Abstract
Alterations in microvasculature represent some of the earliest pathological processes across a wide variety of human diseases. In many organs, however, inaccessibility and difficulty in directly imaging tissues prevent the assessment of microvascular changes, thereby significantly limiting their translation into improved patient care. The eye provides a unique solution by allowing for the non-invasive and direct visualization and quantification of many aspects of the human microvasculature, including biomarkers for structure, function, hemodynamics, and metabolism. Optical coherence tomography angiography (OCTA) studies have specifically identified reduced capillary densities at the level of the retina in several eye diseases including glaucoma. This narrative review examines the published data related to OCTA-assessed microvasculature biomarkers and major systemic cardiovascular disease. While loss of capillaries is being established in various ocular disease, pilot data suggest that changes in the retinal microvasculature, especially within the macula, may also reflect small vessel damage occurring in other organs resulting from cardiovascular disease. Current evidence suggests retinal microvascular biomarkers as potential indicators of major systemic cardiovascular diseases, including systemic arterial hypertension, atherosclerotic disease, and congestive heart failure.
1. Introduction
Cardiovascular disease (CVD) is a leading cause of death globally, accounting for approximately 32% of all fatalities, with myocardial infarctions and strokes accounting for 85% [1]. CVDs are defined as a group of disorders of the heart and its vessels, and include coronary heart disease (CHD), cerebrovascular disease, peripheral arterial disease, rheumatic heart disease, congenital heart disease, deep vein thrombosis, and pulmonary embolism [1]. Despite its high prevalence and significant impact on society, developing novel accurate non-invasive diagnostic and prognostic methods for managing systemic vascular disease remains challenging due to tissue inaccessibility and the related challenges in the direct visualization of small blood vessels.
The eye’s microvasculature is singularly unique in that it can be non-invasively and directly visualized, imaged, and quantified. The integrity and function of the retinal tissue are dependent on adequate perfusion via this microcirculatory network and well-controlled blood flow [2]. Due to the accessibility of the eye’s vascular beds, the use of eye vasculature to assess pathological changes within retinal microvascular beds as an indicator for systemic vascular pathophysiology has been investigated in pilot work [2,3,4,5,6]. Specifically, changes in retinal microvasculature, particularly at the level of the macula, and variations in the thickness and layering of the retina have been associated with various aspects of cardiovascular health [2,7,8,9].
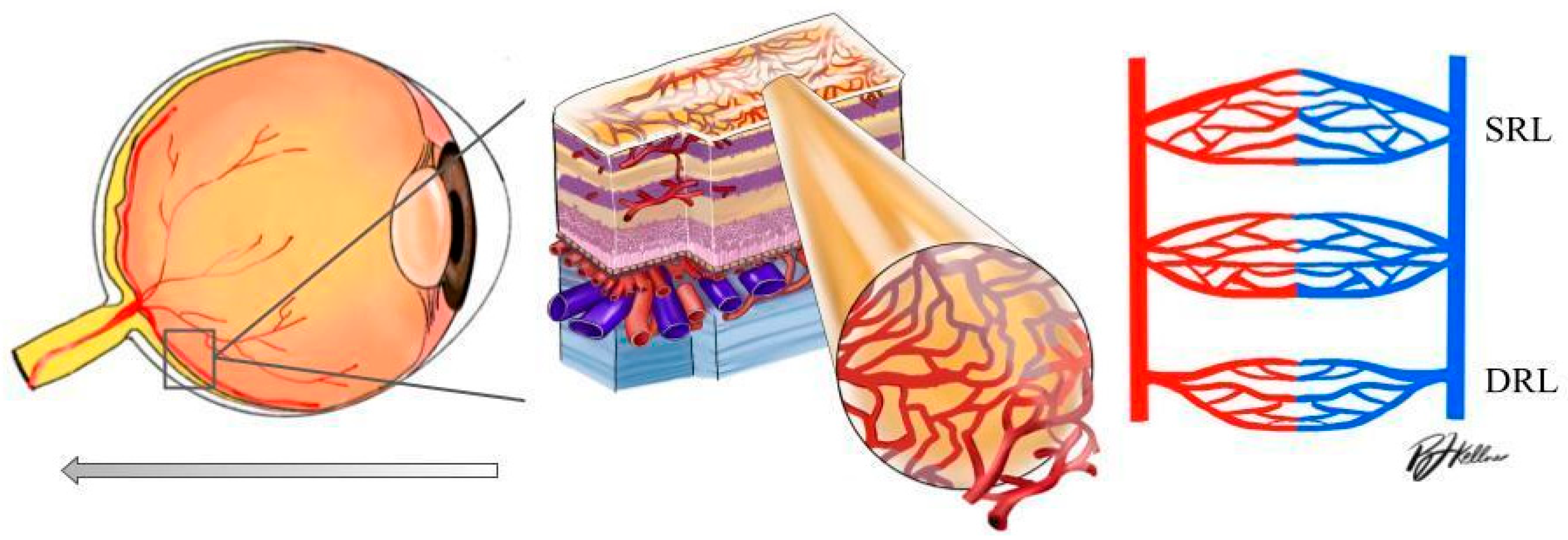
Optical coherence tomography angiography (OCTA) is an advanced ocular imaging platform that provides structural biomarkers and a three-dimensional assessment of large and small retinal capillaries within layers of the retina and choroid. OCTA provides information related to the optic nerve region, measuring VD at the level of the radial peripapillary capillary slab (from the internal limiting membrane to the nerve fiber layer). OCTA is also able to provide a visualization of the macular superficial capillary plexus (SCP) and deep capillary plexus (DCP), which allows for a more detailed study of retinal microvascular changes at their respective layers [10] (Figure 1). OCTA can both delineate the foveal avascular zone (FAZ) and produce depth-resolved images of the superficial and deep vascular plexuses, known as the superficial and deep retinal layer (SRL and DRL) [11,12]. To date, OCTA pilot research has identified a loss of capillary density at the level of the macula, corresponding to changes in vascular density (VD) and signal intensity in hypertension and rhegmatogenous retinal detachment [13,14].
