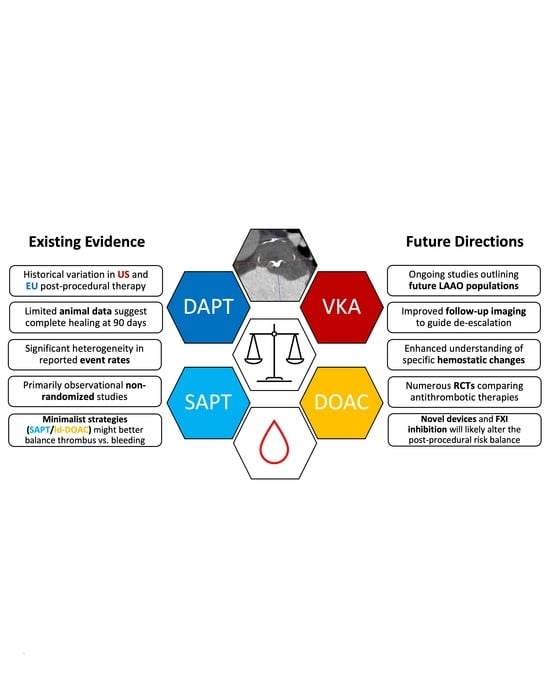
Left Atrial Appendage Occlusion and Post-procedural Antithrombotic Management
Abstract
:1. Introduction
2. Therapeutic Rationale
2.1. Hemostatic Changes
2.2. Additional Risk Factors
3. Antithrombotic Strategies
3.1. Oral Anticoagulation
3.1.1. Vitamin K Antagonists
| Anticoagulation-Specific Studies | DRT * | Ischemic Stroke ** | Major Bleeding ** | CV Mortality ** | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Publ. | n | Device(s) | Random | FU (Months) | DOAC | VKA | DOAC | VKA | DOAC | VKA | DOAC | VKA |
| VKA | |||||||||||||
| PROTECT-AF [14] | 2009 | 463 | WM | Yes | 18 (±10) | 2.2% | 3.5% | 0.7% | |||||
| PREVAIL [15] | 2014 | 269 | WM | Yes | 18 | 1.9% | 2.6% | ||||||
| DOAC | |||||||||||||
| Della-Rocca (DOAC) [58] | 2021 | 357 | WM | No | 14 (IQR; 12, 15) | 3.4% | 1.7% | 3.4% | 2.8% | ||||
| Della-Rocca (ldDOAC) [58] | 2021 | 198 | WM | No | 13 (IQR; 12, 14) | 0.0% | 0.0% | 0.5% | 2.0% | ||||
| Pinnacle FLX ^ [20] | 2021 | 395 | WM FLX | No | 12 | 1.8% | 2.6% | 7.9% | 4.4% | ||||
3.1.2. Direct Oral Anticoagulation
3.2. Antiplatelet Therapy
| Antiplatelet-Specific Studies | DRT * | Ischemic Stroke ** | Major Bleeding ** | CV Mortality ** | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Publ. | n | Device(s) | Random | FU (Months) | SAPT | DAPT | SAPT | DAPT | SAPT | DAPT | SAPT | DAPT |
| DAPT | |||||||||||||
| ASAP study [70] | 2013 | 150 | WM | No | 14.4 (±8.6) | 4.2% | 1.7% | 2.1% | |||||
| Urena et al. [74] | 2013 | 52 | ACP | No | 20.0 (±5.0) | 0.0% | 1.9% | 1.9% | 1.9% | ||||
| Weise et al. [52] | 2016 | 298 | ACP/AM/WM/WC | No | 26.9 (±17.9) | 2.6% | 1.7% | 3.9% | |||||
| Pracon et al. [38] ^ | 2018 | 99 | ACP/AM/WM | No | 12 | 7.1% | 1.0% | 6.1% | |||||
| PRAGUE-17 [75] ^ | 2020 | 201 | AM/WM (FLX) | Yes | 19 (IQR; 12, 28) | 3.4% | 2.6% | 3.8% | 3.2% | ||||
| FLXibility ^ [21] | 2023 | 300 | WM FLX | No | 12 | 2.4% | 2.0% | 8.5% | 5.1% | ||||
| SAPT | |||||||||||||
| Rodriguez-Gabella et al. [71] | 2016 | 31 | ACP/AM/WM | No | 19 (IQR; 12, 24) | 3.3% | 0.0% | 3.2% | 3.2% | ||||
| Korsholm et al. [53] | 2017 | 107 | ACP/AM | No | 28 (IQR; 19, 38) | 1.9% | 2.3% | 3.8% | |||||
3.3. No Therapy
3.4. Comparing Strategies
4. Duration of Treatment
4.1. Device Healing–In Vivo
| Canine Studies | |||||
| Study | Publ. | n | Device Type | Antithrombotic Therapy | Results |
| Bass [25] | 2010 | 10 | ACP | ASA | 90 days: Atrial device surface covered by stable neointima. All animals displayed complete occlusion at both 30 and 90 days follow-up, as assessed by TEE and angiography. |
| Schwartz et al. [13] | 2010 | 9 | WM | VKA + ASA | 3 days (n = 3): Atrial device surface covered by organizing thrombus. 45 days (n = 3): Thin white pannus across the atrial device surface. Endocardial ingrowth covering all exposed surfaces and in continuation with LA surface. 90 days (n = 3): A monolayer of endothelial cells covering healthy neo-endocardium. |
| Kar et al. [24] | 2014 | 6 | ACP (3) WM (3) | VKA + ASA | 28 days: Complete neo-endocardial coverage of WM. Incomplete coverage of ACP disc. Remaining mild peri-device flow on TEE in both WM and ACP cases. |
| Kramer et al. [26] | 2022 | 5 | WM FLX | ASA + Clopidogrel | 45 days: Thin layer of endothelial cells covering the device surface in four out of five cases. One case displayed only partial neo-endothelial coverage. |
| Saliba et al. [80] | 2023 | 24 | WM FLX (12) FLX Pro (12) | None | 3 days (n = 6): Acute inflammation around fabric knots in 5.0% and 33.7% of FLX Pro and FLX cases, respectively. Reduced thrombus thickness in FLX Pro (0.3 mm) vs. FLX (1.5 mm) cases. 14 days (n = 6): Comparable inflammation in the two devices. Less thrombus thickness in FLX Pro (1.2 mm) vs. FLX (4.1 mm)—consistent with TEE findings. 45 days (n = 12): Smooth neo-endocardial coverage of 6/6 FLX Pro and 2/6 FLX devices. |
| Porcine Studies | |||||
| Study | Publ. | n | Device Type | Antithrombotic Therapy | Results |
| Saliba et al. [80] | 2023 | 8 | WM FLX (4) FLX Pro (4) | None | 90 days: Nearly 100% white glistening tissue coverage across atrial surface of both device types. Endothelial coverage of 87.7% and 68.2% was seen across FLX Pro and FLX devices, respectively. |
| Human Cases | |||||
| Study | Publ. | n | Device Type | Antithrombotic Therapy | Results |
| Massarenti et al. [81] | 2012 | 1 | WM | VKA + ASA | 10 months: No significant endothelialization observed. Fibrous connective tissue but no thrombus or neoplastic formation on pathology. Surgery. |
| Schiettekatte et al. [82] | 2014 | 1 | ACP | DAPT | 1.5 years: Small thrombus associated with areas of incomplete endothelialization. Surgery. |
| Prosperi-Porta et al. [83] | 2018 | 1 | WM | DAPT | 1 year: Well-seated but poorly endothelialized surface with associated device thrombus. Post-mortem. |
