Imaging of Pulmonary Sarcoidosis—A Review
Abstract
:1. Introduction
2. Imaging in Sarcoidosis—General Principles
2.1. Imaging in Sarcoidosis: Plain CXR vs. High-Resolution Computed Tomography
2.2. Imaging in Pulmonary Sarcoidosis: Other Imaging Modalities
3. CT Detection and Diagnosis of Sarcoidosis
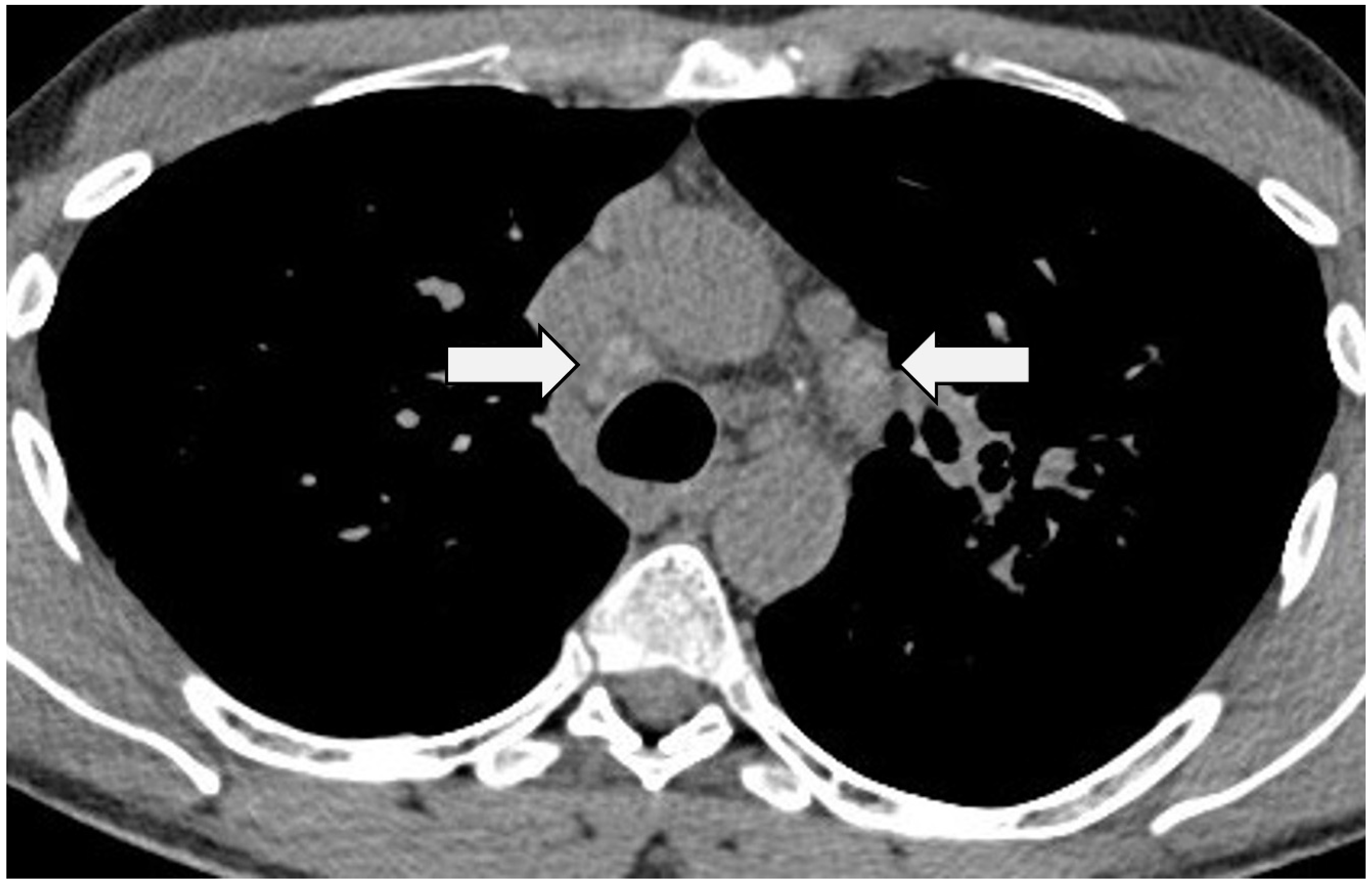
3.1. Intra-Thoracic Nodal Enlargement
3.2. Nodules
3.3. Masses and Consolidation
3.4. Ground-Glass Opacification
3.5. Airway Disease
3.6. Pulmonary Fibrosis
4. Uncommon CT Manifestations and Complications in Pulmonary Sarcoidosis
4.1. Cavitation
4.2. Fungal Colonisation
4.3. Pleural Disease
4.4. Pulmonary Hypertension
4.5. Halo/Reversed-Halo Sign
5. Disease Monitoring in Pulmonary Sarcoidosis
6. CT Phenotypes in Sarcoidosis
7. Disease Quantification and Prognostication in Sarcoidosis
7.1. Morphological–Functional Relationships in Sarcoidosis
7.2. Reversible, Irreversible and Progressive Disease in Sarcoidosis
7.3. Factors Contributing to and Predictors of Mortality in Sarcoidosis
8. Summary
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, O.P.; Shigemitsu, H. A historical sketch; life and time of Jonathan Hutchinson (1828–1913), the first sarcoidologist. Sarcoidosis Vasc. Diffus. Lung Dis. 2008, 25, 71–75. [Google Scholar]
- Neville, E.; Walker, A.N.; James, D.G. Prognostic factors predicting the outcome of sarcoidosis: An analysis of 818 patients. Q. J. Med. 1983, 52, 525–533. [Google Scholar]
- Hunninghake, G.W.; Gilbert, S.; Pueringer, R.; Dayton, C.; Floerchinger, C.; Helmers, R.; Merchant, R.; Wilson, J.; Galvin, J.; Schwartz, D. Outcome of the treatment for sarcoidosis. Am. J. Respir. Crit. Care Med. 1994, 149 Pt 1, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.P.; Teirstein, A.S.; Judson, M.A.; Rossman, Y.H., Jr.; Bresnitz, E.A.; Depalo, L.; Hunninghake, G.; Iannuzzi, M.C.; Johns, C.J. Clinical characteristics of patients in a case control study of sarcoidosis. Am. J. Respir. Crit. Care Med. 2001, 164 Pt 1, 1885–1889. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gal, A.; Koss, M.N. The pathology of pulmonary sarcoidosis: Update. Semin. Diagn. Pathol. 2007, 24, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Ricker, W. Sarcoidosis; a clinico pathological review of 300 cases, including 22 autopsies. Manit. Med. Rev. 1948, 28, 215. [Google Scholar]
- Hansell, D.M.; Milne, D.G.; Wilsher, M.L.; Wells, A.U. Pulmonary sarcoidosis: Morphologic associations of airflow obstruction at thin-section CT. Radiology 1998, 209, 697–704. [Google Scholar] [CrossRef]
- Kouranos, V.; Ward, S.; Kokosi, M.A.; Castillo, D.; Chua, F.; Judge, E.P.; Thomas, S.; Van Tonder, F.; Devaraj, A.; Nicholson, A.G.; et al. Mixed Ventilatory Defects in Pulmonary Sarcoidosis: Prevalence and Clinical Features. Chest 2020, 158, 2007–2014. [Google Scholar] [CrossRef]
- Ungprasert, P.; Ryu, J.H.; Matteson, E.L. Clinical Manifestations, Diagnosis, and Treatment of Sarcoidosis. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 358–375. [Google Scholar] [CrossRef]
- Abehsera, M.; Valeyre, D.; Grenier, P.; Jaillet, H.; Battesti, J.P.; Brauner, M.W. Sarcoidosis with pulmonary fibrosis: CT patterns and correlation with pulmonary function. AJR Am. J. Roentgenol. 2000, 174, 1751–1757. [Google Scholar] [CrossRef]
- Kuznitsky, E.; Bittorf, A. Boecksches Sarkoid mit Beteiligung innerer Organe. Münchener Med. Wochenschr. 1915, 62, 1349–1353. [Google Scholar]
- Wurm, K.; Meier, G. Therapeutic experiences with dexamethasone in pulmonary sarcoidosis (Boeck’s disease). Beitr. Klin. Tuberk. Spezif. Tuberkuloseforsch 1960, 123, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Scadding, J.G. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Br. Med. J. 1961, 2, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Karetzky, M.; McDonough, M. Exercise and resting pulmonary function in sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 1996, 13, 43–49. [Google Scholar]
- Harrison, B.D.; Shaylor, J.M.; Stokes, T.C.; Wilkes, A.R. Airflow limitation in sarcoidosis--a study of pulmonary function in 107 patients with newly diagnosed disease. Respir. Med. 1991, 85, 59–64. [Google Scholar] [CrossRef]
- Yeager, H.; Rossman, M.D.; Baughman, R.P.; Teirstein, A.S.; Judson, M.A.; Rabin, D.L.; Iannuzzi, M.C.; Rose, C.; Bresnitz, E.A.; DePalo, L.; et al. Pulmonary and psychosocial findings at enrollment in the ACCESS study. Sarcoidosis Vasc. Diffus. Lung Dis. 2005, 22, 147–153. [Google Scholar]