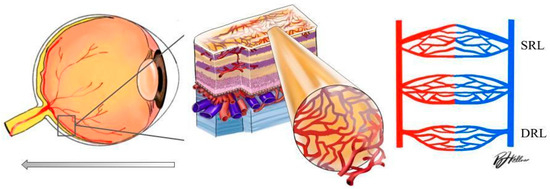
Figure 1.
Left: schematic diagram of the human eye with an enlargement of the retinal tissue. Right: schematic diagram of the significant vascular layers of the retina discussed in this paper (DRL: deep retinal layer; SRL: superficial retinal layer).
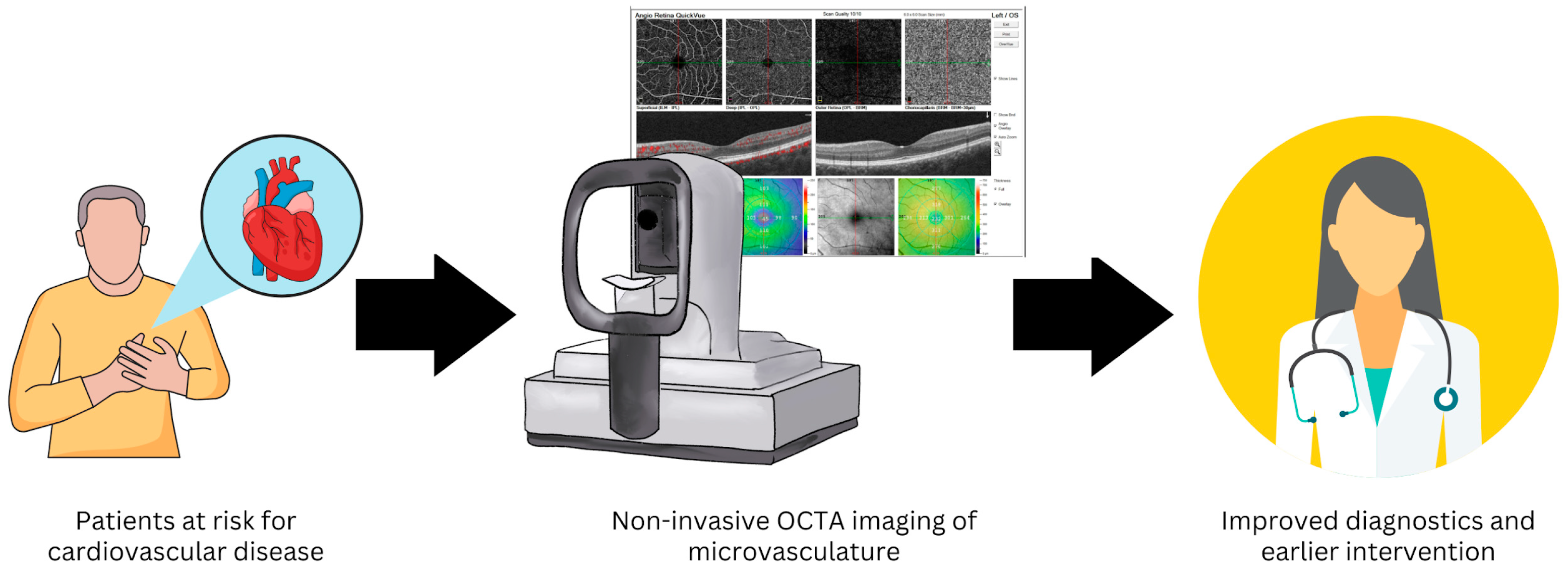
The non-invasive nature and wide accessibility of OCTA in outpatient clinical settings provide high potential for assessing the ocular microvasculature as a window into the health of the systemic cardiovascular system (Figure 2). The purpose of this research article is to analyze the available data related to OCTA-assessed retinal (macular and peripapillary where available) microvascular capillaries as potential prognostic indicators for systemic cardiovascular diseases, including systemic hypertension, atherosclerotic disease, and congestive heart failure.