| McIvor et al. [84] | 2019 | 1 | WM | DOAC | 3 years: Only limited superior endothelium across the atrial device surface. Device not adherent to the atrial wall. Surgery. |
| Sharma et al. [85] | 2019 | 2 | WM | VKA + ASA | 1.5 years (Case 1): Complete lack of endothelialization. Surgery. 2 years (Case 2): Partial and incomplete endothelization. Surgery. |
| Ellis et al. [86] | 2022 | 2 | AM WM | - | 2 years (Amulet): Endocardial growth across 60–75% of disc surface. Surgery. 8 months (Watchman): Endothelialization across 40–55% of atrial device surface. Post-mortem. |
| Vukomanovic et al. [87] | 2022 | 2 | WM | VKA + ASA | 3 years (Case 1): Large mobile thrombus and non-endothelialized central screw hub. Surgery. 2 years (Case 2): Large superior thrombus on device surface. Non-endothelialized central screw hub. Surgery. |
4.2. Device Healing—Imaging
5. Discussion and Future Directions
5.1. Ongoing Trials
5.2. Next-Generation Devices
5.3. Novel Factor XI Anticoagulants
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brundel, B.; Ai, X.; Hills, M.T.; Kuipers, M.F.; Lip, G.Y.H.; de Groot, N.M.S. Atrial fibrillation. Nat. Rev. Dis. Primers 2022, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke Off. J. Int. Stroke Soc. 2021, 16, 217–221. [Google Scholar] [CrossRef]
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.; Franco, O.H.; Hofman, A.; Witteman, J.C.; Stricker, B.H.; Heeringa, J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur. Heart J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.V.; Varosy, P.; Price, M.J.; Slotwiner, D.; Kusumoto, F.M.; Rammohan, C.; Kavinsky, C.J.; Turi, Z.G.; Akar, J.; Koutras, C.; et al. The NCDR Left Atrial Appendage Occlusion Registry. J. Am. Coll. Cardiol. 2020, 75, 1503–1518. [Google Scholar] [CrossRef]
- Clarke, J.D.; Higgins, A.Y.; Wang, Y.; Faridi, K.F.; Curtis, J.A.; Freeman, J.V.; Friedman, D.J. Impact of Preprocedure Imaging for Left Atrial Appendage Occlusion: Insights From the NCDR LAAO Registry. JACC Cardiovasc. Interv. 2023, 16, 1317–1328. [Google Scholar] [CrossRef]
- Writing Committee Members; Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2023, 83, 109–179. [Google Scholar] [CrossRef]
- Simard, T.J.; Hibbert, B.; Alkhouli, M.A.; Abraham, N.S.; Holmes, D.R., Jr. Device-related thrombus following left atrial appendage occlusion. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2022, 18, 224–232. [Google Scholar] [CrossRef]
- Aminian, A.; Schmidt, B.; Mazzone, P.; Berti, S.; Fischer, S.; Montorfano, M.; Lam, S.C.C.; Lund, J.; Asch, F.M.; Gage, R.; et al. Incidence, Characterization, and Clinical Impact of Device-Related Thrombus Following Left Atrial Appendage Occlusion in the Prospective Global AMPLATZER Amulet Observational Study. JACC Cardiovasc. Interv. 2019, 12, 1003–1014. [Google Scholar] [CrossRef]
- Dukkipati, S.R.; Kar, S.; Holmes, D.R.; Doshi, S.K.; Swarup, V.; Gibson, D.N.; Maini, B.; Gordon, N.T.; Main, M.L.; Reddy, V.Y. Device-Related Thrombus after Left Atrial Appendage Closure. Circulation 2018, 138, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Hildick-Smith, D.; Landmesser, U.; Camm, A.J.; Diener, H.C.; Paul, V.; Schmidt, B.; Settergren, M.; Teiger, E.; Nielsen-Kudsk, J.E.; Tondo, C. Left atrial appendage occlusion with the Amplatzer™ Amulet™ device: Full results of the prospective global observational study. Eur. Heart J. 2020, 41, 2894–2901. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Holmes, D.R.; Van Tassel, R.A.; Hauser, R.; Henry, T.D.; Mooney, M.; Matthews, R.; Doshi, S.; Jones, R.M.; Virmani, R. Left atrial appendage obliteration: Mechanisms of healing and intracardiac integration. JACC Cardiovasc. Interv. 2010, 3, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; Reddy, V.Y.; Turi, Z.G.; Doshi, S.K.; Sievert, H.; Buchbinder, M.; Mullin, C.M.; Sick, P. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet 2009, 374, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R., Jr.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R., Jr.; Alkhouli, M. The History of the Left Atrial Appendage Occlusion. Card. Electrophysiol. Clin. 2020, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; De Backer, O.; Nielsen-Kudsk, J.E.; Mazzone, P.; Berti, S.; Fischer, S.; Lund, J.; Montorfano, M.; Lam, S.C.C.; Freixa, X.; et al. Incidence and Clinical Impact of Major Bleeding Following Left Atrial Appendage Occlusion: Insights from the Amplatzer™ Amulet™ LAA Occluder Observational Study. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2021, 17, 774–782. [Google Scholar] [CrossRef]
- Lakkireddy, D.; Thaler, D.; Ellis, C.R.; Swarup, V.; Sondergaard, L.; Carroll, J.; Gold, M.R.; Hermiller, J.; Diener, H.C.; Schmidt, B.; et al. Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE): A Randomized, Controlled Trial. Circulation 2021, 144, 1543–1552. [Google Scholar] [CrossRef]