- Baughman, R.P.; Shipley, R.; Desai, S.; Drent, M.; Judson, M.A.; Costabel, U.; du Bois, R.M.; Kavuru, M.; Schlenker-Herceg, R.; Flavin, S.; et al. Changes in chest roentgenogram of sarcoidosis patients during a clinical trial of infliximab therapy: Comparison of different methods of evaluation. Chest 2009, 136, 526–535. [Google Scholar] [CrossRef]
- Zappala, C.J.; Desai, S.R.; Copley, S.J.; Spagnolo, R.; Cramer, D.; Sen, D.; Alam, S.M.; du Bois, R.M.; Hansell, D.M.; Wells, A.U. Optimal scoring of serial change on chest radiography in sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2011, 28, 130–138. [Google Scholar]
- Siegelman, S.S.; Zerhouni, E.A.; Leo, F.P.; Khouri, N.F.; Stitik, F.P. CT of the solitary pulmonary nodule. AJR Am. J. Roentgenol. 1980, 135, 1–13. [Google Scholar] [CrossRef]
- Murata, K.; Khan, A.; Rojas, K.A.; Herman, P.G. Optimization of computed tomography technique to demonstrate the fine structure of the lung. Investig. Radiol. 1988, 23, 170–175. [Google Scholar] [CrossRef]
- Zerhouni, E.A.; Spivey, J.F.; Morgan, R.H.; Leo, F.P.; Stitik, F.P.; Siegelman, S.S. Factors influencing quantitative CT measurements of solitary pulmonary nodules. J. Comput. Assist. Tomogr. 1982, 6, 1075–1087. [Google Scholar] [CrossRef]
- Mayo, J.R.; Webb, W.R.; Gould, R.; Stein, M.G.; Bass, I.; Gamsu, G.; Goldberg, H.I. High-resolution CT of the lungs: An optimal approach. Radiology 1987, 163, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Mathieson, J.R.; Mayo, J.R.; Staples, C.A.; Müller, N.L. Chronic diffuse infiltrative lung disease: Comparison of diagnostic accuracy of CT and chest radiography. Radiology 1989, 171, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Vock, P.; Soucek, M.; Daepp, M.; Kalender, W.A. Lung: Spiral volumetric CT with single-breath-hold technique. Radiology 1990, 176, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Kalender, W.A.; Seissler, W.; Klotz, E.; Vock, P. Spiral volumetric CT with single-breath-hold technique, continuous transport, and continuous scanner rotation. Radiology 1990, 176, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Mostard, R.L.; Verschakelen, J.A.; van Kroonenburgh, M.J.; Nelemans, P.J.; Wijnen, P.A.; Vöö, S.; Drent, M. Severity of pulmonary involvement and (18)F-FDG PET activity in sarcoidosis. Respir. Med. 2013, 107, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Keijsers, R.G.; Verzijlbergen, E.J.; van den Bosch, J.M.; Zanen, P.; van de Garde, E.M.; Oyen, W.J.; Grutters, J.C. 18F-FDG PET as a predictor of pulmonary function in sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2011, 28, 123–129. [Google Scholar]
- Chareonthaitawee, P.; Beanlands, R.S.; Chen, W.; Dorbala, S.; Miller, E.J.; Murthy, V.L.; Birnie, D.H.; Chen, E.S.; Cooper, L.T.; Tung, R.H.; et al. Joint SNMMI-ASNC expert consensus document on the role of 18F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J. Nucl. Cardiol. 2017, 24, 1741–1758. [Google Scholar] [CrossRef] [PubMed]
- Youssef, G.; Leung, E.; Mylonas, I.; Nery, P.; Williams, K.; Wisenberg, G.; Gulenchyn, K.Y.; Dekemp, R.A.; Dasilva, J.; Birnie, D.; et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: A systematic review and metaanalysis including the Ontario experience. J. Nucl. Med. 2012, 53, 241–248. [Google Scholar] [CrossRef]
- Braun, J.J.; Kessler, R.; Constantinesco, A.; Imperiale, A. 18F-FDG PET/CT in sarcoidosis management: Review and report of 20 cases. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1537–1543. [Google Scholar] [CrossRef]
- Mostard, R.L.; Prompers, L.; Weijers, R.E.; van Kroonenburgh, M.J.; Wijnen, P.A.; Geusens, P.P.; Drent, M. F-18 FDG PET/CT for detecting bone and bone marrow involvement in sarcoidosis patients. Clin. Nucl. Med. 2012, 37, 21–25. [Google Scholar] [CrossRef]
- Ota, K.; Tsunemi, T.; Saito, K.; Yamanami, F.; Watanabe, M.; Irioka, T.; Mizusawa, H. 18F-FDG PET successfully detects spinal cord sarcoidosis. J. Neurol. 2009, 256, 1943–1946. [Google Scholar] [CrossRef]
- Kauczor, H.U.; Ley-Zaporozhan, J.; Ley, S. Imaging of pulmonary pathologies: Focus on magnetic resonance imaging. Proc. Am. Thorac. Soc. 2009, 6, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Hatabu, H.; Ohno, Y.; Gefter, W.B.; Parraga, G.; Madore, B.; Lee, K.S.; Altes, T.A.; Lynch, D.A.; Mayo, J.R.; Seo, J.B.; et al. Expanding Applications of Pulmonary MRI in the Clinical Evaluation of Lung Disorders: Fleischner Society Position Paper. Radiology 2020, 297, 286–301. [Google Scholar] [CrossRef]