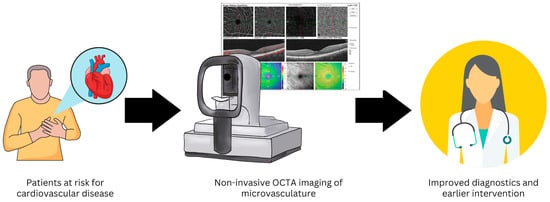
Figure 2.
Non-invasive optical coherence tomography angiography (OCTA) imaging of the retinal microvasculature may provide a method to improve diagnostics and allow earlier intervention in patients at risk for cardiovascular disease.
2. Methods
This analysis includes all published data related to OCTA-assessed microvascular VD biomarkers and major systemic cardiovascular disease. To achieve this, a comprehensive search was conducted through 1 December 2023 using PubMed, Embase, Ovid, Scopus, and Trip searches including the following keywords with AND/OR statements: cardiovascular disease, myocardial infarction, coronary artery disease, ischemic heart disease, optical coherence tomography, optical coherence tomography angiography, enhanced depth imaging, perfusion, blood flow, vascular density, retina, and choroidal imaging. Articles were screened for relevance, and reference lists of relevant articles were also searched and cross-referenced for other relevant articles. Data were collected and organized using Microsoft Word (version 16.30), Microsoft Excel (version 16.30), and EndNote (X8.2).
3. Results and Discussion
3.1. Systemic Arterial Hypertension
Systemic arterial hypertension is highly prevalent and remains the predominant risk factor for cardiovascular disease. Within the eye, elevated blood pressure results in hypertensive retinopathy characterized by several retinal microvascular changes [15]. Various population-based studies have demonstrated that smaller retinal arteriolar calibers and larger retinal venular calibers precede the clinical stage of systemic hypertension and can predict up to a 5-year risk of clinical hypertension in initially normotensive individuals [5,6]. A study measuring capillary microvasculature by invasive fundus fluorescein angiography showed an increase in the perifoveal inter-capillary area and a decrease in capillary blood velocity in patients with hypertension [16].
Recent studies have used OCTA to investigate retinal vascularity in patients with systemic hypertension, finding a significant reduction in VD in hypertensive patients [17,18]. As seen in Table 1, multiple studies demonstrate a decrease in VD in both the deep and superficial vascular plexi [13,17,18,19,20,21,22,23,24,25,26,27]. As shown in Table 1, different studies reported the VD values of both the parafoveal region alone and the entire macular region analyzed, with VD values averaged as a mean. Lee et al. [13] examined the SCP and found that in patients with chronic hypertension and hypertensive retinopathy, there was a reduction in the superficial macular VD and perfusion density, and an increase in the FAZ, compared to those in their normotensive control group [13]. A similar study by Peng et al. [19] found that the deep vascular plexus (DVP) VD was significantly decreased in essential hypertensive patients with and without hypertensive retinopathy compared to that of controls. Of note, VD in the superficial vascular plexus (SVP) of patients with hypertensive retinopathy was significantly decreased compared to that of patients with essential hypertension without hypertensive retinopathy and that of the control group [19].

Table 1.
Summary of studies investigating retinal optical coherence tomography angiography (OCTA) parameters in subjects with systemic hypertension. Percent change of vascular density (VD) was estimated by subtracting the reported mean VD of the experimental group by that of the control group and dividing it by the VD of the control group. DCP: deep capillary plexus; DRL: deep retinal layer; DVP: deep vascular plexus; HTN: hypertension; SCP: superficial capillary plexus; SRL: superficial retinal layer; SVP: superficial vascular plexus.
