Early Impact of Mobilization Process on Cardiac Function and Size in Patients Undergoing Autologous Hematopoietic Stem Cell Transplantation
Abstract
:1. Introduction
2. Materials and Methods
- Written consent to participate in the study;
- Patients over the age of 18 years scheduled for autologous HSCT for various reasons.
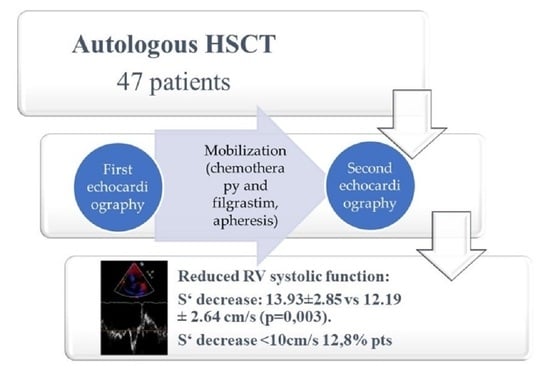
3. Results
Sub-Analysis of Patients with Cyclophosphamide-Based Chemotherapy
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devine, H.; Tierney, D.K.; Schmit-Pokorny, K.; McDermott, K. Mobilization of hematopoietic stem cells for use in autologous transplantation. Clin. J. Oncol. Nurs. 2010, 14, 212–222. [Google Scholar] [CrossRef]
- Cardinale, D.; Biasillo, G.; Salvatici, M.; Sandri, M.T.; Cipolla, C.M. Using biomarkers to predict and to prevent cardiotoxicity of cancer therapy. Expert Rev. Mol. Diagn. 2017, 17, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Karakukcu, M.; Unal, E. Stem cell mobilization and collection from pediatric patients and healthy children. Transfus. Apher. Sci. 2015, 53, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, W.I.; DiPersio, J.F.; McCarty, J.M. Improving stem cell mobilization strategies: Future directions. Bone Marrow Transpl. 2009, 43, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Niederwieser, D.; Baldomero, H.; Atsuta, Y.; Aljurf, M.; Seber, A.; Greinix, H.T.; Koh, M.; Worel, N.; Galeano, S.; Jaimovich, G.; et al. One and Half Million Hematopoietic Stem Cell Transplants (HSCT). Dissemination, Trends and Potential to Improve Activity by Telemedicine from the Worldwide Network for Blood and Marrow Transplantation (WBMT). Blood 2019, 134, 2035. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Gratwohl, A.; Bregni, M.; Cesaro, S.; Dreger, P.; de Witte, T.; Farge-Bancel, D.; Gaspar, B.; Marsh, J.; et al. European Group for Blood and Marrow Transplantation (EBMT). The EBMT activity survey: 1990–2010. Bone Marrow Transpl. 2012, 47, 906–923. [Google Scholar] [CrossRef]
- Blaes, A.H.; Konety, S.H.; Hurley, P. Cardiovascular complications of hematopoietic stem cell transplantation. Curr. Treat. Options Cardiovasc. Med. 2016, 18, 25. [Google Scholar] [CrossRef]
- Tuzovic, M.; Mead, M.; Young, P.A.; Schiller, G.; Yang, E.H. Cardiac Complications in the Adult Bone Marrow Transplant Patient. Curr. Oncol. Rep. 2019, 21, 28. [Google Scholar] [CrossRef]
- Armenian, S.H.; Sun, C.L.; Shannon, T.; Mills, G.; Francisco, L.; Venkataraman, K.; Wong, F.L.; Forman, S.J.; Bhatia, S. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood 2011, 118, 6023–6029. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Ohmoto, A.; Fuji, S. Cardiac complications associated with hematopoietic stem-cell transplantation. Bone Marrow Transplant. 2021, 56, 2637–2643. [Google Scholar] [CrossRef]
- Wu, V.C.C.; Takeuchi, M. Echocardiographic assessment of right ventricular systolic function. Cardiovasc. Diagn. Ther. 2018, 8, 70–79. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [PubMed]
- Rotz, S.J.; Ryan, T.J.; Hlavaty, J.; George, S.A.; El-Bietar, J.; Dandoy, C.E. Cardiotoxicity and cardiomyopathy in children and young adult survivors of hematopoietic stem cell transplant. Pediatr. Blood Cancer 2017, 64, e26600. [Google Scholar] [CrossRef]
- Muckiene, G.; Vaitiekus, D.; Žaliaduonytė, D.; Zabiela, V.; Verseckaite-Costa, R.; Vaiciuliene, D.; Juozaityte, E.; Jurkevicius, R. Prognostic Impact of Global Longitudinal Strain and NT-proBNP on Early Development of Cardiotoxicity in Breast Cancer Patients Treated with Anthracycline-Based Chemotherapy. Medicina 2023, 59, 953. [Google Scholar] [CrossRef] [PubMed]
