Valve Surgery in Acute Endocarditis: A Randomized Controlled Study. J. Cardiothorac. Vasc. Anesth. 2022, 36, 3015–3020. [Google Scholar] [CrossRef]
- Asch, S.; Kaufmann, T.P.; Walter, M.; Leistner, M.; Danner, B.C.; Perl, T.; Kutschka, I.; Niehaus, H. The effect of perioperative hemadsorption in patients operated for acute infective endocarditis-A randomized controlled study. Artif. Organs 2021, 45, 1328–1337. [Google Scholar] [CrossRef]
- Santer, D.; Miazza, J.; Koechlin, L.; Gahl, B.; Rrahmani, B.; Hollinger, A.; Eckstein, F.S.; Siegemund, M.; Reuthebuch, O.T. Hemoadsorption during Cardiopulmonary Bypass in Patients with Endocarditis Undergoing Valve Surgery: A Retrospective Single-Center Study. J. Clin. Med. 2021, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Haidari, Z.; Wendt, D.; Thielmann, M.; Mackowiak, M.; Neuhaeuser, M.; Jakob, H.; Ruhparwar, A.; El-Gabry, M. Intraoperative Hemoadsorption in Patients with Native Mitral Valve Infective Endocarditis. Ann. Thorac. Surg. 2020, 110, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Geisler, D.; Arleth, N.; Grabenwöger, J.; Arnold, Z.; Aschacher, T.; Winkler, B.; Mach, M.; Grabenwöger, M. Impact of CytoSorb® on interleukin-6 in cardiac surgery. Front. Cardiovasc. Med. 2023, 10, 1166093. [Google Scholar] [CrossRef] [PubMed]
- Zakkar, M.; Ascione, R.; James, A.F.; Angelini, G.D.; Suleiman, M.S. Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol. Ther. 2015, 154, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, J.M.; Peelen, L.M.; Coulson, T.G.; Tran, L.; Reid, C.M.; Smith, J.A.; Myles, P.S.; Pilcher, D. Age and other perioperative risk factors for postoperative systemic inflammatory response syndrome after cardiac surgery. Br. J. Anaesth. 2017, 119, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Squiccimarro, E.; Labriola, C.; Malvindi, P.G.; Margari, V.; Guida, P.; Visicchio, G.; Kounakis, G.; Favale, A.; Dambruoso, P.; Mastrototaro, G.; et al. Prevalence and Clinical Impact of Systemic Inflammatory Reaction after Cardiac Surgery. J Cardiothorac. Vasc. Anesth. 2019, 33, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Reis, T.; Ronco, C.; Soranno, D.E.; Clark, W.; De Rosa, S.; Forni, L.G.; Lorenzin, A.; Ricci, Z.; Villa, G.; Kellum, J.A.; et al. Standardization of Nomenclature for the Mechanisms and Materials Utilized for Extracorporeal Blood Purification. Blood Purif. 2023, 949–962. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Yang, T.; Chang, K.; Deng, N.; Zhao, W.; Su, B. Targeting circulating high mobility group box-1 and histones by extracorporeal blood purification as an immunomodulation strategy against critical illnesses. Crit. Care 2023, 27, 77. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Wei, Y.; Chen, B. Application of Hemoperfusion in the Treatment of Acute Poisoning. Blood Purif. 2024, 53, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Hayanga, J.W.A.; Song, T.; Durham, L.; Garrison, L.; Smith, D.; Molnar, Z.; Scheier, J.; Deliargyris, E.N.; Moazami, N. Extracorporeal hemoadsorption in critically ill COVID-19 patients on VV ECMO: The CytoSorb therapy in COVID-19 (CTC) registry. Crit. Care 2023, 27, 243. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.; David, C.; Marcu, A.; Olita, M.R.; Mihaila, M.; Tomescu, D. Artificial Liver Support with CytoSorb and MARS in Liver Failure: A Retrospective Propensity Matched Analysis. J. Clin. Med. 2023, 12, 2258. [Google Scholar] [CrossRef] [PubMed]
- Szigetváry, C.E.; Turan, C.; Kovács, E.H.; Kói, T.; Engh, M.A.; Hegyi, P.; Csukly, G.; Ruszkai, Z.; Molnár, Z. Hemoadsorption as Adjuvant Therapy in Acute Respiratory Distress Syndrome (ARDS): A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 3068. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R. Hemoperfusion: Technical aspects and state of the art. Crit. Care 2022, 26, 135. [Google Scholar] [CrossRef] [PubMed]