- Boersma, L.V.; Ince, H.; Kische, S.; Pokushalov, E.; Schmitz, T.; Schmidt, B.; Gori, T.; Meincke, F.; Protopopov, A.V.; Betts, T.; et al. Evaluating Real-World Clinical Outcomes in Atrial Fibrillation Patients Receiving the WATCHMAN Left Atrial Appendage Closure Technology: Final 2-Year Outcome Data of the EWOLUTION Trial Focusing on History of Stroke and Hemorrhage. Circ. Arrhythm. Electrophysiol. 2019, 12, e006841. [Google Scholar] [CrossRef]
- Kar, S.; Doshi, S.K.; Sadhu, A.; Horton, R.; Osorio, J.; Ellis, C.; Stone, J., Jr.; Shah, M.; Dukkipati, S.R.; Adler, S.; et al. Primary Outcome Evaluation of a Next-Generation Left Atrial Appendage Closure Device: Results From the PINNACLE FLX Trial. Circulation 2021, 143, 1754–1762. [Google Scholar] [CrossRef]
- Betts, T.R.; Grygier, M.; Nielsen Kudsk, J.E.; Schmitz, T.; Sandri, M.; Casu, G.; Bergmann, M.; Hildick-Smith, D.; Christen, T.; Allocco, D.J. Real-world clinical outcomes with a next-generation left atrial appendage closure device: The FLXibility Post-Approval Study. Europace 2023, 25, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Busu, T.; Shah, K.; Osman, M.; Alqahtani, F.; Raybuck, B. Incidence and Clinical Impact of Device-Related Thrombus Following Percutaneous Left Atrial Appendage Occlusion: A Meta-Analysis. JACC Clin. Electrophysiol. 2018, 4, 1629–1637. [Google Scholar] [CrossRef]
- Lempereur, M.; Aminian, A.; Freixa, X.; Gafoor, S.; Kefer, J.; Tzikas, A.; Legrand, V.; Saw, J. Device-associated thrombus formation after left atrial appendage occlusion: A systematic review of events reported with the Watchman, the Amplatzer Cardiac Plug and the Amulet. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2017, 90, E111–E121. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Hou, D.; Jones, R.; Werner, D.; Swanson, L.; Tischler, B.; Stein, K.; Huibregtse, B.; Ladich, E.; Kutys, R.; et al. Impact of Watchman and Amplatzer devices on left atrial appendage adjacent structures and healing response in a canine model. JACC Cardiovasc. Interv. 2014, 7, 801–809. [Google Scholar] [CrossRef]
- Bass, J.L. Transcatheter occlusion of the left atrial appendage--experimental testing of a new Amplatzer device. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2010, 76, 181–185. [Google Scholar] [CrossRef]
- Kramer, A.D.; Korsholm, K.; Jensen, J.M.; Nørgaard, B.L.; Peelukhana, S.; Herbst, T.; Horton, R.; Kar, S.; Saw, J.; Alkhouli, M.; et al. Cardiac computed tomography following Watchman FLX implantation: Device-related thrombus or device healing? Eur. Heart J. Cardiovasc. Imaging 2022, 24, 250–259. [Google Scholar] [CrossRef]
- Jaffer, I.H.; Fredenburgh, J.C.; Hirsh, J.; Weitz, J.I. Medical device-induced thrombosis: What causes it and how can we prevent it? J. Thromb. Haemost. JTH 2015, 13 (Suppl. S1), S72–S81. [Google Scholar] [CrossRef]
- Aarnink, E.W.; Huijboom, M.F.M.; Bor, W.L.; Maarse, M.; Zheng, K.L.; Ten Cate, H.; Ten Berg, J.M.; Boersma, L.V.A. Hemostatic biomarkers and antithrombotic strategy in percutaneous left atrial interventions: State-of-the-art review. Thromb. Res. 2022, 215, 41–51. [Google Scholar] [CrossRef]
- Asmarats, L.; O’Hara, G.; Champagne, J.; Paradis, J.M.; Bernier, M.; O’Connor, K.; Beaudoin, J.; Junquera, L.; Del Val, D.; Muntané-Carol, G.; et al. Short-Term Oral Anticoagulation Versus Antiplatelet Therapy Following Transcatheter Left Atrial Appendage Closure. Circ. Cardiovasc. Interv. 2020, 13, e009039. [Google Scholar] [CrossRef]
- Rodes-Cabau, J.; O’Hara, G.; Paradis, J.M.; Bernier, M.; Rodriguez-Gabella, T.; Regueiro, A.; O’Connor, K.; Beaudoin, J.; Puri, R.; Cote, M.; et al. Changes in Coagulation and Platelet Activation Markers Following Transcatheter Left Atrial Appendage Closure. Am. J. Cardiol. 2017, 120, 87–91. [Google Scholar] [CrossRef]
- Duthoit, G.; Silvain, J.; Marijon, E.; Ducrocq, G.; Lepillier, A.; Frere, C.; Dimby, S.F.; Popovic, B.; Lellouche, N.; Martin-Toutain, I.; et al. Reduced Rivaroxaban Dose Versus Dual Antiplatelet Therapy after Left Atrial Appendage Closure: ADRIFT a Randomized Pilot Study. Circ. Cardiovasc. Interv. 2020, 13, e008481. [Google Scholar] [CrossRef]
- Schmidt, B.; Nielsen-Kudsk, J.E.; Ellis, C.R.; Thaler, D.; Sabir, S.A.; Gambhir, A.; Landmesser, U.; Shah, N.; Gray, W.; Swarup, V.; et al. Incidence, Predictors, and Clinical Outcomes of Device-Related Thrombus in the Amulet IDE Trial. JACC Clin. Electrophysiol. 2023, 9, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xiong, S.H.; Guan, Y.G.; Zhao, X.X.; Qin, Y.W.; Guo, Z.F.; Bai, Y. An updated meta-analysis of device related thrombus following left atrial appendage closure in patients with atrial fibrillation. Front. Cardiovasc. Med. 2022, 9, 1088782. [Google Scholar] [CrossRef]