- Brady, D.; Lavelle, L.P.; McEvoy, S.H.; Murphy, D.J.; Gallagher, A.; Gibney, B.; Butler, M.W.; Shortt, F.; McMullan, M.; Fabre, A.; et al. Assessing fibrosis in pulmonary sarcoidosis: Late-enhanced MRI compared to anatomic HRCT imaging. QJM 2016, 109, 257–264. [Google Scholar] [CrossRef]
- Aitken, M.; Chan, M.V.; Urzua Fresno, C.; Farrell, A.; Islam, N.; McInnes, M.D.F.; Iwanochko, M.; Balter, M.; Moayedi, Y.; Thavendiranathan, P.; et al. Diagnostic Accuracy of Cardiac MRI versus FDG PET for Cardiac Sarcoidosis: A Systematic Review and Meta-Analysis. Radiology 2022, 304, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Ginat, D.T.; Dhillon, G.; Almast, J. Magnetic resonance imaging of neurosarcoidosis. J. Clin. Imaging Sci. 2011, 1, 15. [Google Scholar] [CrossRef]
- Niimi, H.; Kang, E.Y.; Kwong, J.S.; Carignan, S.; Müller, N.L. CT of chronic infiltrative lung disease: Prevalence of mediastinal lymphadenopathy. J. Comput. Assist. Tomogr. 1996, 20, 305–308. [Google Scholar] [CrossRef]
- Bhalla, A.S.; Das, A.; Naranje, P.; Goyal, A.; Guleria, R.; Khilnani, G.C. Dilemma of diagnosing thoracic sarcoidosis in tuberculosis-endemic regions: An imaging-based approach. Part 2. Indian J. Radiol. Imaging 2017, 27, 380–388. [Google Scholar] [CrossRef]
- Trisolini, R.; Anevlavis, S.; Tinelli, C.; Orlandi, P.; Patelli, M. CT pattern of lymphadenopathy in untreated patients undergoing bronchoscopy for suspected sarcoidosis. Respir. Med. 2013, 107, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, J.; Müller, N.L. Pulmonary sarcoidosis: Changes on follow-up CT examination. AJR Am. J. Roentgenol. 1992, 159, 473–477. [Google Scholar] [CrossRef]
- Garland, L.H. Pulmonary sarcoidosis; the early roentgen findings. Radiology 1947, 48, 333–352, discussion 352–334. [Google Scholar] [CrossRef]
- Sider, L.; Horton, E.S. Hilar and mediastinal adenopathy in sarcoidosis as detected by computed tomography. J. Thorac. Imaging 1990, 5, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Gawne-Cain, M.L.; Hansell, D.M. The pattern and distribution of calcified mediastinal lymph nodes in sarcoidosis and tuberculosis: A CT study. Clin. Radiol. 1996, 51, 263–267. [Google Scholar] [CrossRef]
- Gross, B.H.; Schneider, H.J.; Proto, A.V. Eggshell calcification of lymph nodes: An update. AJR Am. J. Roentgenol. 1980, 135, 1265–1268. [Google Scholar] [CrossRef]
- Eckardt, J.; Olsen, K.E.; Jørgensen, O.D.; Licht, P.B. Minimally invasive diagnosis of sarcoidosis by EBUS when conventional diagnostics fail. Sarcoidosis Vasc. Diffus. Lung Dis. 2010, 27, 43–48. [Google Scholar]
- Brauner, M.W.; Grenier, P.; Mompoint, D.; Lenoir, S.; de Crémoux, H. Pulmonary sarcoidosis: Evaluation with high-resolution CT. Radiology 1989, 172, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.L.; Kullnig, P.; Miller, R.R. The CT findings of pulmonary sarcoidosis: Analysis of 25 patients. AJR Am. J. Roentgenol. 1989, 152, 1179–1182. [Google Scholar] [CrossRef]
- Remy-Jardin, M.; Giraud, F.; Remy, J.; Wattinne, L.; Wallaert, B.; Duhamel, A. Pulmonary sarcoidosis: Role of CT in the evaluation of disease activity and functional impairment and in prognosis assessment. Radiology 1994, 191, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Itoh, H.; Kitaichi, M.; Nagai, S.; Izumi, T. CT and pathological correlation of pulmonary sarcoidosis. Semin. Ultrasound CT MRI 1995, 16, 361–370. [Google Scholar] [CrossRef]
- Dawson, W.B.; Müller, N.L. High-resolution computed tomography in pulmonary sarcoidosis. Semin. Ultrasound CT MRI 1990, 11, 423–429. [Google Scholar]
- Johkoh, T.; Ikezoe, J.; Tomiyama, N.; Nagareda, T.; Kohno, N.; Takeuchi, N.; Yamagami, H.; Kido, S.; Takashima, S.; Arisawa, J. CT findings in lymphangitic carcinomatosis of the lung: Correlation with histologic findings and pulmonary function tests. AJR Am. J. Roentgenol. 1992, 158, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Ikezoe, J.; Godwin, J.D.; Hunt, K.J.; Marglin, S.I. Pulmonary lymphangitic carcinomatosis: Chronicity of radiographic findings in long-term survivors. AJR Am. J. Roentgenol. 1995, 165, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.L.; Miller, R.R. Ground-glass attenuation, nodules, alveolitis, and sarcoid granulomas. Radiology 1993, 189, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.A.; Webb, W.R.; Gamsu, G.; Stulbarg, M.; Golden, J. Computed tomography in pulmonary sarcoidosis. J. Comput. Assist. Tomogr. 1989, 13, 405–410. [Google Scholar] [CrossRef]