A larger cohort study by Chua et al. [20] examined the SVP and DVP and demonstrated that patients with poorly controlled blood pressure (BP ≥ 140/90 mmHg) had reduced DRL VD [20]. Bridging this concept, Donati et al. [21] showed a significant reduction in the VD of the DRL and an enlargement of the deep FAZ area in hypertensive patients, thus suggesting the presence of an early modification of the microvascular network at the level of the deep retinal plexus [21]. Parafoveal VD in the choriocapillaris, DCP, and total capillary plexus was also found to be decreased in hypertensive patients versus those of controls [22]. Conversely, no significant difference was found in the SCP and middle capillary plexus between hypertensive and control patients [22]. Similar results were obtained in another study in which there was a significant decrease in VD between hypertensive and control eyes in the DRL, but no significant difference in the VD of the SRL [23]. Other studies, however, found statistically significant capillary dropout in both the DRL and SRL in patients with hypertension [24,25]. Significant capillary dropout in both the SRL and DRL has also been reported in patients with recent hypertensive crises (systolic BP > 180 mmHg and diastolic BP > 110 mmHg) within 7 days [25]. These results point to a consistency of capillary dropout in poorly controlled blood pressure; however, exact mechanistic pathways and the level of tissue insult require a targeted approach for confirmation in larger controlled samples.
The length of hypertensive diagnosis has also been associated with retinal vascular changes in hypertensive patients [17,27,28]. Specifically, one study found that patients with hypertension for more than 10 years have lower a peripapillary VD and perfusion density in the SCP. The authors did not find significant differences between the group with hypertension for less than 10 years and the control group [28]. Lim et al. [27] divided hypertensive patients by diagnosis for less than 5 years or greater than 5 years and found that patients with more than 5 years of hypertension had a thinner ganglion cell inner plexiform layer and peripapillary retinal nerve fiber layer compared to those of controls [27].
These preliminary findings suggest that the detection of capillary densities in the macula and peripapillary regions may serve as early pathologic markers of hypertensive damage and may ultimately improve abilities to monitor disease progression and better guide therapeutic treatment. For example, a study utilizing foveal OCTA in hypertensive patients found that foveal VD was significantly correlated with the Keith–Wagener–Barker grade of hypertensive retinopathy [29]. Of importance is that one study has found a positive moderate to strong correlation between retinal capillary density and cardiac remodeling markers in hypertensive patients [30]. It is important to note that, while consistent in the theme of outcomes, the current available data are limited and there is a lack of controlled studies accounting for treatments and comorbidities. The variation between studies on hypertension and OCTA biomarkers is likely due to differing OCTA machines and algorithms, and variations in patient populations, sample sizes, and outcome parameters (Table 1).
3.2. Atherosclerotic Disease
Atherosclerotic disease, a form of arteriosclerosis, is characterized by the thickening and hardening of artery walls due to the accumulation of fatty deposits, cholesterol, and other substances. This process leads to two major clinical categories: ischemic stroke and coronary artery disease (CAD). Ischemic stroke occurs when atherosclerotic plaques block blood vessels in the brain, leading to impaired blood flow and oxygen deprivation to brain tissues. This can result in brain damage and a loss of neurological functions. On the other hand, CAD involves the narrowing or blockage of the coronary arteries, which supply blood to the heart muscle. This can lead to angina (chest pain), myocardial infarction (heart attack), and other serious heart conditions. Both conditions are major contributors to morbidity and mortality worldwide and share common risk factors such as hypertension, high cholesterol, diabetes, obesity, and smoking.
The retina and choroid are affected by both CAD and ischemic stroke. Retinal and choroidal vascular biomarkers may act as powerful surrogates for measuring coronary circulation. Original studies [31,32] used fundus photography and retinal imaging to show a positive fair correlation between retinal arterial atherosclerosis and the severity of CAD [31].
Recent studies using OCTA have further characterized CAD’s effects on ophthalmic vasculature by identifying capillary dropout in the macula. Specifically, Wang et al. [33] found in a prospective, cross-sectional observational study that the mean VD of several layers, including both the SRL and DRL, was significantly lower in patients with CAD. Specifically, decreased VD and blood flow were associated with coronary artery and branch stenosis [33]. Another study examined 45 patients undergoing elective coronary angiography and OCTA. Patients with coronary heart disease confirmed using elective coronary angiography had a significantly lower VD in the SCP, DCP, and choriocapillaris [34]. Additionally, an observational study by Matuleviciute et al. [35] demonstrated a significantly decreased risk of three-vessel CAD with increased VD [35]. More recently, a study in patients with ST elevation myocardial infarctions found that decreased VD in the SCP, DCP, and choriocapillaris was positively correlated to left ventricular ejection fraction (LVEF), and SCP and DCP central fovea (1 mm) VD were negatively correlated to the number of affected coronary vessels in the studied patients [36].
While most studies (Table 2) demonstrated changes in both the SRL and DRL in patients with CAD, some studies demonstrated changes in the SRL alone. In 2018, Arounould et al. [37] studied changes only in the SRL. The study included 237 patients hospitalized for acute coronary syndrome and found that retinal VD in the SCP had a negative moderate correlation with higher American Heart Association risk and Global Registry of Acute Coronary scores, and lower left ventricular ejection fraction (LVEF) [37].

Table 2.