- Roziakova, L.E.B.; Mistrik, M.; Dubrava, J.; Gergel, J.; Lenkova, N.; Mladosievicova, B. Serial measurements of cardiac biomarkers in patients after allogeneic hematopoietic stem cell transplantation. J. Exp. Clin. Cancer Res. 2012, 31, 13. [Google Scholar] [CrossRef]
- Michel, L.; Rassaf, T.; Totzeck, M. Biomarkers for the detection of apparent and subclinical cancer therapy-related cardiotoxicity. J. Thorac. Dis. 2018, 10 (Suppl. 35), S4282–S4295. [Google Scholar] [CrossRef] [PubMed]
- Di Lisi, D.; Manno, G.; Novo, G. Subclinical cardiotoxicity: The emerging role of myocardial work and other imaging techniques. Curr. Probl. Cardiol. 2021, 46, 100818. [Google Scholar] [CrossRef] [PubMed]
- Vaitiekus, D.; Muckienė, G.; Vaitiekiene, A.; Sereikaitė, L.; Inčiūraitė, R.; Insodaite, R.; Čepulienė, D.; Kupčinskas, J.; Ugenskienė, R.; Jurkevičius, R.; et al. HFE gene variants’ impact on Anthracycline-Based Chemotherapy-Induced subclinical cardiotoxicity. Cardiovasc. Toxicol. 2021, 21, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Harvanová, Ľ.; Lábska, V.E.B.; Hrubiško, M.; Bátorová, A.; Dúbrava, J.; Gergeľ, J.; Mladosievičová, B. Cardiovascular complications among hematopoietic cell transplantation survivors-the role of cardiomarkers. Klin. Onkol. 2022, 35, 454–460. [Google Scholar] [CrossRef]
- Vaitiekus, D.; Muckiene, G.; Vaitiekiene, A.; Maciuliene, D.; Vaiciuliene, D.; Ambrazeviciute, G.; Sereikaite, L.; Verikas, D.; Jurkevičius, R.; Juozaityte, E. Impact of arterial hypertension on Doxorubicin-Based Chemotherapy-Induced subclinical cardiac damage in breast cancer patients. Cardiovasc. Toxicol. 2020, 20, 321–327. [Google Scholar] [CrossRef]
- Edward, J.; Banchs, J.; Parker, H.; Cornwell, W. Right ventricular function across the spectrum of health and disease. Heart 2023, 109, 349–355. [Google Scholar] [CrossRef]
- Ghio, S.; Gavazzi, A.; Campana, C.; Inserra, C.; Klersy, C.; Sebastiani, R.; Arbustini, E.F.R.; Tavazzi, L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J. Am. Coll. Cardiol. 2001, 37, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Tanındı, A.; Demirci, U.; Taçoy, G.; Buyukberber, S.; Alsancak, Y.; Coskun, U.; Yalcin, R.; Benekli, M. Assessment of right ventricular functions during cancer chemotherapy. Eur. J. Echocardiogr. 2011, 12, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Tekiner, F.; Yildiz, C.; Güner, Ş.İ. Evaluation of right ventricle functions might be a method for early recognizing cardiotoxicity in hematopoietic stem cell transplantation. Int. J. Cardiovasc. Acad. 2020, 6, 46. [Google Scholar] [CrossRef]
- Rotz, S.J.; Ryan, T.J.; Hayek, S.S. Cardiovascular disease and its management in children and adults undergoing hematopoietic stem cell transplantation. J. Thromb. Thrombolysis 2021, 51, 854–869. [Google Scholar] [CrossRef]
- Nishikawa, T.; Miyahara, E.; Kurauchi, K.; Watanabe, E.; Ikawa, K.; Asaba, K.; Tanabe, T.; Okamoto, Y.; Kawano, Y. Mechanisms of Fatal Cardiotoxicity following High-Dose Cyclophosphamide Therapy and a Method for Its Prevention. PLoS ONE 2015, 10, e0131394. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rahman, T.; Dunham, A.; Huang, H.; Bukhari, S.M.A.; Mehta, A.; Awuah, W.A.; Ede-Imafidon, D.; Cantu-Herrera, E.; Talukder, S.; Joshi, A.; et al. Chemotherapy Induced Cardiotoxicity: A state of the art review on general mechanisms, prevention, treatment and recent advances in novel therapeutics. Curr. Probl. Cardiol. 2023, 48, 101591. [Google Scholar] [CrossRef]