- Gruden, J.F.; Webb, W.R.; Warnock, M. Centrilobular opacities in the lung on high-resolution CT: Diagnostic considerations and pathologic correlation. AJR Am. J. Roentgenol. 1994, 162, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Rajagopala, S.; Sankari, S.; Kancherla, R.; Ramanathan, R.P.; Balalakshmoji, D. Miliary Sarcoidosis: Does it exist? A case series and systematic review of literature. Sarcoidosis Vasc. Diffus. Lung Dis. 2020, 37, 53–65. [Google Scholar] [CrossRef]
- Bergin, C.J.; Bell, D.Y.; Coblentz, C.L.; Chiles, C.; Gamsu, G.; MacIntyre, N.R.; Coleman, R.E.; Putman, C.E. Sarcoidosis: Correlation of pulmonary parenchymal pattern at CT with results of pulmonary function tests. Radiology 1989, 171, 619–624. [Google Scholar] [CrossRef]
- Grenier, P.; Valeyre, D.; Cluzel, P.; Brauner, M.W.; Lenoir, S.; Chastang, C. Chronic diffuse interstitial lung disease: Diagnostic value of chest radiography and high-resolution CT. Radiology 1991, 179, 123–132. [Google Scholar] [CrossRef]
- Malaisamy, S.; Dalal, B.; Bimenyuy, C.; Soubani, A.O. The clinical and radiologic features of nodular pulmonary sarcoidosis. Lung 2009, 187, 9–15. [Google Scholar] [CrossRef]
- Desai, S.; Devaraj, A.; Lynch, D.; Sverzellati, N.; Elicker, B. Webb, Müller and Naidich’s High-Resolution CT of the Lung, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2021; pp. 777–829. [Google Scholar]
- Herráez Ortega, I.; Alonso Orcajo, N.; López González, L. The “sarcoid cluster sign”. A new sign in high resolution chest CT. Radiologia 2009, 51, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, M.; Hatabu, H.; Morikawa, K.; Uematsu, H.; Ohno, Y.; Nishimura, K.; Nagai, S.; Izumi, T.; Konishi, J.; Itoh, H. Large coalescent parenchymal nodules in pulmonary sarcoidosis: “sarcoid galaxy” sign. AJR Am. J. Roentgenol. 2002, 178, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Marchiori, E.; Zanetti, G.; Mano, C.M. Pulmonary tuberculosis with the sarcoid cluster sign in high-resolution chest CT. Radiologia 2010, 52, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.R.; Sivarasan, N.; Johannson, K.A.; George, P.M.; Culver, D.A.; Devaraj, A.; Lynch, D.A.; Milne, D.; Renzoni, E.; Nunes, H.; et al. High-resolution CT phenotypes in pulmonary sarcoidosis: A multinational Delphi consensus study. Lancet Respir. Med. 2023. [Google Scholar] [CrossRef]
- Lenique, F.; Brauner, M.W.; Grenier, P.; Battesti, J.P.; Loiseau, A.; Valeyre, D. CT assessment of bronchi in sarcoidosis: Endoscopic and pathologic correlations. Radiology 1995, 194, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Chambellan, A.; Turbie, P.; Nunes, H.; Brauner, M.; Battesti, J.P.; Valeyre, D. Endoluminal stenosis of proximal bronchi in sarcoidosis: Bronchoscopy, function, and evolution. Chest 2005, 127, 472–481. [Google Scholar] [CrossRef]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of terms for Thoracic Imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef]
- Davies, C.W.; Tasker, A.D.; Padley, S.P.; Davies, R.J.; Gleeson, F.V. Air trapping in sarcoidosis on computed tomography: Correlation with lung function. Clin. Radiol. 2000, 55, 217–221. [Google Scholar] [CrossRef]
- Magkanas, E.; Voloudaki, A.; Bouros, D.; Prassopoulos, P.; Alexopoulou, C.; Tzanakis, N.; Linardakis, M.; Gourtsoyiannis, N. Pulmonary sarcoidosis. Correlation of expiratory high-resolution CT findings with inspiratory patterns and pulmonary function tests. Acta Radiol. 2001, 42, 494–501. [Google Scholar]
- Bartz, R.R.; Stern, E.J. Airways obstruction in patients with sarcoidosis: Expiratory CT scan findings. J. Thorac. Imaging 2000, 15, 285–289. [Google Scholar] [CrossRef]
- Iannuzzi, M.C.; Rybicki, B.A.; Teirstein, A.S. Sarcoidosis. N. Engl. J. Med. 2007, 357, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Traill, Z.C.; Maskell, G.F.; Gleeson, F.V. High-resolution CT findings of pulmonary sarcoidosis. AJR Am. J. Roentgenol. 1997, 168, 1557–1560. [Google Scholar] [CrossRef] [PubMed]
