Diagnosis, Clinical Presentation and Management of Celiac Disease in Children and Adolescents in Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
- Department of Gastroenterology, Hepatology, Nutrition Disorders, and Pediatrics and the Department of Pathomorphology, The Children’s Memorial Health Institute, Warsaw (POL-1)
- Department of Paediatric Endoscopy and Gastrointestinal Function Testing, Ludwik Rydygier Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Torun, Bydgoszcz (POL-2)
- Department of Pediatrics, Faculty of Medical Sciences, Medical University of Silesia in Katowice, Katowice (POL-3)
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Patients
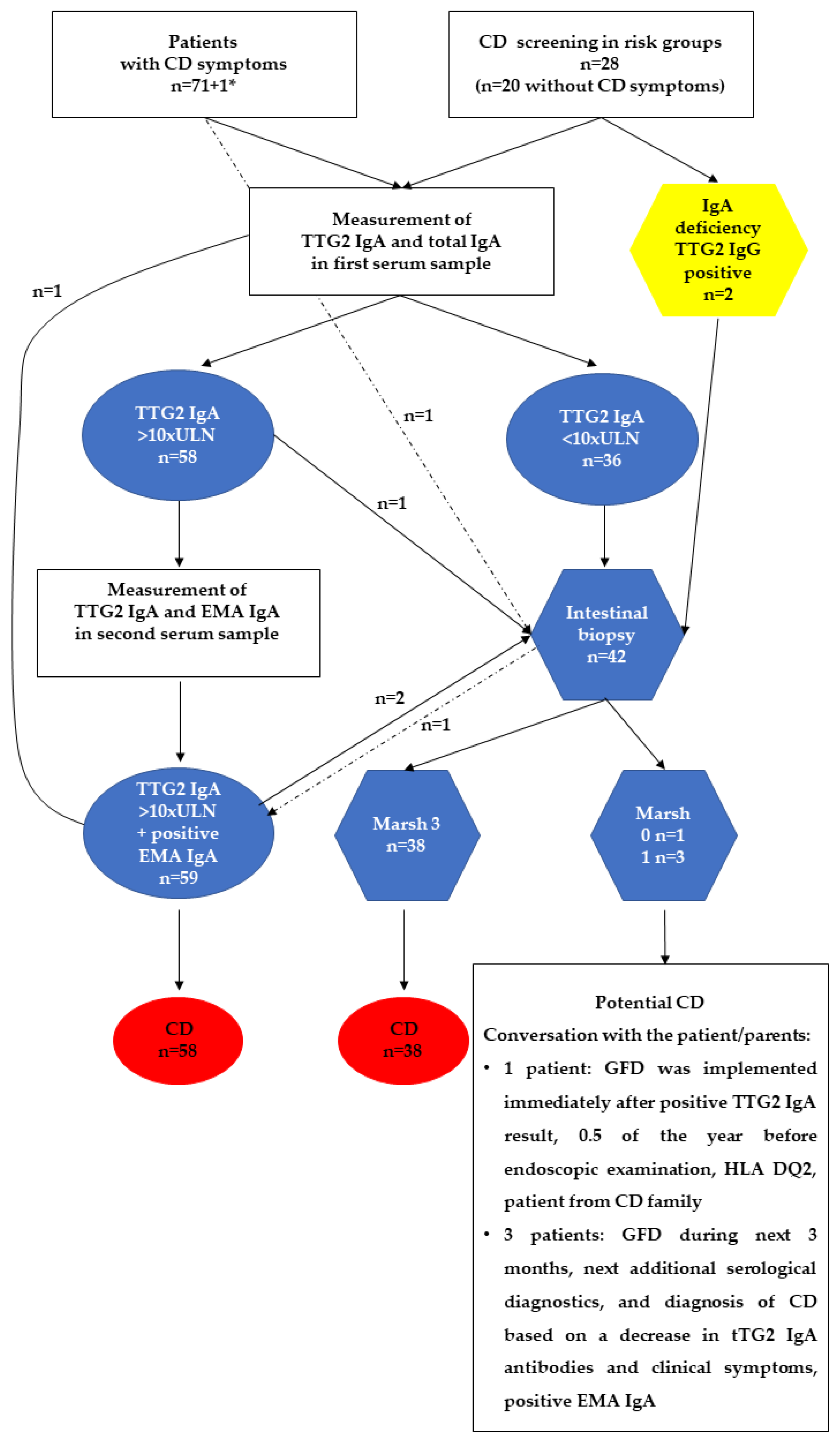
3.2. CD Diagnostic Approach
3.3. CD Screening in At-Risk Groups
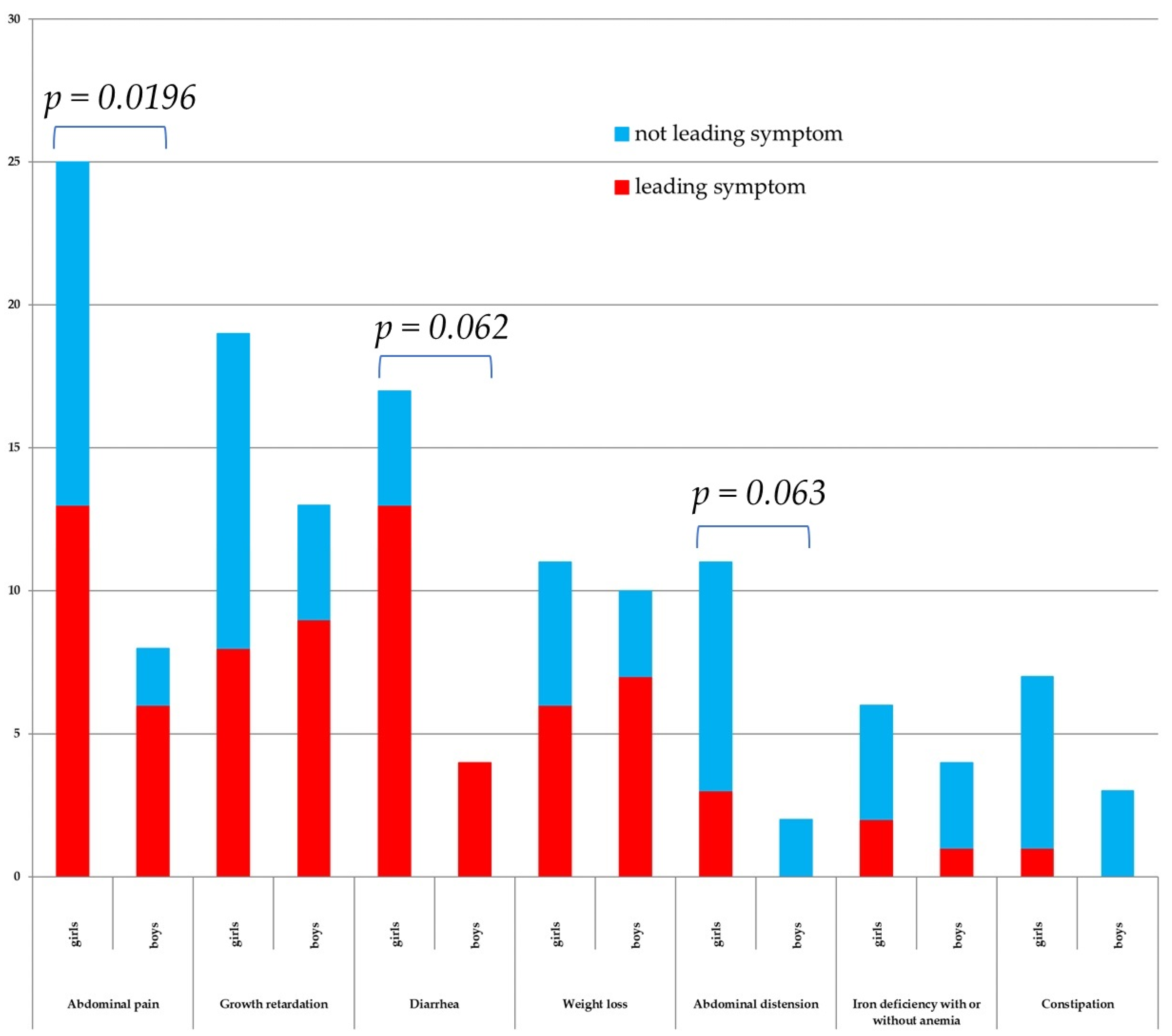
3.4. CD Symptoms
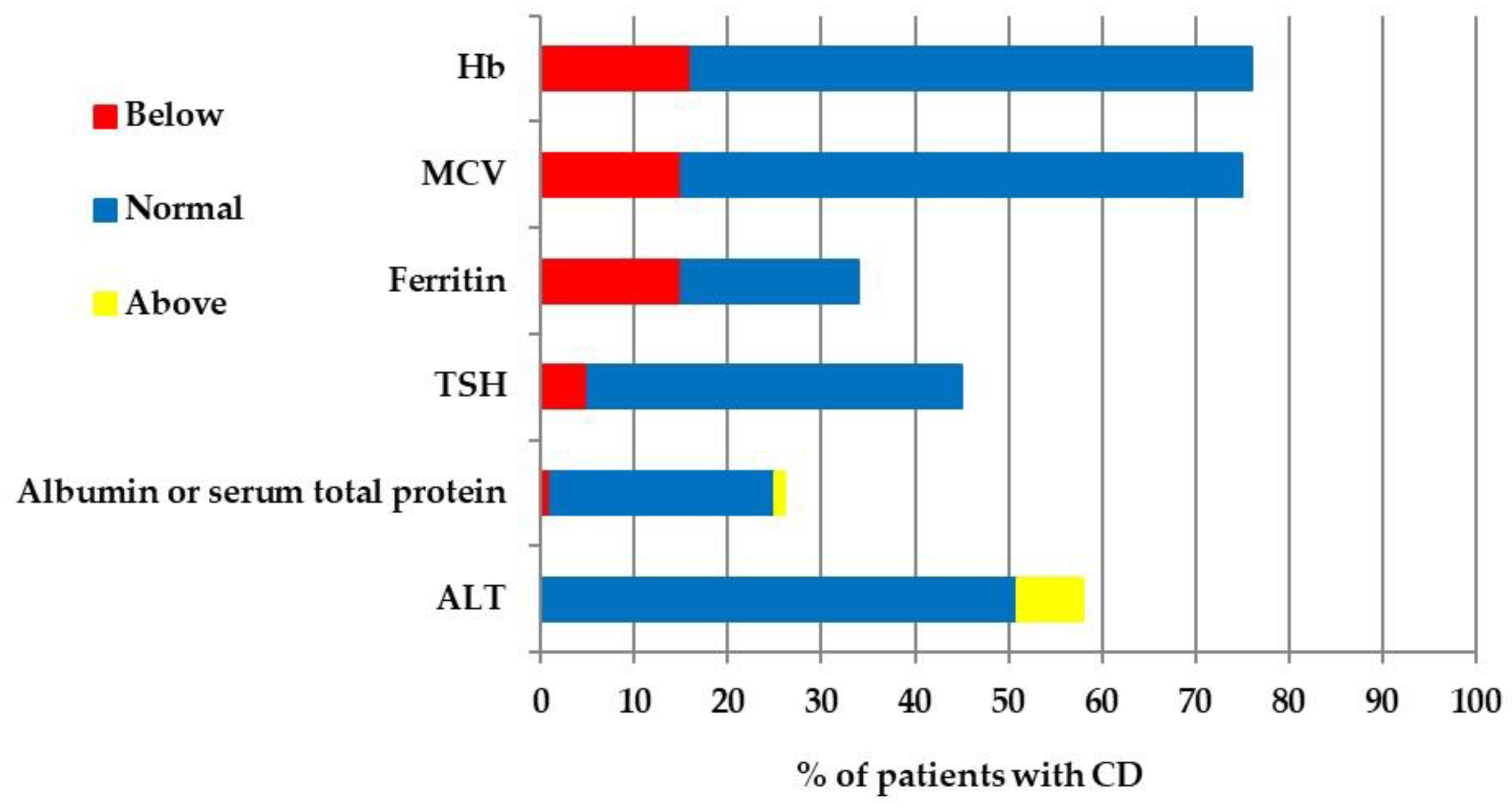
3.5. CD Management
4. Discussion