Summary of studies investigating retinal optical coherence tomography angiography (OCTA) parameters in subjects with coronary artery disease. Percent change of vascular density (VD) was estimated by subtracting the reported mean VD of the experimental group by that of the control group and dividing it by the VD of the control group. CAD: coronary artery disease; CTO: coronary total occlusion; DCP: deep capillary plexus; DVP: deep vascular plexus; MI: myocardial infarction; OCAD: obstructive coronary artery disease; SCP: superficial capillary plexus; SRL: superficial retinal layer; SVP: superficial vascular plexus.
Some studies have sought to characterize retinal microvascular changes in patients with various levels of severity of coronary occlusion. Zhong et al. [38] observed retinal changes in patients with total coronary artery occlusion CAD (TCAO) on coronary angiography compared to those in patients with non-total coronary artery occlusion CAD (NTCAO) (>50% stenosis in at least one major artery). They found that VD of the SCP was significantly lower in TCAO patients versus those in NTCAO patients [38]. A different study in 2023 found that patients with obstructive coronary artery disease (OCAD) (>50% stenosis in at least one major coronary artery) and non-obstructive coronary artery disease (NOCAD) (20–50% stenosis in all major coronary arteries) had significantly reduced VD in all regions of the superficial vessel plexus and in all regions except for the fovea in the deep vessel plexus compared to the control. Additionally, a more significant decrease in VD was found in OCAD patients versus that in NOCAD patients [39].
The preponderance of study results suggests that significant changes in the retinal and choroidal microstructure and capillary loss occur in patients with CAD; thus, there is potential to use retinal vascular biomarkers for CAD early diagnosis, follow-up, and management. One study, however, by Arnould et al. [40] with 30 participants found no significant association between retinal VD and hemodynamic variables in the acute phase of myocardial infarction [40]. The scarce literature necessitates further investigation of the relationships between VD and CAD to elicit the specific prognostic and diagnostic abilities of each biomarker to disease outcomes.
The retinal and cerebral vasculatures share many similarities from embryology to functionality, thus allowing the non-invasive assessment of retinal vasculature to provide an estimation of changes in the cerebral vasculature. Previous studies have shown that changes in retinal blood vessels can be a window for evaluating cerebral vasculopathy [41,42]. With the advent of digital image processing, image analysis can be used to quantitatively measure retinal vasculature parameters such as central retinal artery equivalent diameter (CRAE), central retinal vein equivalent diameter (CRVE), and arteriovenous ratio (AVR), which is the ratio of the arteriole diameter to the venule diameter. These measurements have been used in many population-based studies to determine the relationship between retinal vessel characteristics and ischemic stroke [43,44,45].
Studies have shown that retinal vascular caliber has been independently correlated with stroke and can be viewed as an important marker for ischemic stroke detection [46,47]. Retinal caliber in patients with previous ischemic stroke was compared to that in control patients, and CRAE and subfoveal choroidal thickness in ischemic stroke patients were found to be significantly reduced compared to those in the control [43]. Highlighting the clinical potential of these findings, Zhao et al. [48] in 2021 developed a risk assessment model for ischemic stroke using measured retinal caliber and the Fazekas classification of white matter lesions along with traditional stroke risk factors in patients at risk for ischemic stroke. The authors found that the assessment model that included CRAE, the Fazekas classification of white matter lesions, and traditional risk factors was better than the single-index models they compared it to [48].
Studies investigated retinal structural and microvascular changes in ischemic strokes in patients with large artery atherosclerosis or small vessel occlusion (see Table 3). The superior peripapillary retinal nerve fiber layer thickness in large artery atherosclerosis patients was significantly thinner than that in small vessel occlusion patients [49]. A study by Duan et al. [50] found that vascular dropout may be a potential biomarker of ischemic stroke and its subtypes including lacunar and non-lacunar. The study demonstrated specific damage patterns in retinal microvascular and macular morphology in different subtypes of ischemic stroke. They found that lower vascular orientation distribution in the SCP was associated with lacunar infarction compared to non-lacunar infarction. In the DCP, however, they found that a higher FAZ axis ratio and lower FAZ circularity were associated with ischemic stroke, thus suggesting that the deep microvasculature could be more sensitive to ischemic stroke [50]. One study assessed patients with recent small subcortical infarcts and found that VD was significantly reduced in the superficial retinal capillary plexus and DCP of the macula compared to that in controls. They suggested that the DCP may be more susceptible to hypoperfusion because it is at the border of the inner plexiform layer and outer plexiform layer and therefore has a lower level of oxygen [51]. In contrast, a study in 2022 found that patients with thalamic infarcts had a significantly lower density in the SCP and intermediate capillary plexus compared to those in controls. No significant difference was found in the DCP between the thalamic infarct and control patients [52].

Table 3.