- Heymann, M.; Schorer, R.; Putzu, A. The Effect of CytoSorb on Inflammatory Markers in Critically Ill Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Care Med. 2023, 51, 1659–1673. [Google Scholar] [CrossRef]
- Albrecht, F.; Schunk, S.; Fuchs, M.; Volk, T.; Geisel, J.; Fliser, D.; Meiser, A. Rapid and Effective Elimination of Myoglobin with CytoSorb® Hemoadsorber in Patients with Severe Rhabdomyolysis. Blood Purif. 2023, 1–8. [Google Scholar] [CrossRef]
- Gräfe, C.; Paal, M.; Winkels, M.; Irlbeck, M.; Liebchen, U.; Scharf, C. Correlation between Bilirubin Elimination with the Cytokine Adsorber CytoSorb® and Mortality in Critically Ill Patients with Hyperbilirubinemia. Blood Purif. 2023, 52, 849–856. [Google Scholar] [CrossRef]
- Greimel, A.; Habler, K.; Gräfe, C.; Maciuga, N.; Brozat, C.I.; Vogeser, M.; Zoller, M.; Happich, F.L.; Liebchen, U.; Frank, S.; et al. Extracorporeal adsorption of protective and toxic bile acids and bilirubin in patients with cholestatic liver dysfunction: A prospective study. Ann. Intensive Care 2023, 13, 110. [Google Scholar] [CrossRef]
- Gruda, M.C.; Ruggeberg, K.G.; O’Sullivan, P.; Guliashvili, T.; Scheirer, A.R.; Golobish, T.D.; Capponi, V.J.; Chan, P.P. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb® sorbent porous polymer beads. PLoS ONE 2018, 13, e0191676. [Google Scholar] [CrossRef]
- Malard, B.; Lambert, C.; Kellum, J.A. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med. Exp. 2018, 6, 12. [Google Scholar] [CrossRef]
- Pieri, M.; Bonizzoni, M.A.; Belletti, A.; Calabrò, M.G.; Fominskiy, E.; Nardelli, P.; Ortalda, A.; Scandroglio, A.M. Extracorporeal Blood Purification with CytoSorb in 359 Critically Ill Patients. Blood Purif. 2023, 52, 759–767. [Google Scholar] [CrossRef]
- Jansen, A.; Waalders, N.J.B.; van Lier, D.P.T.; Kox, M.; Pickkers, P. CytoSorb hemoperfusion markedly attenuates circulating cytokine concentrations during systemic inflammation in humans in vivo. Crit. Care 2023, 27, 117. [Google Scholar] [CrossRef] [PubMed]
- Persic, V.; Jerman, A.; Malgaj Vrecko, M.; Berden, J.; Gorjup, V.; Stecher, A.; Lukic, M.; Jereb, M.; Taleska Stupica, G.; Gubensek, J. Effect of CytoSorb Coupled with Hemodialysis on Interleukin-6 and Hemodynamic Parameters in Patients with Systemic Inflammatory Response Syndrome: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 7500. [Google Scholar] [CrossRef]
- Garau, I.; Maerz, A.; Sehner, S.; Reuter, D.A.; Reichenspurner, H.; Zoellner, C.; Kubitz, J.C. Hemadsorption during cardiopulmonary bypass reduces interleukin 8 and tumor necrosis factor alpha serum levels in cardiac surgery: A randomized controlled trial. Minerva Anestesiol. 2019, 85, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Poli, E.C.; Alberio, L.; Bauer-Doerries, A.; Marcucci, C.; Roumy, A.; Kirsch, M.; De Stefano, E.; Liaudet, L.; Schneider, A.G. Cytokine clearance with CytoSorb® during cardiac surgery: A pilot randomized controlled trial. Crit. Care 2019, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Lang, H.; Vollmer Barbosa, C.; Tian, Z.; Melk, A.; Schmidt, B.M.W. Efficacy of CytoSorb (R): A systematic review and meta-analysis. Crit. Care 2023, 27, 215. [Google Scholar] [CrossRef]
- Träger, K.; Skrabal, C.; Fischer, G.; Datzmann, T.; Schroeder, J.; Fritzler, D.; Hartmann, J.; Liebold, A.; Reinelt, H. Hemoadsorption treatment of patients with acute infective endocarditis during surgery with cardiopulmonary bypass—A case series. Int. J. Artif. Organs 2017, 40, 240–249. [Google Scholar] [CrossRef]