- Nagler, A.; Rocha, V.; Labopin, M.; Ünal, A.; Cesaro, S.; Campos, A.; Volin, L.; Poiré, X.; Aljurf, M.; Masszi, T.; et al. Allogeneic Hematopoietic Stem-Cell Transplantation for Acute Myeloid Leukemia in Remission: Comparison of Intravenous Busulfan Plus Cyclophosphamide (Cy) Versus Total-Body Irradiation Plus Cy As Conditioning Regimen—A Report From the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J. Clin. Oncol. 2013, 31, 3549–3556. [Google Scholar] [CrossRef]
- O’Donnell, P.V.; Luznik, L.; Jones, R.J.; Vogelsang, G.B.; Leffell, M.S.; Phelps, M.; Rhubart, P.; Cowan, K.H.; Piantadosi, S.; Fuchs, E.J. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol. Blood Marrow Transplant. 2002, 8, 377–386. [Google Scholar] [CrossRef]
- Martin, M.A.; Fornecker, L.M.; Marcellin, L.; Mousseaux, E.; Hij, A.; Snowden, J.A.; Farge, D.; Martin, T. Acute and fatal cardiotoxicity following high-dose cyclophosphamide in a patient undergoing autologous stem cell transplantation for systemic sclerosis despite satisfactory cardiopulmonary screening. Bone Marrow Transplant. 2017, 52, 1674–1677. [Google Scholar] [CrossRef] [PubMed]
- Poręba, M.; Gać, P.; Usnarska-Zubkiewicz, L.; Pilecki, W.; Kuliczkowski, K.; Mazur, G.; Sobieszczańska, M.; Poręba, R. Echocardiographic evaluation of the early cardiotoxic effect of hematopoietic stem cell transplantation in patients with hematologic malignancies. Leuk. Lymphoma 2016, 57, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Ishida, M.; Nakatani, T.; Fukuhara, S.; Hisashi, Y.; Ohtsu, Y.; Suga, M.; Yutani, C.; Yahihara, T.; Yamada, K.; et al. Bone marrow is a source of regenerated cardiomyocytes in doxorubicin-induced cardiomyopathy and granulocyte colony-stimulating factor enhances migration of bone marrow cells and attenuates cardiotoxicity of doxorubicin under electron microscopy. J. Heart Lung Transplant. 2004, 23, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.H.; Joe, J.H.; Jang, K.S.; Song, Y.S.; So, B.I.; Fang, C.H.; Shin, J.; Kim, J.H.; Lim, H.K.; Kim, K.S. Effects of granulocyte-colony stimulating factor (G-CSF) on diabetic cardiomyopathy in Otsuka Long-Evans Tokushima fatty rats. Cardiovasc. Diabetol. 2011, 10, 92. [Google Scholar] [CrossRef]
- Pourtaji, A.; Sahebkar, A.; Poorzand, H.; Moshiri, M.; Mohammadpour, A.H.; Mousavi, S.R. Evaluation of the Cardioprotective Effect of Granulocyte Colony Stimulating Factor in Patients with Carbon Monoxide Poisoning. Protein Pept. Lett. 2021, 28, 589–601. [Google Scholar] [CrossRef]
- Haybar, H.; Shahrabi, S.; Zayeri, Z.D.; Pezeshki, S.S. Strategies to increase cardioprotection through cardioprotective chemokines in chemotherapy-induced cardiotoxicity. Int. J. Cardiol. 2018, 269, 276–282. [Google Scholar] [CrossRef]

| Sex | |
| Male, n (%) | 27 (57.4) |
| Female, n (%) | 20 (42.6) |
| Age, years (median (min–max)) | 61 (18–74) |
| Main disease | |
| Multiple Myeloma, n (%) | 35 (74.5) |
| Mantle cell lymphoma, n (%) | 4 (8.5) |
| Hodgkin‘s lymphoma, n (%) | 3 (6.4) |
| PCNS diffuse large B cell lymphoma, n (%) | 2 (4.3) |
| Anaplastic large cell lymphoma, n (%) | 1 (2.1) |
| Peripheral T cell lymphoma, n (%) | 1 (2.1) |
| Ewing sarcoma, n (%) | 1 (2.1) |
| Echocardiographic Values | Before Mobilization | After Mobilization | p |
|---|---|---|---|
| LVEDD, mm | 46.48 ± 4.05 | 46.02 ± 5.25 | 0.633 |