- Fauchier, L.; Cinaud, A.; Brigadeau, F.; Lepillier, A.; Pierre, B.; Abbey, S.; Fatemi, M.; Franceschi, F.; Guedeney, P.; Jacon, P.; et al. Device-Related Thrombosis after Percutaneous Left Atrial Appendage Occlusion for Atrial Fibrillation. J. Am. Coll. Cardiol. 2018, 71, 1528–1536. [Google Scholar] [CrossRef]
- Simard, T.; Jung, R.G.; Lehenbauer, K.; Piayda, K.; Pracon, R.; Jackson, G.G.; Flores-Umanzor, E.; Faroux, L.; Korsholm, K.; Chun, J.K.R.; et al. Predictors of Device-Related Thrombus Following Percutaneous Left Atrial Appendage Occlusion. J. Am. Coll. Cardiol. 2021, 78, 297–313. [Google Scholar] [CrossRef]
- Sedaghat, A.; Nickenig, G.; Schrickel, J.W.; Ince, H.; Schmidt, B.; Protopopov, A.V.; Betts, T.R.; Gori, T.; Sievert, H.; Mazzone, P.; et al. Incidence, predictors and outcomes of device-related thrombus after left atrial appendage closure with the WATCHMAN device-Insights from the EWOLUTION real world registry. Catheter. Cardiovasc. Interv. 2021, 97, E1019–E1024. [Google Scholar] [CrossRef] [PubMed]
- Vij, V.; Piayda, K.; Nelles, D.; Gloekler, S.; Galea, R.; Fürholz, M.; Meier, B.; Valgimigli, M.; O’Hara, G.; Arzamendi, D.; et al. Clinical and echocardiographic risk factors for device-related thrombus after left atrial appendage closure: An analysis from the multicenter EUROC-DRT registry. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2022, 111, 1276–1285. [Google Scholar] [CrossRef]
- Pracon, R.; Bangalore, S.; Dzielinska, Z.; Konka, M.; Kepka, C.; Kruk, M.; Kaczmarska-Dyrda, E.; Petryka-Mazurkiewicz, J.; Bujak, S.; Solecki, M.; et al. Device Thrombosis after Percutaneous Left Atrial Appendage Occlusion Is Related to Patient and Procedural Characteristics but Not to Duration of Postimplantation Dual Antiplatelet Therapy. Circ. Cardiovasc. Interv. 2018, 11, e005997. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, A.; Schrickel, J.W.; Andrie, R.; Schueler, R.; Nickenig, G.; Hammerstingl, C. Thrombus Formation after Left Atrial Appendage Occlusion with the Amplatzer Amulet Device. JACC Clin. Electrophysiol. 2017, 3, 71–75. [Google Scholar] [CrossRef]
- Cepas-Guillen, P.L.; Flores-Umanzor, E.; Sanchis, L.; Regueiro, A.; Freixa, X. Pulmonary Ridge Coverage as a Potential Modifiable Risk Factor of Device-Related Thrombosis. JACC Clin. Electrophysiol. 2023, 9, 261. [Google Scholar] [CrossRef]
- Korsholm, K.; Jensen, J.M.; Nørgaard, B.L.; Nielsen-Kudsk, J.E. Detection of Device-Related Thrombosis Following Left Atrial Appendage Occlusion: A Comparison Between Cardiac Computed Tomography and Transesophageal Echocardiography. Circ. Cardiovasc. Interv. 2019, 12, e008112. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Neuss, M.; Weissenborn, J.; Butter, C. Predictors of thrombus formation after percutaneous left atrial appendage closure using the WATCHMAN device. Heart Vessel. 2017, 32, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Cepas-Guillén, P.; Flores-Umanzor, E.; Leduc, N.; Bajoras, V.; Perrin, N.; Farjat-Pasos, J.; McInerney, A.; Lafond, A.; Millán, X.; Zendjebil, S.; et al. Impact of Device Implant Depth after Left Atrial Appendage Occlusion. JACC Cardiovasc. Interv. 2023, 16, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Calabro, P.; Gragnano, F.; Niccoli, G.; Marcucci, R.; Zimarino, M.; Spaccarotella, C.; Renda, G.; Patti, G.; Ando, G.; Moscarella, E.; et al. Antithrombotic Therapy in Patients Undergoing Transcatheter Interventions for Structural Heart Disease. Circulation 2021, 144, 1323–1343. [Google Scholar] [CrossRef]
- Friberg, L.; Rosenqvist, M.; Lip, G.Y. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: The Swedish Atrial Fibrillation cohort study. Eur. Heart J. 2012, 33, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Tzikas, A.; Shakir, S.; Gafoor, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; Nielsen-Kudsk, J.E.; Cruz-Gonzalez, I.; et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: Multicentre experience with the AMPLATZER Cardiac Plug. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2016, 11, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.W.; Betts, T.R.; Sievert, H.; Schmidt, B.; Pokushalov, E.; Kische, S.; Schmitz, T.; Meincke, F.; Stein, K.M.; Boersma, L.V.A.; et al. Safety and efficacy of early anticoagulation drug regimens after WATCHMAN left atrial appendage closure: Three-month data from the EWOLUTION prospective, multicentre, monitored international WATCHMAN LAA closure registry. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2017, 13, 877–884. [Google Scholar] [CrossRef]
- Enomoto, Y.; Gadiyaram, V.K.; Gianni, C.; Horton, R.P.; Trivedi, C.; Mohanty, S.; Di Biase, L.; Al-Ahmad, A.; Burkhardt, J.D.; Narula, A.; et al. Use of non-warfarin oral anticoagulants instead of warfarin during left atrial appendage closure with the Watchman device. Heart Rhythm. 2017, 14, 19–24. [Google Scholar] [CrossRef]