- Hennebicque, A.S.; Nunes, H.; Brillet, P.Y.; Moulahi, H.; Valeyre, D.; Brauner, M.W. CT findings in severe thoracic sarcoidosis. Eur. Radiol. 2005, 15, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Padley, S.P.; Padhani, A.R.; Nicholson, A.; Hansell, D.M. Pulmonary sarcoidosis mimicking cryptogenic fibrosing alveolitis on CT. Clin. Radiol. 1996, 51, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.F.; McClelland, R.L.; Ho, L.A.; Mikacenic, C.R.; Hayes, J.; Spada, C.; Raghu, G. Sarcoidosis and IPF in the same patient-a coincidence, an association or a phenotype? Respir. Med. 2018, 144S, S20–S27. [Google Scholar] [CrossRef]
- Bianchi, F.; Piccioli, C.; Rosi, E.; Carobene, L.; Spina, D.; Mazzei, M.A.; Bartolucci, M.; Moroni, C.; Novelli, L.; Rottoli, P.; et al. Combined sarcoidosis and idiopathic pulmonary fibrosis (CSIPF): A novel disease phenotype? Respir. Med. 2019, 160, 105650. [Google Scholar] [CrossRef]
- Rockoff, S.D.; Rohatgi, P.K. Unusual manifestations of thoracic sarcoidosis. AJR Am. J. Roentgenol. 1985, 144, 513–528. [Google Scholar] [CrossRef]
- Hours, S.; Nunes, H.; Kambouchner, M.; Uzunhan, Y.; Brauner, M.W.; Valeyre, D.; Brillet, P.Y. Pulmonary cavitary sarcoidosis: Clinico-radiologic characteristics and natural history of a rare form of sarcoidosis. Medicine 2008, 87, 142–151. [Google Scholar] [CrossRef]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of chronic pulmonary aspergillosis complicating sarcoidosis. Eur. Respir. J. 2013, 41, 621–626. [Google Scholar] [CrossRef]
- Denning, D.W.; Cadranel, J.; Beigelman-Aubry, C.; Ader, F.; Chakrabarti, A.; Blot, S.; Ullmann, A.J.; Dimopoulos, G.; Lange, C.; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 2016, 47, 45–68. [Google Scholar] [CrossRef]
- Wollschlager, C.; Khan, F. Aspergillomas complicating sarcoidosis. A prospective study in 100 patients. Chest 1984, 86, 585–588. [Google Scholar] [CrossRef]
- Fujita, S. Serologic diagnosis of fungal infections. Nihon Rinsho 2008, 66, 2313–2318. [Google Scholar] [PubMed]
- Bouza, E.; Almirante, B.; García Rodríguez, J.; Garnacho-Montero, J.; Salavert, M.; Muñoz, P.; Sanguinetti, M. Biomarkers of fungal infection: Expert opinion on the current situation. Rev. Esp. Quimioter. 2020, 33, 1–10. [Google Scholar] [CrossRef]
- Szwarcberg, J.B.; Glajchen, N.; Teirstein, A.S. Pleural involvement in chronic sarcoidosis detected by thoracic CT scanning. Sarcoidosis Vasc. Diffus. Lung Dis. 2005, 22, 58–62. [Google Scholar]
- Huggins, J.T.; Doelken, P.; Sahn, S.A.; King, L.; Judson, M.A. Pleural effusions in a series of 181 outpatients with sarcoidosis. Chest 2006, 129, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Froudarakis, M.E.; Bouros, D.; Voloudaki, A.; Papiris, S.; Kottakis, Y.; Constantopoulos, S.H.; Siafakas, N.M. Pneumothorax as a first manifestation of sarcoidosis. Chest 1997, 112, 278–280. [Google Scholar] [CrossRef]
- Gomm, S.A. An unusual presentation of sarcoidosis--spontaneous haemopneumothorax. Postgrad. Med. J. 1984, 60, 621–623. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.P.; Xu, L.L.; Li, X. Recurrent pneumothorax as a presenting manifestation of active sarcoidosis: A case report and literature review. Chin. Med. J. 2010, 123, 1615–1616. [Google Scholar]
- Omori, H.; Asahi, H.; Irinoda, T.; Itabashi, T.; Saito, K. Pneumothorax as a presenting manifestation of early sarcoidosis. Jpn. J. Thorac. Cardiovasc. Surg. 2004, 52, 33–35. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. G. Ital. Cardiol. 2023, 24, 1e–116e. [Google Scholar] [CrossRef]
- Handa, T.; Nagai, S.; Miki, S.; Fushimi, Y.; Ohta, K.; Mishima, M.; Izumi, T. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest 2006, 129, 1246–1252. [Google Scholar] [CrossRef]
- Bourbonnais, J.M.; Samavati, L. Clinical predictors of pulmonary hypertension in sarcoidosis. Eur. Respir. J. 2008, 32, 296–302. [Google Scholar] [CrossRef]
- Rapti, A.; Kouranos, V.; Gialafos, E.; Aggeli, K.; Moyssakis, J.; Kallianos, A.; Kostopoulos, C.; Anagnostopoulou, O.; Sfikakis, P.P.; Wells, A.U.; et al. Elevated pulmonary arterial systolic pressure in patients with sarcoidosis: Prevalence and risk factors. Lung 2013, 191, 61–67. [Google Scholar] [CrossRef]
- Sulica, R.; Teirstein, A.S.; Kakarla, S.; Nemani, N.; Behnegar, A.; Padilla, M.L. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest 2005, 128, 1483–1489. [Google Scholar] [CrossRef]
- Hoffstein, V.; Ranganathan, N.; Mullen, J.B. Sarcoidosis simulating pulmonary veno-occlusive disease. Am. Rev. Respir. Dis. 1986, 134, 809–811. [Google Scholar] [CrossRef]