Study Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lundin, K.E.A.; Qiao, S.W.; Snir, O.; Sollid, L.M. Coeliac disease—From genetic and immunological studies to clinical applications. Scand. J. Gastroenterol. 2015, 50, 708–717. [Google Scholar] [CrossRef]
- Lurie, Y.; Landau, D.A.; Pfeffer, J.; Oren, R. Celiac disease diagnosed in the elderly. J. Clin. Gastroenterol. 2008, 42, 59–61. [Google Scholar] [CrossRef]
- Husby, S.; Murray, J.A. Diagnosing coeliac disease and the potential for serological markers. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 655–663. [Google Scholar] [CrossRef]
- Majsiak, E.; Choina, M.; Golicki, D.; Gray, A.M.; Cukrowska, B. The impact of symptoms on quality of life before and after diagnosis of coeliac disease: The results from a Polish population survey and comparison with the results from the United Kingdom. BMC Gastroenterol. 2021, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- Majsiak, E.; Choina, M.; Gray, A.M.; Wysokiński, M.; Cukrowska, B. Clinical manifestation and diagnostic process of celiac disease in Poland—Comparison of pediatric and adult patients in retrospective study. Nutrients 2022, 14, 491. [Google Scholar] [CrossRef] [PubMed]
- Rybak, A.; Socha, P.; Stolarczyk, A.; Cukrowska, B.; Obrycki, Ł.; Wierzbicka, A.; Oralewska, B.; Szaflarska-Popławska, A.; Iwańczak, B.; Jarocka-Cyrta, E.; et al. Clinical picture of celiac disease in children in Poland. Standardy Med. Pediatria 2014, 11, 297–304. (In Polish) [Google Scholar]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Card, T.R.; Kaukinen, K.; Bai, J.; Zingone, F.; Sanders, D.S.; Murray, J.A. Screening for celiac disease in the general population and in high-risk groups. United Eur. Gastroenterol. J. 2015, 3, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Parzanese, I.; Qehajaj, D.; Patrinicola, F.; Aralica, M.; Chiriva-Internati, M.; Stifter, S.; Elli, L.; Grizzi, F. Celiac disease: From pathophysiology to treatment. World J. Gastrointest. Pathophysiol. 2017, 8, 27–38. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef]
- Riznik, P.; De Leo, L.; Dolinsek, J.; Gyimesi, J.; Klemenak, M.; Koletzko, B.; Koletzko, S.; Korponay-Szabó, I.R.; Krencnik, T.; Not, T.; et al. Diagnostic Delays in Children with Coeliac Disease in the Central European Region. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Riznik, P.; Carnohorski, I.; Dolinsek, J.; Dragutinovic, N.; Gyimesi, J.; Hauer, A.H.; Kamhi Trop, T.; Klemenak, M.; Korponay-Szabo, I.R.; Krencnik, T.; et al. Diagnostic approach in children with newly diagnosed coeliac disease—Are the ESPGHAN guidelines followed appropriately? In Proceedings of the ESPGHAN 55th Annual Meeting, Vienna, Austria, 17–20 May 2023. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 211. [Google Scholar] [CrossRef]
- Serwis JAKICENTYL.PL. Available online: https://www.jakicentyl.pl/ (accessed on 20 August 2023).