- Landmesser, U.; Tondo, C.; Camm, J.; Diener, H.C.; Paul, V.; Schmidt, B.; Settergren, M.; Teiger, E.; Nielsen-Kudsk, J.E.; Hildick-Smith, D. Left atrial appendage occlusion with the AMPLATZER Amulet device: One-year follow-up from the prospective global Amulet observational registry. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2018, 14, e590–e597. [Google Scholar] [CrossRef]
- Patti, G.; Sticchi, A.; Verolino, G.; Pasceri, V.; Vizzi, V.; Brscic, E.; Casu, G.; Golino, P.; Russo, V.; Rapacciuolo, A.; et al. Safety and Efficacy of Single Versus Dual Antiplatelet Therapy after Left Atrial Appendage Occlusion. Am. J. Cardiol. 2020, 134, 83–90. [Google Scholar] [CrossRef]
- Freeman, J.V.; Higgins, A.Y.; Wang, Y.; Du, C.; Friedman, D.J.; Daimee, U.A.; Minges, K.E.; Pereira, L.; Goldsweig, A.M.; Price, M.J.; et al. Antithrombotic Therapy after Left Atrial Appendage Occlusion in Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2022, 79, 1785–1798. [Google Scholar] [CrossRef]
- Weise, F.K.; Bordignon, S.; Perrotta, L.; Konstantinou, A.; Bologna, F.; Nagase, T.; Chen, S.; Chun, K.R.J.; Schmidt, B. Short-term dual antiplatelet therapy after interventional left atrial appendage closure with different devices. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2018, 13, e2138–e2146. [Google Scholar] [CrossRef]
- Korsholm, K.; Nielsen, K.M.; Jensen, J.M.; Jensen, H.K.; Andersen, G.; Nielsen-Kudsk, J.E. Transcatheter left atrial appendage occlusion in patients with atrial fibrillation and a high bleeding risk using aspirin alone for post-implant antithrombotic therapy. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2017, 12, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Main, M.L.; Fan, D.; Reddy, V.Y.; Holmes, D.R.; Gordon, N.T.; Coggins, T.R.; House, J.A.; Liao, L.; Rabineau, D.; Latus, G.G.; et al. Assessment of Device-Related Thrombus and Associated Clinical Outcomes with the WATCHMAN Left Atrial Appendage Closure Device for Embolic Protection in Patients with Atrial Fibrillation (from the PROTECT-AF Trial). Am. J. Cardiol. 2016, 117, 1127–1134. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Doshi, S.K.; Kar, S.; Price, M.J.; Sanchez, J.M.; Sievert, H.; Valderrabano, M.; Reddy, V.Y. Left Atrial Appendage Closure as an Alternative to Warfarin for Stroke Prevention in Atrial Fibrillation: A Patient-Level Meta-Analysis. J. Am. Coll. Cardiol. 2015, 65, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R., Jr.; Reddy, V.Y.; Gordon, N.T.; Delurgio, D.; Doshi, S.K.; Desai, A.J.; Stone, J.E., Jr.; Kar, S. Long-Term Safety and Efficacy in Continued Access Left Atrial Appendage Closure Registries. J. Am. Coll. Cardiol. 2019, 74, 2878–2889. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, V.J.; Brouwer, J.; Delewi, R.; Hermanides, R.S.; Holvoet, W.; Dubois, C.L.F.; Frambach, P.; De Bruyne, B.; van Houwelingen, G.K.; Van Der Heyden, J.A.S.; et al. Anticoagulation with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N. Engl. J. Med. 2020, 382, 1696–1707. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; Magnocavallo, M.; Di Biase, L.; Mohanty, S.; Trivedi, C.; Tarantino, N.; Gianni, C.; Lavalle, C.; Van Niekerk, C.J.; Romero, J.; et al. Half-Dose Direct Oral Anticoagulation Versus Standard Antithrombotic Therapy after Left Atrial Appendage Occlusion. JACC Cardiovasc. Interv. 2021, 14, 2353–2364. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Dangas, G.D.; Tijssen, J.G.P.; Wöhrle, J.; Søndergaard, L.; Gilard, M.; Möllmann, H.; Makkar, R.R.; Herrmann, H.C.; Giustino, G.; Baldus, S.; et al. A Controlled Trial of Rivaroxaban after Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 120–129. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Brueckmann, M.; Granger, C.B.; Kappetein, A.P.; Mack, M.J.; Blatchford, J.; Devenny, K.; Friedman, J.; Guiver, K.; et al. Dabigatran versus warfarin in patients with mechanical heart valves. N. Engl. J. Med. 2013, 369, 1206–1214. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Weitz, J.I. Warfarin faring better: Vitamin K antagonists beat rivaroxaban and apixaban in the INVICTUS and PROACT Xa trials. J. Thromb. Haemost. 2023, 21, 3067–3071. [Google Scholar] [CrossRef]
- Joosten, L.P.T.; van Doorn, S.; van de Ven, P.M.; Köhlen, B.T.G.; Nierman, M.C.; Koek, H.L.; Hemels, M.E.W.; Huisman, M.V.; Kruip, M.; Faber, L.M.; et al. Safety of Switching from a Vitamin K Antagonist to a Non-Vitamin K Antagonist Oral Anticoagulant in Frail Older Patients with Atrial Fibrillation: Results of the FRAIL-AF Randomized Controlled Trial. Circulation 2023, 149, 279–289. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Coylewright, M.; Holmes, D.R., Jr.; Kapadia, S.R.; Hsu, J.C.; Gibson, D.N.; Freeman, J.V.; Yeh, R.W.; Piccini, J.P.; Price, M.J.; Allocco, D.J.; et al. DAPT Is Comparable to OAC Following LAAC with WATCHMAN FLX: A National Registry Analysis. JACC Cardiovasc. Interv. 2023, 16, 2708–2718. [Google Scholar] [CrossRef] [PubMed]