- Takemura, T.; Matsui, Y.; Saiki, S.; Mikami, R. Pulmonary vascular involvement in sarcoidosis: A report of 40 autopsy cases. Hum. Pathol. 1992, 23, 1216–1223. [Google Scholar] [CrossRef]
- Ratanawatkul, P.; Oh, A.; Richards, J.C.; Swigris, J.J. Performance of pulmonary artery dimensions measured on high-resolution computed tomography scan for identifying pulmonary hypertension. ERJ Open Res. 2020, 6, 00232–02019. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.T.; Kuzo, R.; Goodman, L.R.; Siegel, R.; Haasler, G.B.; Presberg, K.W. Utility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Medical College of Wisconsin Lung Transplant Group. Chest 1998, 113, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Nunes, H.; Humbert, M.; Capron, F.; Brauner, M.; Sitbon, O.; Battesti, J.P.; Simonneau, G.; Valeyre, D. Pulmonary hypertension associated with sarcoidosis: Mechanisms, haemodynamics and prognosis. Thorax 2006, 61, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, J.E.; Fishman, E.K.; Siegelman, S.S. Invasive pulmonary aspergillosis in acute leukemia: Characteristic findings on CT, the CT halo sign, and the role of CT in early diagnosis. Radiology 1985, 157, 611–614. [Google Scholar] [CrossRef]
- Primack, S.L.; Hartman, T.E.; Lee, K.S.; Müller, N.L. Pulmonary nodules and the CT halo sign. Radiology 1994, 190, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, M.; Blandino, A.; Scribano, E.; Minutoli, F.; Volta, S.; Pandolfo, I. Computed tomography halo sign in pulmonary nodules: Frequency and diagnostic value. J. Thorac. Imaging 1999, 14, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Nabeshima, K.; Matsumoto, T.; Akagi, T.; Fujita, M.; Watanabe, K. Histological findings of the computed tomography halo in pulmonary sarcoidosis. Eur. Respir. J. 2009, 34, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, K.S.; Ryu, Y.H.; Yoon, Y.C.; Choe, K.O.; Kim, T.S.; Sung, K.J. Reversed halo sign on high-resolution CT of cryptogenic organizing pneumonia: Diagnostic implications. AJR Am. J. Roentgenol. 2003, 180, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Marchiori, E.; Zanetti, G.; Escuissato, D.L.; Souza, A.S.; de Souza Portes Meirelles, G.; Fagundes, J.; Souza, C.A.; Hochhegger, B.; Marom, E.M.; Godoy, M.C.B. Reversed halo sign: High-resolution CT scan findings in 79 patients. Chest 2012, 141, 1260–1266. [Google Scholar] [CrossRef]
- Marchiori, E.; Zanetti, G.; Duarte Guimarães, M.; Hochhegger, B. The reversed halo sign extending the spectrum of atypical radiological manifestations in sarcoidosis. Ann. Thorac. Med. 2014, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Chappell, A.G.; Cheung, W.Y.; Hutchings, H.A. Sarcoidosis: A long-term follow up study. Sarcoidosis Vasc. Diffus. Lung Dis. 2000, 17, 167–173. [Google Scholar]
- Kirkil, G.; Lower, E.E.; Baughman, R.P. Predictors of Mortality in Pulmonary Sarcoidosis. Chest 2018, 153, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Nardi, A.; Brillet, P.Y.; Letoumelin, P.; Girard, F.; Brauner, M.; Uzunhan, Y.; Naccache, J.M.; Valeyre, D.; Nunes, H. Stage IV sarcoidosis: Comparison of survival with the general population and causes of death. Eur. Respir. J. 2011, 38, 1368–1373. [Google Scholar] [CrossRef]
- Primack, S.L.; Hartman, T.E.; Hansell, D.M.; Müller, N.L. End-stage lung disease: CT findings in 61 patients. Radiology 1993, 189, 681–686. [Google Scholar] [CrossRef]
- Ungprasert, P.; Crowson, C.S.; Carmona, E.M.; Matteson, E.L. Outcome of pulmonary sarcoidosis: A population-based study 1976-2013. Sarcoidosis Vasc. Diffus. Lung Dis. 2018, 35, 123–128. [Google Scholar] [CrossRef]
- Hambly, N.; Farooqi, M.M.; Dvorkin-Gheva, A.; Donohoe, K.; Garlick, K.; Scallan, C.; Chong, S.G.; MacIsaac, S.; Assayag, D.; Johannson, K.A.; et al. Prevalence and characteristics of progressive fibrosing interstitial lung disease in a prospective registry. Eur. Respir. J. 2022, 60, 2102571. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Winterbauer, R.H.; Hutchinson, J.F. Use of pulmonary function tests in the management of sarcoidosis. Chest 1980, 78, 640–647. [Google Scholar] [CrossRef]
- Cortes-Telles, A.; Forkert, L.; O’Donnell, D.E.; Moran-Mendoza, O. Idiopathic pulmonary fibrosis: New insights on functional characteristics at diagnosis. Can. Respir. J. 2014, 21, 825606. [Google Scholar] [CrossRef]