| LVEDDi, mm/m2 | 24.85 ± 2.78 | 24.75 ± 2.93 | 0.863 |
| LV EF, % | 60.49 ± 7.66 | 59.74 ± 7.08 | 0.622 |
| GLS, % | −17.45 ± 3.62 | −17.59 ± 3.65 | 0.309 |
| E/E‘ | 6.58 ± 3.28 | 6.91 ± 2.65 | 0.973 |
| RVEDD, mm | 36.02 ± 3.83 | 34.96 ± 4.44 | 0.217 |
| S‘, cm/s | 13.93 ± 2.85 | 12.19 ± 2.64 | 0.003 |
| Cardiovascular Risk Factors | N of Risk Factors among All Patients (%) | Patients with S’ after Mobilization ≥ 10 (n (%)) | Patients with S’ after Mobilization < 10 (n (%)) | p |
|---|---|---|---|---|
| Coronary artery disease (CAD) 1 | 4 (8.5) | 3 (75.0) | 1 (25.0) | 0.432 |
| Arterial hypertension | 23 (48.9) | 19 (82.6) | 4 (17.4) | 0.416 |
| Diabetes mellitus | 6 (12.8) | 5 (83.3) | 1 (16.7) | 1.000 |
| Family history of CAD | 10 (21.3) | 8 (80.0) | 2 (20.0) | 0.594 |
| Dyslipidaemia | 41 (87.2) | 36 (87.8) | 5 (12.2) | 1.000 |
| Smoking | 0 (0) | 0 (0) | 0 (0) | |
| Previous smoking | 7 (14.9) | 6 (85.7) | 1 (14.3) | 1.000 |
| Cardiovascular Risk Factors | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Coronary artery disease (CAD) | 2.533 | 0.219–29.290 | 0.457 |
| Arterial hypertension | 2.316 | 0.381–14.079 | 0.362 |
| Diabetes mellitus | 1.440 | 0.138–14.978 | 0.760 |
| Family history of CAD | 2.062 | 0.320–13.313 | 0.447 |
| Dyslipidaemia | 0.694 | 0.067–7.223 | 0.760 |
| Previous smoking | 1.167 | 0.115–11.814 | 0.896 |
| Echocardiographic Values | Before Mobilization | After Mobilization | p |
|---|---|---|---|
| LVEDD, mm | 45.87 ± 3.91 | 45.08 ± 5.14 | 0.469 |
| LVEDDi, mm/m2 | 24.49 ± 2.76 | 24.29 ± 2.90 | 0.768 |
| LV EF, % | 61.67 ± 6.82 | 60.40 ± 7.09 | 0.449 |
| GLS, % | −17.87 ± 3.89 | −18.00 ± 3.59 | 0.886 |
| E/E‘ | 7.39 ± 3.29 | 7.05 ± 2.77 | 0.634 |
| RVEDD, mm | 36.26 ± 3.97 | 35.14 ± 4.74 | 0.290 |
| S‘, cm/s | 13.89 ± 3.12 | 12.20 ± 2.68 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaitiekiene, A.; Kulboke, M.; Bieseviciene, M.; Bartnykaite, A.; Kireilis, B.; Rinkuniene, D.; Jankauskas, A.; Zemaitis, J.; Gaidamavicius, I.; Gerbutavicius, R.; et al. Early Impact of Mobilization Process on Cardiac Function and Size in Patients Undergoing Autologous Hematopoietic Stem Cell Transplantation. J. Clin. Med. 2024, 13, 773. https://doi.org/10.3390/jcm13030773
Vaitiekiene A, Kulboke M, Bieseviciene M, Bartnykaite A, Kireilis B, Rinkuniene D, Jankauskas A, Zemaitis J, Gaidamavicius I, Gerbutavicius R, et al. Early Impact of Mobilization Process on Cardiac Function and Size in Patients Undergoing Autologous Hematopoietic Stem Cell Transplantation. Journal of Clinical Medicine. 2024; 13(3):773. https://doi.org/10.3390/jcm13030773
Chicago/Turabian StyleVaitiekiene, Audrone, Migle Kulboke, Monika Bieseviciene, Agne Bartnykaite, Benas Kireilis, Diana Rinkuniene, Antanas Jankauskas, Justinas Zemaitis, Ignas Gaidamavicius, Rolandas Gerbutavicius, and et al. 2024. "Early Impact of Mobilization Process on Cardiac Function and Size in Patients Undergoing Autologous Hematopoietic Stem Cell Transplantation" Journal of Clinical Medicine 13, no. 3: 773. https://doi.org/10.3390/jcm13030773
APA StyleVaitiekiene, A., Kulboke, M., Bieseviciene, M., Bartnykaite, A., Kireilis, B., Rinkuniene, D., Jankauskas, A., Zemaitis, J., Gaidamavicius, I., Gerbutavicius, R., Vaitiekus, D., Vaskelyte, J. J., & Sakalyte, G. (2024). Early Impact of Mobilization Process on Cardiac Function and Size in Patients Undergoing Autologous Hematopoietic Stem Cell Transplantation. Journal of Clinical Medicine, 13(3), 773. https://doi.org/10.3390/jcm13030773