Summary of studies investigating retinal optical coherence tomography angiography (OCTA) parameters in subjects with ischemic stroke. The percent change of vascular density (VD) was estimated by subtracting the reported mean VD of the experimental group by that of the control group and dividing it by the VD of the control group. DCP: deep capillary plexus; DRL: deep retinal layer; DVP: deep vascular plexus; IPR: inner retina plexus; RPCP: radial peripapillary capillary plexus; SCP: superficial capillary plexus; SVP: superficial vascular plexus.
Multiple studies have found significant changes in both the SRL and DRL in patients with stroke. A case–control study with 15 ischemic stroke patients found that VD was significantly reduced at the level of all vascular plexuses in stroke patients [53]. Additionally, a study by Liang et al. [54] in 2022 found that the VD in the SCP and DCP was significantly reduced in ischemic stroke patients compared to those in the control [54]. Lu et al. [55] compared retinal microvasculature between ischemic stroke patients with large artery atherosclerosis (LAA) and small artery disease (SAD). They showed that LAA patients had significant capillary dropout in the deep vasculature compared with those with SAD; anterior LAA patients had significant capillary loss in the superficial retina compared with posterior LAA patients [55].
While the current literature regarding changes to the macular and peripapillary microvascular and ischemic stroke are variable and without consensus, the majority of OCTA studies suggest potential for its use to detect early retinal microvasculature changes in specific subtypes of ischemic stroke. However, only longitudinal studies inclusive of patients at high risk for various subtypes of ischemic stroke can provide clarity on the predictability of retinal VD outcomes for improved risk modeling and ultimately enhanced stroke patient care.
3.3. Congestive Heart Failure
Congestive heart failure (CHF) is a serious condition in which the heart fails to adequately perfuse the body. Although the pathogenesis of CHF is multifactorial, many studies have implicated a significant role in microvascular dysfunction [56,57,58]. Specifically, low cardiac output with CHF has been associated with compensatory mechanisms of peripheral vasoconstriction to maintain adequate BP and perfusion to essential tissues such as the heart and brain [59,60]. Some studies have found a decrease in cerebral blood flow in patients with CHF and have hypothesized a reduction in retinal blood flow as well [61,62].
Using Doppler ultrasound, Meira-Freitas et al. [63] evaluated the hemodynamics of the ophthalmic artery in patients with heart failure (HF) and proposed that HF could be a risk factor for low ocular perfusion [63]. In one of the first cross-sectional clinical studies to evaluate CHF and ocular blood flow via OCT, Altinkaynak et al. [64] demonstrated that the choroidal thickness in the macular area decreased in patients with CHF. Additionally, the authors found that the mean subfoveal choroidal thickness value was significantly lower in patients with CHF when compared with that in demographically matched healthy controls [64]. Using OCTA, Topaloglu et al. [65] found a significant lower VD in HF patients compared to that in controls (see Table 4) [65]. Although unable to show a statistically significant difference in flow densities between the study and control group, Alnawaiseh et al. [66] demonstrated a correlation between reduced macular flow density (whole en face) and LVEF, and New York Heart Association HF class, and showed a reduction in the ocular perfusion of chronic systolic HF when compared with that of healthy controls [66]. One study observed retinal VD using OCTA in children with HF due to dilated cardiomyopathy compared to that in controls and found that VD in the SCP was significantly lower in the HF group. No significant difference was found in the DCP or FAZ [67].

Table 4.
Summary of studies investigating retinal optical coherence tomography angiography (OCTA) parameters in subjects with congestive heart failure (CHF). The percent change of vascular density (VD) was estimated by subtracting the reported mean VD of the experimental group by that of the control group and dividing it by the VD of the control group. DCP: deep capillary plexus; NYHA: New York Heart Association; SCP: superficial capillary plexus.
While not highly prevalent, some HF clinical studies are using retinal microvascular measurements as outcomes in patients [68]. The current findings indicate that OCTA and quantitative analyses of VD indeed may be useful as novel and non-invasive techniques to monitor microcirculation and central perfusion in patients with CHF. Long-term data are missing, however, and the prediction of OCTA biomarkers for CHF progression requires confirmation in significantly larger and well-controlled studies that specifically account for the impact of BP within this patient population.
4. Limitations
The limitations of this analysis include the diverse patient populations, variability in the inclusion of one or both eyes, limited OCTA data linked to cardiovascular disease, especially outside of the macular region, and lack of standardization in OCTA methodologies and data presentation. In addition, this analysis includes reports of average values for the whole region of interest assessed in the different studies (macula/peripapillary region) as means/ averages, and also regional differences (i.e., hemispheric/sectorial). Overall, well-controlled longitudinal studies with larger sample sizes are required to reach specificity in the location, significance, and pattern of retinal microvascular capillary dropout using OCTA for each one of these systemic diseases. Due to the complex nature of cardiovascular disease and BP, risk models should address and account for differences in BP and potentially hypertensive medication use among patient populations.