- Kułaga, Z.; Różdżyńska, A.; Palczewska, A.; Grajda, A.; Gurzkowska, B.; Napieralska, E.; Litwin, M. Siatki centylowe wysokości, masy ciała i wskaźnika masy ciała dzieci i młodzieży w Polsce—Wyniki badania OLAF. Stand. Med. Pediatr. 2010, 7, 690–700. [Google Scholar]
- Kułaga, Z.; Litwin, M.; Tkaczyk, M.; Palczewska, I.; Zajączkowska, M.; Zwolińska, D.; Krynicki, T.; Wasilewska, A.; Moczulska, A.; Morawiec-Knysak, A.; et al. Polish 2010 growth references for school-aged children and adolescents. Eur. J. Pediatr. 2011, 170, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Kułaga, Z.; Grajda, A.; Gurzkowska, B.; Góźdź, M.; Wojtyło, M.; Swiąder, A.; Różdżyńska-Świątkowska, A.; Litwin, M. Polish 2012 growth references for preschool children. Eur. J. Pediatr. 2013, 172, 753–761. [Google Scholar] [CrossRef] [PubMed]
- de Onis, M.; Blössner, M. The World Health Organization Global Database on Child Growth and Malnutrition: Methodology and applications. Int. J. Epidemiol. 2003, 32, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.N. Gluten, major histocompatibility complex and the small intestine: A molecular and immunobiologic approach to the spectrum of gluten sensitivity (celiac sprue). Gastroenterology 1992, 102, 330–354. [Google Scholar] [CrossRef]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The histopathology of coeliac disease time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef]
- Ravikumara, M.; Tuthill, D.P.; Jenkins, H.R. The changing clinical presentation of coeliac disease. Arch. Dis. Child. 2006, 91, 969–971. [Google Scholar] [CrossRef]
- Tapsas, D.; Holle´n, E.; Stenhammar, L.; Fälth-Magnusson, K. The clinical presentation of coeliac disease in 1030 Swedish children: Changing features over the past four decades. Dig. Liver Dis. 2016, 48, 16–22. [Google Scholar] [CrossRef]
- Kivelä, L.; Kaukinen, K.; Lähdeaho, M.L.; Huhtala, H.; Ashorn, M.; Ruuska, T.; Hiltunen, P.; Visakorpi, J.; Mäki, M.; Kurppa, K. Presentation of celiac disease in Finnish children is no longer changing: A 50-year perspective. J. Pediatr. 2015, 167, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Riznik, P.; De Leo, L.; Dolinsek, J.; Gyimesi, J.; Klemenak, M.; Koletzko, B.; Koletzko, S.; Korponay-Szabó, I.R.; Krencnik, T.; Not, T.; et al. Clinical Presentation in Children with Coeliac Disease in Central Europe. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Van Kalleveen, M.W.; De Meij, T.; Plötz, F.B. Clinical spectrum of paediatric coeliac disease: A 10-year single-centre experience. Eur. J. Pediatr. 2018, 177, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Mearin, M.L.; Agardh, D.; Antunes, H.; Al-Toma, A.; Auricchio, R.; Castillejo, G.; Catassi, C.; Ciacci, C.; Discepolo, V.; Dolinsek, J.; et al. Position Paper on Management and Follow-up of Children and Adolescents With Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.C.; Corkins, M.R.; Malone, A.; Miller, S.; Mogensen, K.M.; Guenter, P.; Jensen, G.L.; ASPEN Malnutrition Committee. The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr. Clin. Pract. 2021, 36, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and clinical significance. J. Parenter. Enteral. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Jańczyk, W.; Bierła, J.B.; Trojanowska, I.; Wierzbicka-Rucińska, A.; Cukrowska, B.; Socha, P. Prevalence and significance of autoantibody seropositivity in children with Wilson’s disease. Diagnostics 2023, 13, 768. [Google Scholar] [CrossRef]
- Coeliac Section of the Polish Society of Pediatric Gastroenterology, Hepatology and Nutrition. Available online: https://ptghizd.pl/sekcje/sekcja-celiakalna/ (accessed on 29 November 2023). (In Polish).
- Bierła, J.B.; Cukrowska, B. News about celiac disease—Report from the celiac session of the 51st congress ESPGHAN 2018. Stand. Med. Pediatr. 2018, 15, 566–568. (In Polish) [Google Scholar]
- Bierła, J.B.; Majsiak, E.; Cukrowska, B. Report of the 18th International Celiac Disease Symposium. Stand. Med. Pediatr. 2019, 16, 592–595. (In Polish) [Google Scholar]
- Bierła, J.B. Celiac disease news—Guidelines from the 54th annual meeting of ESPGHAN (2022). Stand. Med. Pediatr. 2022, 19, 576–584. (In Polish) [Google Scholar]
- Krzyżanowska-Sołtysiak, M. Tests to Detect Celiac Disease Are Free in Poland. Bezglutenowa.mama.pl. 21 May 2017. Available online: https://bezglutenowamama.pl/badania-wykrywajace-celiakie-sa-w-polsce-bezplatne (accessed on 29 November 2023). (In Polish).