- Calaras, D.; Munteanu, O.; Scaletchi, V.; Simionica, I.; Botnaru, V. Ventilatory disturbances in patients with intrathoracic sarcoidosis—A study from a functional and histological perspective. Sarcoidosis Vasc. Diffus. Lung Dis. 2017, 34, 58–67. [Google Scholar] [CrossRef]
- Baughman, R.P.; Engel, P.J.; Taylor, L.; Lower, E.E. Survival in sarcoidosis-associated pulmonary hypertension: The importance of hemodynamic evaluation. Chest 2010, 138, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.U.; Desai, S.R.; Rubens, M.B.; Goh, N.S.; Cramer, D.; Nicholson, A.G.; Colby, T.V.; du Bois, R.M.; Hansell, D.M. Idiopathic pulmonary fibrosis: A composite physiologic index derived from disease extent observed by computed tomography. Am. J. Respir. Crit. Care Med. 2003, 167, 962–969. [Google Scholar] [CrossRef]
- Wells, A.U.; Cullinan, P.; Hansell, D.M.; Rubens, M.B.; Black, C.M.; Newman-Taylor, A.J.; Du Bois, R.M. Fibrosing alveolitis associated with systemic sclerosis has a better prognosis than lone cryptogenic fibrosing alveolitis. Am. J. Respir. Crit. Care Med. 1994, 149, 1583–1590. [Google Scholar] [CrossRef]
- Wells, A.U.; Hansell, D.M.; Rubens, M.B.; King, A.D.; Cramer, D.; Black, C.M.; du Bois, R.M. Fibrosing alveolitis in systemic sclerosis: Indices of lung function in relation to extent of disease on computed tomography. Arthritis Rheum. 1997, 40, 1229–1236. [Google Scholar] [CrossRef]
- Hansell, D.M.; Wells, A.U.; Padley, S.P.; Müller, N.L. Hypersensitivity pneumonitis: Correlation of individual CT patterns with functional abnormalities. Radiology 1996, 199, 123–128. [Google Scholar] [CrossRef]
- Goh, N.S.; Desai, S.R.; Veeraraghavan, S.; Hansell, D.M.; Copley, S.J.; Maher, T.M.; Corte, T.J.; Sander, C.R.; Ratoff, J.; Devaraj, A.; et al. Interstitial lung disease in systemic sclerosis: A simple staging system. Am. J. Respir. Crit. Care Med. 2008, 177, 1248–1254. [Google Scholar] [CrossRef]
- Walsh, S.L.; Sverzellati, N.; Devaraj, A.; Wells, A.U.; Hansell, D.M. Chronic hypersensitivity pneumonitis: High resolution computed tomography patterns and pulmonary function indices as prognostic determinants. Eur. Radiol. 2012, 22, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Bartholmai, B.J.; Rajagopalan, S.; Kokosi, M.; Nair, A.; Karwoski, R.; Raghunath, S.M.; Walsh, S.L.; Wells, A.U.; Hansell, D.M. Automated Quantitative Computed Tomography Versus Visual Computed Tomography Scoring in Idiopathic Pulmonary Fibrosis: Validation Against Pulmonary Function. J. Thorac. Imaging 2016, 31, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Bartholmai, B.J.; Rajagopalan, S.; Kokosi, M.; Nair, A.; Karwoski, R.; Walsh, S.L.; Wells, A.U.; Hansell, D.M. Mortality prediction in idiopathic pulmonary fibrosis: Evaluation of computer-based CT analysis with conventional severity measures. Eur. Respir. J. 2017, 49, 1601011. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kim, G.H.; Salisbury, M.L.; Barber, D.; Bartholmai, B.J.; Brown, K.K.; Conoscenti, C.S.; De Backer, J.; Flaherty, K.R.; Gruden, J.F.; et al. Computed Tomographic Biomarkers in Idiopathic Pulmonary Fibrosis. The Future of Quantitative Analysis. Am. J. Respir. Crit. Care Med. 2019, 199, 12–21. [Google Scholar] [CrossRef]
- Müller, N.L.; Mawson, J.B.; Mathieson, J.R.; Abboud, R.; Ostrow, D.N.; Champion, P. Sarcoidosis: Correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology 1989, 171, 613–618. [Google Scholar] [CrossRef]
- Lopes, A.J.; de Menezes, S.L.; Dias, C.M.; de Oliveira, J.F.; Mainenti, M.R.; Guimarães, F.S. Comparison between cardiopulmonary exercise testing parameters and computed tomography findings in patients with thoracic sarcoidosis. Lung 2011, 189, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Brauner, M.W.; Lenoir, S.; Grenier, P.; Cluzel, P.; Battesti, J.P.; Valeyre, D. Pulmonary sarcoidosis: CT assessment of lesion reversibility. Radiology 1992, 182, 349–354. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Machado, R.F.; Schraufnagel, D.; Sweiss, N.J.; Baughman, R.P. Racial difference in sarcoidosis mortality in the United States. Chest 2015, 147, 438–449. [Google Scholar] [CrossRef]
- Tukey, M.H.; Berman, J.S.; Boggs, D.A.; White, L.F.; Rosenberg, L.; Cozier, Y.C. Mortality among African American women with sarcoidosis: Data from the Black Women’s Health Study. Sarcoidosis Vasc. Diffus. Lung Dis. 2013, 30, 128–133. [Google Scholar]
- Sartwell, P.E.; Edwards, L.B. Epidemiology of sarcoidosis in the U.S. Navy. Am. J. Epidemiol. 1974, 99, 250–257. [Google Scholar] [CrossRef]