5. Future Directions
As cardiovascular disease is a leading and rising cause of death globally, the improved non-invasive and highly accessible imaging of microvascular health will prevent a significant number of cardiovascular-related events, costs, and deaths. Well-controlled studies that account for the significant differences in blood and perfusion pressure across study populations and among cardiovascular disease groups are needed. In addition, long-term studies are needed to clarify acute versus chronic microvascular changes to reveal the strength and prognostic abilities over time. Artificial intelligence applications, including machine and transfer learning approaches to imagery, may reduce noise and increase the specificity and translatability of OCTA vascular biomarkers toward improved risk modeling and the enhanced management of systemic vascular disease.
6. Conclusions
The high prevalence and societal impact of systemic cardiovascular diseases necessitate improved diagnostic and predictive risk modeling approaches, with a focus on fast non-invasive acquisition and high accessibility. The eye is unique because it allows for the non-invasive and direct visualization and quantification of the earliest changes to human microvasculature. Advancements in OCTA imaging provide estimates of blood VD in specific regions of the retina and provide surrogate assessments of capillary loss and microvascular dropout. Although highly variable in the current literature, a preponderance of current studies has found lower retinal VD, suggesting that a reduction in microvascular capillaries may be associated with HTN, CAD, ischemic stroke, and CHF.
The vast majority of studies have specifically identified microvascular changes occurring within the macular region, while significantly less is known about peripapillary and peripheral tissues. In addition, acute versus chronic relationships between retinal and systemic microvascular changes are almost entirely unexplored, and longitudinal data on disease progression are scarce. Well-controlled longitudinal studies are therefore needed to confirm these preliminary data, identify areas of earliest detectability, differentiate acute versus chronic changes to the retinal microvasculature, and clarify the exact prognostic abilities for each biomarker and its relationship to systemic cardiovascular disease. Additionally, as seen in Table 1, Table 2, Table 3 and Table 4, the calculation of the percent changes in OCTA VD was not possible in all the studies cited due to the limited available raw data, thus highlighting the need for well-designed, larger prospective controlled studies over time.
Author Contributions
Conceptualization, R.L.K., L.C., G.G., B.S. and A.H.; methodology, R.L.K.; formal analysis, R.L.K.; investigation, R.L.K.; data curation, R.L.K., L.C., G.G., B.S. and A.H.; writing—original draft preparation, R.L.K. and L.C.; writing—review and editing, R.L.K., L.C., A.V.V., F.O., C.C., M.Z., G.A., G.G., B.S., J.T.K. and A.H.; visualization, R.L.K.; supervision, G.G., B.S. and A.H. G.G. and B.S. share the last author position for this work. All authors have read and agreed to the published version of the manuscript.
Funding
Alon Harris is supported by NIH grants (NIH R01EY030851 and NIH R01EY034718) and NYEE Foundation grants, as well as in part by a Departmental Challenge Grant award from Research to Prevent Blindness, NY. Giovanna Guidoboni is supported by NIH R01EY034718 and NSF-DMS 2108711/2327640. The sponsors/funders play did not play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Alon Harris would like to disclose that he received remuneration from AdOM, Qlaris, and Cipla for serving as a consultant, and he serves on the board of AdOM and Qlaris. Professor Alon Harris holds an ownership interest in AdOM, Oxymap, Qlaris, and SlitLed. All relationships listed above are pursuant to Icahn School of Medicine’s policy on outside activities. Giovanna Guidoboni would like to disclose that she received remuneration from Qlaris, Foresite Healthcare LLC for serving as a consultant, and she is owner of Gspace LLC. The contribution of the authors Francesco Oddone and Carmela Carnevale was supported by Fondazione Roma and by the Italian Ministry of Health. None of the other authors listed have any financial disclosures.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AVR | Arteriovenous Ratio |
| CAD | Coronary Artery Disease |
| CHD | Coronary Heart Disease |
| CHF | Congestive Heart Failure |
| CRAE | Central Retinal Artery Equivalent |
| CRVE | Central Retinal Vein Equivalent |
| CTO | Coronary Total Occlusion |
| CVD | Cardiovascular Disease |
| DCP | Deep Capillary/Vascular Plexus |
| DRL | Deep Retinal Layer |
| DVP | Deep Vascular Plexus |
| FAZ | Foveal Avascular Zone |
| HF | Heart Failure |
| HTN | Hypertension |
| IS | Ischemic Stroke |
| LAA | Large Artery Atherosclerosis |
| LVEF | Left Ventricular Ejection Fraction |
| MI | Myocardial Infarction |
| NYHA | New York Heart Association |
| OCTA | Optical Coherence Tomography Angiography |
| SAD | Small Artery Disease |
| SCP | Superficial Capillary Plexus |
| SRL | Superficial Retinal Layer |
| SVP | Superficial Vascular Plexus |
| VD | Vascular Density |
References
- World Heath Organization. Cardiovascular Diseases (CVDs). 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 1 July 2023).