- Krzyżanowska-Sołtysiak, M. Can a Family Doctor Order Blood Tests to Detect Celiac Disease? Bezglutenowa.mama.pl. 23 September 2022. Available online: https://bezglutenowamama.pl/czy-lekarz-rodzinny-moze-zlecic-badania-krwi-wykrywajace-celiakie (accessed on 29 November 2023). (In Polish).
| Girls n or Range | Boys n or Range | |
|---|---|---|
| Number of patients | 56 | 44 |
| Age (years) | 1.6–18.0 | 3.4–18.0 |
| Growth (cm) | 58–173 | 92–177 |
| Body weight (kg) | 11–57 | 12–70 |
| BMI | 12.65–32.70 | 11.42–26.87 |
| CD risk groups | 22 | 18 |
| Asymptomatic | 8 | 11 |
| “No-biopsy” approach | 30 | 28 |
| Endoscopy/intestinal biopsies | 26 Marsh 3 (n = 1 Marsh 0; n = 2 Marsh 1) | 16 Marsh 3 (n = 1 Marsh 1) |
| CD Risk Groups | |||||||
|---|---|---|---|---|---|---|---|
| CD in Family |
IgA Deficiency | Thyroiditis | DMT1 |
Down’s Syndrome | Turner Syndrome | Other: | |
| Since birth: | 7 | 0 | 0 | 0 | 1 | 1 | 1 |
| <1 year: | 0 | 0 | 1 | 9 | 0 | ||
| 1–5 years: | 8 | 2 | 4 | 5 | 0 | ||
| 5–10 years: | 3 | 0 | 1 | 2 | 0 | ||
| SUM = 46 * | 18 | 2 | 6 | 16 | 1 | 1 | 1 |
|
Tested (% of risk group) ** | 10 (55.56%) | 1 (50.00%) | 2 (33.33%) | 7 (43.75%) | 1 (100%) | 1 (100%) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bierła, J.B.; Szaflarska-Popławska, A.; Grzybowska-Chlebowczyk, U.; Oralewska, B.; Cyba, M.; Oracz, G.; Konopka, E.; Cukrowska, B.; Syczewska, M.; Kołodziejczyk, H.; et al. Diagnosis, Clinical Presentation and Management of Celiac Disease in Children and Adolescents in Poland. J. Clin. Med. 2024, 13, 765. https://doi.org/10.3390/jcm13030765
Bierła JB, Szaflarska-Popławska A, Grzybowska-Chlebowczyk U, Oralewska B, Cyba M, Oracz G, Konopka E, Cukrowska B, Syczewska M, Kołodziejczyk H, et al. Diagnosis, Clinical Presentation and Management of Celiac Disease in Children and Adolescents in Poland. Journal of Clinical Medicine. 2024; 13(3):765. https://doi.org/10.3390/jcm13030765
Chicago/Turabian StyleBierła, Joanna B., Anna Szaflarska-Popławska, Urszula Grzybowska-Chlebowczyk, Beata Oralewska, Marta Cyba, Grzegorz Oracz, Ewa Konopka, Bożena Cukrowska, Małgorzata Syczewska, Honorata Kołodziejczyk, and et al. 2024. "Diagnosis, Clinical Presentation and Management of Celiac Disease in Children and Adolescents in Poland" Journal of Clinical Medicine 13, no. 3: 765. https://doi.org/10.3390/jcm13030765
APA StyleBierła, J. B., Szaflarska-Popławska, A., Grzybowska-Chlebowczyk, U., Oralewska, B., Cyba, M., Oracz, G., Konopka, E., Cukrowska, B., Syczewska, M., Kołodziejczyk, H., Rižnik, P., & Dolinšek, J. (2024). Diagnosis, Clinical Presentation and Management of Celiac Disease in Children and Adolescents in Poland. Journal of Clinical Medicine, 13(3), 765. https://doi.org/10.3390/jcm13030765