- Cepas-Guillen, P.L.; Flores-Umanzor, E.; Regueiro, A.; Brugaletta, S.; Ibañez, C.; Sanchis, L.; Sitges, M.; Rodés-Cabau, J.; Sabaté, M.; Freixa, X. Low Dose of Direct Oral Anticoagulants after Left Atrial Appendage Occlusion. J. Cardiovasc. Dev. Dis. 2021, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Möbius-Winkler, S.; Miller, M.A.; Neuzil, P.; Schuler, G.; Wiebe, J.; Sick, P.; Sievert, H. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: The ASAP study (ASA Plavix Feasibility Study with Watchman Left Atrial Appendage Closure Technology). J. Am. Coll. Cardiol. 2013, 61, 2551–2556. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gabella, T.; Nombela-Franco, L.; Regueiro, A.; Jimenez-Quevedo, P.; Champagne, J.; O’Hara, G.; Bernier, M.; Macaya, C.; Rodes-Cabau, J. Single Antiplatelet Therapy Following Left Atrial Appendage Closure in Patients with Contraindication to Anticoagulation. J. Am. Coll. Cardiol. 2016, 68, 1920–1921. [Google Scholar] [CrossRef]
- Mhanna, M.; Beran, A.; Al-Abdouh, A.; Jabri, A.; Al-Aaraj, A.; Sajdeya, O.; Abuhelwa, Z.; Khokher, W.; Bhuta, S.; Burmeister, C.J.; et al. Single Versus Dual Antiplatelet Therapy following Left Atrial Appendage Occlusion in Patients with High Bleeding Risk. Curr. Probl. Cardiol. 2022, 47, 101269. [Google Scholar] [CrossRef]
- Brouwer, J.; Nijenhuis, V.J.; Delewi, R.; Hermanides, R.S.; Holvoet, W.; Dubois, C.L.F.; Frambach, P.; De Bruyne, B.; van Houwelingen, G.K.; Van Der Heyden, J.A.S.; et al. Aspirin with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N. Engl. J. Med. 2020, 383, 1447–1457. [Google Scholar] [CrossRef]
- Urena, M.; Rodés-Cabau, J.; Freixa, X.; Saw, J.; Webb, J.G.; Freeman, M.; Horlick, E.; Osten, M.; Chan, A.; Marquis, J.-F.; et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J. Am. Coll. Cardiol. 2013, 62, 96–102. [Google Scholar] [CrossRef]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 3122–3135. [Google Scholar] [CrossRef] [PubMed]
- Darmon, A.; Couture, E.L.; Stein, G.; Cormier, B.; Chevalier, B.; Lefèvre, T.; Sanguineti, A.; Horvilleur, J.; Garot, P. Left Atrial Appendage Closure in Patients with Atrial Fibrillation at Very High Bleeding Risk without Postimplantation Antithrombotic Therapy. J. Invasive Cardiol. 2020, 32, 385–391. [Google Scholar] [PubMed]
- Li, S.Y.; Wang, J.; Hui, X.; Zhu, H.J.; Wang, B.Y.; Xu, H. Meta-analysis of postoperative antithrombotic therapy after left atrial appendage occlusion. J. Int. Med. Res. 2020, 48, 300060520966478. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, L.; Wong, Y.H.; Reddy, V.Y.; Boersma, L.V.A.; Bergmann, M.W.; Doshi, S.; Kar, S.; Sievert, H.; Wehrenberg, S.; Stein, K.; et al. Propensity-Matched Comparison of Oral Anticoagulation Versus Antiplatelet Therapy after Left Atrial Appendage Closure with WATCHMAN. JACC Cardiovasc. Interv. 2019, 12, 1055–1063. [Google Scholar] [CrossRef]
- Carvalho, P.E.P.; Gewehr, D.M.; Miyawaki, I.A.; Nogueira, A.; Félix, N.; Garot, P.; Darmon, A.; Mazzone, P.; Preda, A.; Nascimento, B.R.; et al. Comparison of Initial Antithrombotic Regimens after Left Atrial Appendage Occlusion: A Systematic Review and Network Meta-analysis. J. Am. Coll. Cardiol. 2023, 82, 1765–1773. [Google Scholar] [CrossRef]
- Saliba, W.I.; Kawai, K.; Sato, Y.; Kopesky, E.; Cheng, Q.; Ghosh, S.K.B.; Herbst, T.J.; Kawakami, R.; Konishi, T.; Virmani, R.; et al. Enhanced Thromboresistance and Endothelialization of a Novel Fluoropolymer-Coated Left Atrial Appendage Closure Device. JACC Clin. Electrophysiol. 2023, 9, 1555–1567. [Google Scholar] [CrossRef]
- Massarenti, L.; Yilmaz, A. Incomplete endothelialization of left atrial appendage occlusion device 10 months after implantation. J. Cardiovasc. Electrophysiol. 2012, 23, 1384–1385. [Google Scholar] [CrossRef]
- Schiettekatte, S.; Czapla, J.; Nijs, J.; La Meir, M. Unmasking a naked left atrial appendage closure device: A case of a silent embolic threat. Heart Rhythm. 2014, 11, 2314–2315. [Google Scholar] [CrossRef]
- Prosperi-Porta, G.; Schnell, G.; Colbert, J.; Franko, A.; Wilton, S.B.; Kuriachan, V.P. Multiple Thromboembolic Events from a Left Atrial Appendage Occlusion Device. Can. J. Cardiol. 2018, 34, 342.e13–342.e15. [Google Scholar] [CrossRef]
- McIvor, F.; Wall, D. Who watches the WATCHMAN™? A case of incomplete endothelialization at 3 years after device implantation. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2019, 56, 1194–1195. [Google Scholar] [CrossRef]