- Reich, J.M. Mortality of intrathoracic sarcoidosis in referral vs population-based settings: Influence of stage, ethnicity, and corticosteroid therapy. Chest 2002, 121, 32–39. [Google Scholar] [CrossRef]
- Swigris, J.J.; Olson, A.L.; Huie, T.J.; Fernandez-Perez, E.R.; Solomon, J.; Sprunger, D.; Brown, K.K. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am. J. Respir. Crit. Care Med. 2011, 183, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Boucly, A.; Cottin, V.; Nunes, H.; Jaïs, X.; Tazi, A.; Prévôt, G.; Reynaud-Gaubert, M.; Dromer, C.; Viacroze, C.; Horeau-Langlard, D.; et al. Management and long-term outcomes of sarcoidosis-associated pulmonary hypertension. Eur. Respir. J. 2017, 50, 1700465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tong, X.; Zhang, T.; Wang, D.; Liu, S.; Wang, L.; Fan, H. Prevalence of Sarcoidosis-Associated Pulmonary Hypertension: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 809594. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.L.; Wells, A.U.; Sverzellati, N.; Keir, G.J.; Calandriello, L.; Antoniou, K.M.; Copley, S.J.; Devaraj, A.; Maher, T.M.; Renzoni, E.; et al. An integrated clinicoradiological staging system for pulmonary sarcoidosis: A case-cohort study. Lancet Respir. Med. 2014, 2, 123–130. [Google Scholar] [CrossRef]







| Principal Reasons for CT Monitoring in Pulmonary Sarcoidosis |
|---|
| To chart disease behaviour in patients with an initial ‘low confidence, provisional’ diagnosis of sarcoidosis in whom integration with serial PFTs and clinical features may modify diagnostic likelihoods. |
| To ascertain the likelihood of reversibility at baseline and/or during the natural course of the disease. |
| For the assessment of treatment response (including drug trials in sarcoidosis). |
| Prognostication based on the presence/absence of CT features (e.g., disease extent, traction bronchiectasis/bronchiolectasis and honeycombing). |
| CT Phenotype | Description |
|---|---|
| Non-fibrotic | Micronodular—peri-bronchovascular, peri-fissural and/or subpleural predilection, predominantly in the mid/upper zones, with or without a minority component of larger nodules with surrounding micronodules (i.e., ‘galaxy sign’), architectural distortion or volume loss |
| Nodular (>3 mm but <3 cm)—peri-bronchovascular, peri-fissural and/or subpleural predilection, predominantly in the mid/upper zones, with or without a minority component of larger nodules with surrounding micronodules (i.e., ‘galaxy sign’), architectural distortion or volume loss | |
| Nodular (>3 mm but <3 cm)—random distribution | |
| Consolidation as the dominant or sole pattern | |
| Likely to be fibrotic | Bronchocentric reticulation without cavitation and/or fibro-bullous destruction and with or without dense parenchymal opacification and/or a minority component of other CT abnormalities (e.g., delicate bands of ‘loose’ reticulation; enlarged peripheral pulmonary arteries, central pulmonary artery enlargement or a mosaic attenuation pattern) |
| Bronchocentric reticulation with cavitation and/or fibro-bullous destruction and with or without dense parenchymal opacification and/or a minority component of other CT abnormalities (e.g., delicate bands of ‘loose’ reticulation; enlarged peripheral pulmonary arteries, central pulmonary artery enlargement or a mosaic attenuation pattern) | |
| Bronchocentric masses (‘progressive massive fibrosis [PMF]-lookalike’) with or without a minority component of other CT abnormalities (e.g., delicate bands of ‘loose’ reticulation; enlarged peripheral pulmonary arteries, central pulmonary artery enlargement or a mosaic attenuation pattern) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailey, G.L.; Wells, A.U.; Desai, S.R. Imaging of Pulmonary Sarcoidosis—A Review. J. Clin. Med. 2024, 13, 822. https://doi.org/10.3390/jcm13030822
Bailey GL, Wells AU, Desai SR. Imaging of Pulmonary Sarcoidosis—A Review. Journal of Clinical Medicine. 2024; 13(3):822. https://doi.org/10.3390/jcm13030822
Chicago/Turabian StyleBailey, Georgina L., Athol U. Wells, and Sujal R. Desai. 2024. "Imaging of Pulmonary Sarcoidosis—A Review" Journal of Clinical Medicine 13, no. 3: 822. https://doi.org/10.3390/jcm13030822
APA StyleBailey, G. L., Wells, A. U., & Desai, S. R. (2024). Imaging of Pulmonary Sarcoidosis—A Review. Journal of Clinical Medicine, 13(3), 822. https://doi.org/10.3390/jcm13030822