- Haidari, Z.; Leiler, S.; Mamdooh, H.; Fittkau, M.; Boss, K.; Tyczynski, B.; Thielmann, M.; Bagaev, E.; El Gabry, M.; Wendt, D.; et al. Effect of intraoperative haemoadsorption therapy on cardiac surgery for active infective endocarditis with confirmed Staphylococcus aureus bacteraemia. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad010. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, L.-U.; Binczyk, R.; Riess, F.-C. Comparison of intraoperative versus intraoperative plus postoperative hemoadsorption therapy in cardiac surgery patients with endocarditis. Int. J. Artif. Organs 2019, 42, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Massoth, C.; Zarbock, A.; Meersch, M. Acute Kidney Injury in Cardiac Surgery. Crit. Care Clin. 2021, 37, 267–278. [Google Scholar] [CrossRef] [PubMed]
- König, C.; Röhr, A.C.; Frey, O.R.; Brinkmann, A.; Roberts, J.A.; Wichmann, D.; Braune, S.; Kluge, S.; Nierhaus, A. In vitro removal of anti-infective agents by a novel cytokine adsorbent system. Int. J. Artif. Organs 2019, 42, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Scandroglio, A.M.; Pieri, M.; Nardelli, P.; Fominskiy, E.; Calabrò, M.G.; Melisurgo, G.; Ajello, S.; Pappalardo, F. Impact of CytoSorb on kinetics of vancomycin and bivalirudin in critically ill patients. Artif. Organs 2021, 45, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Røed-Undlien, H.; Schultz, N.H.; Lunnan, A.; Husebråten, I.M.; Wollmann, B.M.; Molden, E.; Bjørnstad, J.L. In Vitro Apixaban Removal by CytoSorb Whole Blood Adsorber: An Experimental Study. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Scharf, C.; Weinelt, F.; Schroeder, I.; Paal, M.; Weigand, M.; Zoller, M.; Irlbeck, M.; Kloft, C.; Briegel, J.; Liebchen, U. Does the cytokine adsorber CytoSorb(®) reduce vancomycin exposure in critically ill patients with sepsis or septic shock? a prospective observational study. Ann. Intensive Care 2022, 12, 44. [Google Scholar] [CrossRef]
- Hassan, K.; Brüning, T.; Caspary, M.; Wohlmuth, P.; Pioch, H.; Schmoeckel, M.; Geidel, S. Hemoadsorption of Rivaroxaban and Ticagrelor during Acute Type A Aortic Dissection Operations. Ann. Thorac. Cardiovasc. Surg. 2022, 28, 186–192. [Google Scholar] [CrossRef]
- Mair, H.; Jilek, C.; Haas, B.; Lamm, P. Ticagrelor and Rivaroxaban Elimination with CytoSorb Adsorber before Urgent Off-Pump Coronary Bypass. Ann. Thorac. Surg. 2020, 110, e369–e370. [Google Scholar] [CrossRef]
- Rao, C.; Preissing, F.; Thielmann, M.; Wendt, D.; Haidari, Z.; Kalisnik, J.M.; Daake, L.; Traeger, K. Hemoadsorption Using CytoSorb(®) in Patients with Infective Endocarditis: A German-Based Budget Impact Analysis. J. Cardiovasc. Dev. Dis. 2023, 10, 366. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, A.; Li, Y.; Yang, M.; Wang, S.; Su, B. A Contemporary Review of the Use of Extracorporeal CytoSorb® Hemoadsorption Therapy in Patients with Infective Endocarditis. J. Clin. Med. 2024, 13, 763. https://doi.org/10.3390/jcm13030763
Gong A, Li Y, Yang M, Wang S, Su B. A Contemporary Review of the Use of Extracorporeal CytoSorb® Hemoadsorption Therapy in Patients with Infective Endocarditis. Journal of Clinical Medicine. 2024; 13(3):763. https://doi.org/10.3390/jcm13030763
Chicago/Turabian StyleGong, Anan, Yupei Li, Mei Yang, Shujing Wang, and Baihai Su. 2024. "A Contemporary Review of the Use of Extracorporeal CytoSorb® Hemoadsorption Therapy in Patients with Infective Endocarditis" Journal of Clinical Medicine 13, no. 3: 763. https://doi.org/10.3390/jcm13030763
APA StyleGong, A., Li, Y., Yang, M., Wang, S., & Su, B. (2024). A Contemporary Review of the Use of Extracorporeal CytoSorb® Hemoadsorption Therapy in Patients with Infective Endocarditis. Journal of Clinical Medicine, 13(3), 763. https://doi.org/10.3390/jcm13030763