- Sharma, S.P.; Singh, D.; Nakamura, D.; Gopinathannair, R.; Lakkireddy, D. Incomplete endothelialization of WatchmanTM Device: Predictors and Implications from Two Cases. J. Atr. Fibrillation 2019, 11, 2162. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.R.; Alkhouli, M.; Anderson, J.A.; Swarup, V. Comparative Endothelialization of Amulet LAA Occluder and Watchman 2.5 LAA Device: Observations From Explanted Hearts. JACC Clin. Electrophysiol. 2022, 8, 828–829. [Google Scholar] [CrossRef]
- Vukomanovic, D.; Unzek, S.; Malik, K.; Taase, A.; Zawaneh, M.; Weiss, P.; Fang, K.; Tung, R. Massive Device-Related Thrombus after LAA Occlusion: Intraoperative Insights Into Mechanism. JACC Case Rep. 2022, 4, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Korsholm, K.; Jensen, J.M.; Nørgaard, B.L.; Samaras, A.; Saw, J.; Berti, S.; Tzikas, A.; Nielsen-Kudsk, J.E. Peridevice Leak Following Amplatzer Left Atrial Appendage Occlusion: Cardiac Computed Tomography Classification and Clinical Outcomes. JACC Cardiovasc. Interv. 2021, 14, 83–93. [Google Scholar] [CrossRef]
- Iriart, X.; Blanc, G.; Bouteiller, X.P.; Legghe, B.; Bouyer, B.; Sridi-Cheniti, S.; Bustin, A.; Vasile, C.; Thambo, J.B.; Elbaz, M.; et al. Clinical Implications of CT-detected Hypoattenuation Thickening on Left Atrial Appendage Occlusion Devices. Radiology 2023, 308, e230462. [Google Scholar] [CrossRef] [PubMed]
- Galea, R.; Mahmoudi, K.; Gräni, C.; Elhadad, S.; Huber, A.T.; Heg, D.; Siontis, G.C.M.; Brugger, N.; Sebag, F.; Windecker, S.; et al. Watchman FLX vs. Watchman 2.5 in a Dual-Center Left Atrial Appendage Closure Cohort: The WATCH-DUAL study. Europace 2022, 24, 1441–1450. [Google Scholar] [CrossRef]
- Miller, T.; Hana, D.; Patibandla, S.; Guzman, D.B.; Avalon, J.C.; Zeb, I.; Kadiyala, M.; Mills, J.; Balla, S.; Kim, C.; et al. Cardiac Computed Tomography Angiography for Device-Related Thrombus Assessment after WATCHMAN FLX™ Occluder Device Implantation: A Single-Center Retrospective Observational Study. Cardiovasc. Revasc. Med. 2022, 41, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Granier, M.; Laugaudin, G.; Massin, F.; Cade, S.; Winum, P.F.; Freitag, C.; Pasquie, J.L. Occurrence of Incomplete Endothelialization Causing Residual Permeability after Left Atrial Appendage Closure. J. Invasive Cardiol. 2018, 30, 245–250. [Google Scholar] [PubMed]
- Lindner, S.; Behnes, M.; Wenke, A.; Sartorius, B.; Akin, M.; Mashayekhi, K.; Gawlitza, J.; Weidner, K.J.; Ansari, U.; Haubenreisser, H.; et al. Incomplete neo-endothelialization of left atrial appendage closure devices is frequent after 6 months: A pilot imaging study. Int. J. Cardiovasc. Imaging 2021, 37, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Doshi, S.K.; Alkhouli, M.; Camm, A.J.; Coylewright, M.; Gibson, M.C.; Granger, C.B.; Gurol, M.E.; Huber, K.; Mansour, M.; et al. Rationale and design of a randomized study comparing the Watchman FLX device to DOACs in patients with atrial fibrillation. Am. Heart J. 2023, 264, 123–132. [Google Scholar] [CrossRef]
- Emmert, M.Y.; Puippe, G.; Baumüller, S.; Alkadhi, H.; Landmesser, U.; Plass, A.; Bettex, D.; Scherman, J.; Grünenfelder, J.; Genoni, M.; et al. Safe, effective and durable epicardial left atrial appendage clip occlusion in patients with atrial fibrillation undergoing cardiac surgery: First long-term results from a prospective device trial. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2014, 45, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.; Byrne, T.; Pershad, A. Early results with the LARIAT device for left atrial appendage exclusion in patients with atrial fibrillation at high risk for stroke and anticoagulation. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2015, 86, 121–127. [Google Scholar] [CrossRef]
- Whitlock, R.P.; Belley-Cote, E.P.; Paparella, D.; Healey, J.S.; Brady, K.; Sharma, M.; Reents, W.; Budera, P.; Baddour, A.J.; Fila, P.; et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N. Engl. J. Med. 2021, 384, 2081–2091. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef]
- Sommer, R.J.; Lamport, R.; Melanson, D.; Devellian, C.; Levine, A.; Cain, C.M.; Kaplan, A.V.; Gray, W.A. Preclinical Assessment of a Novel Conformable Foam-Based Left Atrial Appendage Closure Device. Biomed. Res. Int. 2021, 2021, 4556400. [Google Scholar] [CrossRef]
- Sommer, R.J.; Kim, J.H.; Szerlip, M.; Chandhok, S.; Sugeng, L.; Cain, C.; Kaplan, A.V.; Gray, W.A. Conformal Left Atrial Appendage Seal Device for Left Atrial Appendage Closure: First Clinical Use. JACC Cardiovasc. Interv. 2021, 14, 2368–2374. [Google Scholar] [CrossRef]
- Turagam, M.K.; Neuzil, P.; Hala, P.; Mraz, T.; Dukkipati, S.R.; Reddy, V.Y. Intracardiac Echocardiography-Guided Left Atrial Appendage Closure with a Novel Foam-Based Conformable Device: Safety and 1-Year Outcomes. JACC Clin. Electrophysiol. 2022, 8, 197–207. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Prisco, D.; Eikelboom, J.W. Factor XI inhibitors: Cardiovascular perspectives. Eur. Heart J. 2023, 44, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Duga, S.; Salomon, O. Congenital factor XI deficiency: An update. Semin. Thromb. Hemost. 2013, 39, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Kanefendt, F.; Brase, C.; Unger, S.; Kubitza, D. Effects of Tablet Formulation, Food, or Gastric pH on the Bioavailability of Asundexian. Clin. Pharmacol. Drug Dev. 2023, 12, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Lorentz, C.U.; Verbout, N.G.; Wallisch, M.; Hagen, M.W.; Shatzel, J.J.; Olson, S.R.; Puy, C.; Hinds, M.T.; McCarty, O.J.T.; Gailani, D.; et al. Contact Activation Inhibitor and Factor XI Antibody, AB023, Produces Safe, Dose-Dependent Anticoagulation in a Phase 1 First-In-Human Trial. Arter. Thromb. Vasc. Biol. 2019, 39, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Lorentz, C.U.; Tucker, E.I.; Verbout, N.G.; Shatzel, J.J.; Olson, S.R.; Markway, B.D.; Wallisch, M.; Ralle, M.; Hinds, M.T.; McCarty, O.J.T.; et al. The contact activation inhibitor AB023 in heparin-free hemodialysis: Results of a randomized phase 2 clinical trial. Blood 2021, 138, 2173–2184. [Google Scholar] [CrossRef]
- Piel, I.; Engelen, A.; Lang, D.; Schulz, S.I.; Gerisch, M.; Brase, C.; Janssen, W.; Fiebig, L.; Heitmeier, S.; Kanefendt, F. Metabolism and Disposition of the Novel Oral Factor XIa Inhibitor Asundexian in Rats and in Humans. Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 411–425. [Google Scholar] [CrossRef]
- Piccini, J.P.; Caso, V.; Connolly, S.J.; Fox, K.A.A.; Oldgren, J.; Jones, W.S.; Gorog, D.A.; Durdil, V.; Viethen, T.; Neumann, C.; et al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): A multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet 2022, 399, 1383–1390. [Google Scholar] [CrossRef]



| Specific Factors | |
|---|---|
| Patient factors | Age [32] Female sex [32] History of stroke/TIA [33,34] High CHA2DS2-VASc [33] Non-paroxysmal AF [35,36] Hypercoagulable disorder [35] Chronic kidney disease [35] Echocardiographic parameters with LA low-flow
|
| Procedural/device factors | Deep device implant [35,37,40,41] Large LAAO device size [37,38] Exposed metal screw on the device surface Iatrogenic pericardial effusion [35] |
| AT-Mixed Studies | DRT * | Ischemic Stroke ** | Major Bleeding ** | CV Mortality ** | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Publ. | n | Device(s) | Random | FU (Months) | SAPT | DAPT | DOAC | VKA | SAPT | DAPT | DOAC | VKA | SAPT | DAPT | DOAC | VKA | SAPT | DAPT | DOAC | VKA |
| ACP registry [46] | 2016 | 1047 | ACP | No | 13 (IQR; 6, 25) | 4.4% | 2.3% | 2.1% | |||||||||||||
| EWOLUTION registry [47] | 2017 | 893 | WM | No | 3 | 3.8% | 3.1% | 1.3% | 0.8% | 1.4% | 0.5% | 0.0% | 0.0% | 2.9% | 1.6% | 1.9% | 2.0% | ||||
| Enomoto et al. [48] | 2017 | 426 | WM | No | 4 | 1.0% | 0.5% | 0.0% | 0.5% | 0.9% | 1.4% | ||||||||||
| Fauchier et al. [34] | 2018 | 469 | ACP/AM/WM | No | 13 (±13) | 10.8% | 1.2% | 7.3% | |||||||||||||
| AMULET Registry [49] | 2018 | 1078 | AM | No | 12 | 0.8% | 1.6% | 2.5% | 2.9% | 6.6% | 8.4% | 5.8% | 8.3% | ||||||||
| ADRIFT Trial [31] | 2020 | 105 | ACP/AM/WM | Yes | 3 | 6.1% | 0.0% | 0.0% | 0.0% | 21.2% | 14.1% | ||||||||||
| Patti et al. [50] | 2020 | 610 | ACP/AM/WM | No | 12 | 1.4% | 0.9% | 1.8% | 2.1% | 2.9% | 6.7% | 6.0% | 5.5% | ||||||||
| Faroux et al. [35] | 2021 | 592 | ACP/AM/WM | No | 22 (IQR; 8, 38) | 2.6% | 0.0% | 1.1% | 1.7% | 7.4% | 3.2% | ||||||||||
| Cepas-Guillén et al. [43] | 2021 | 139 | ACP/AM/WM/LB | No | 3 | 7.7% | 4.1% | 0.0% | 0.0% | 1.4% | 0.0% | 0.0% | 9.6% | 0.0% | |||||||
| AMULET IDE [18] | 2021 | 1878 | AM/WM(FLX) | No | 18 | 3.3% | 4.5% | 1.7% | 1.9% | 10.6% | 10.0% | 3.1% | 4.8% | ||||||||
| Freeman et al. [51] | 2022 | 31.994 | WM | No | 6 | 3.3% | 1.8% | 1.8% | 0.6% | 0.6% | 0.5% | 3.3% | 3.7% | 4.4% | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kramer, A.; Patti, G.; Nielsen-Kudsk, J.E.; Berti, S.; Korsholm, K. Left Atrial Appendage Occlusion and Post-procedural Antithrombotic Management. J. Clin. Med. 2024, 13, 803. https://doi.org/10.3390/jcm13030803
Kramer A, Patti G, Nielsen-Kudsk JE, Berti S, Korsholm K. Left Atrial Appendage Occlusion and Post-procedural Antithrombotic Management. Journal of Clinical Medicine. 2024; 13(3):803. https://doi.org/10.3390/jcm13030803
Chicago/Turabian StyleKramer, Anders, Giuseppe Patti, Jens Erik Nielsen-Kudsk, Sergio Berti, and Kasper Korsholm. 2024. "Left Atrial Appendage Occlusion and Post-procedural Antithrombotic Management" Journal of Clinical Medicine 13, no. 3: 803. https://doi.org/10.3390/jcm13030803
APA StyleKramer, A., Patti, G., Nielsen-Kudsk, J. E., Berti, S., & Korsholm, K. (2024). Left Atrial Appendage Occlusion and Post-procedural Antithrombotic Management. Journal of Clinical Medicine, 13(3), 803. https://doi.org/10.3390/jcm13030803