- Leung, H.; Wang, J.J.; Rochtchina, E.; Wong, T.Y.; Klein, R.; Mitchell, P. Impact of current and past blood pressure on retinal arteriolar diameter in an older population. J. Hypertens. 2004, 22, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.; Wang, J.J.; Rochtchina, E.; Tan, A.G.; Wong, T.Y.; Klein, R.; Hubbard, L.D.; Mitchell, P. Relationships between age, blood pressure and retinal vessel diameters in an older population. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2900–2904. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, J.J.; Mackey, D.A.; Wong, T.Y. Retinal vascular caliber: Systemic, environmental, and genetic associations. Surv. Ophthalmol. 2009, 54, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Kawasaki, R.; Wang, J.J.; Wong, T.Y.; Mitchell, P.; Daimon, M.; Oizumi, T.; Kato, T.; Kawata, S.; Kayama, T. Retinal arteriolar narrowing predicts 5-year risk of hypertension in Japanese people: The Funagata study. Microcirculation 2010, 17, 94–102. [Google Scholar] [CrossRef]
- Jumar, A.; Harazny, J.M.; Ott, C.; Friedrich, S.; Kistner, I.; Striepe, K.; Schmieder, R.E. Retinal capillary rarefaction in patients with type 2 diabetes mellitus. PLoS ONE 2016, 11, e0162608. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.; Wang, J.J.; Wong, T.Y.; Rochtchina, E.; Klein, R.; Leeder, S.R.; Mitchell, P. Retinal arteriolar narrowing is associated with 5-year incident severe hypertension: The Blue Mountains Eye Study. Hypertension 2004, 44, 442–447. [Google Scholar] [CrossRef]
- Peng, X.Y.; Wang, F.H.; Liang, Y.B.; Wang, J.J.; Sun, L.P.; Peng, Y.; Friedman, D.S.; Liew, G.; Wang, N.L.; Wong, T.Y. Retinopathy in persons without diabetes: The Handan Eye Study. Ophthalmology 2010, 117, 531–537. [Google Scholar] [CrossRef]
- Ding, J.; Wai, K.L.; McGeechan, K.; Ikram, M.K.; Kawasaki, R.; Xie, J.; Klein, R.; Klein, B.B.; Cotch, M.F.; Wang, J.J.; et al. Retinal vascular caliber and the development of hypertension: A meta-analysis of individual participant data. J. Hypertens. 2014, 32, 207–215. [Google Scholar] [CrossRef]
- Chen, H.S.; Liu, C.H.; Wu, W.C.; Tseng, H.J.; Lee, Y.S. Optical coherence tomography angiography of the superficial microvasculature in the macular and peripapillary areas in glaucomatous and healthy eyes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3637–3645. [Google Scholar] [CrossRef]
- Ang, M.; Tan, A.C.S.; Cheung, C.M.G. Optical coherence tomography angiography: A review of current and future clinical applications. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 256, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.S.; Tan, G.S.; Denniston, A.K.; Keane, P.A.; Ang, M.; Milea, D.; Chakravarthy, U.; Cheung, C.M.G. An overview of the clinical applications of optical coherence tomography angiography. Eye 2018, 32, 262–286. [Google Scholar] [CrossRef]
- Lee, W.H.; Park, J.H.; Won, Y.; Lee, M.W.; Shin, Y.I.; Jo, Y.J.; Kim, J.Y. Retinal microvascular change in hypertension as measured by optical coherence tomography angiography. Sci. Rep. 2019, 9, 156. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Shoji, T.; Kanno, J.; Ibuki, H.; Ozaki, K.; Ishii, H.; Ichikawa, Y.; Kimura, I.; Shinoda, K. Evaluation of microvascular changes in the macular area of eyes with rhegmatogenous retinal detachment without macular involvement using swept-source optical coherence tomography angiography. Clin. Ophthalmol. 2018, 12, 2059–2067. [Google Scholar] [CrossRef]
- Wong, T.; Mitchell, P. Hypertensive Retinopathy. N. Engl. J. Med. 2004, 351, 2310–2317. [Google Scholar] [CrossRef]
- Wolf, S.; Arend, O.; Schulte, K.; Ittel, T.H.; Reim, M. Quantification of Retinal Capillary Density and Flow Velocity in Patients with Essential Hypertension. Hypertension 1994, 4, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Xu, Y.; Zeng, X.; Yang, N.; Jiang, M.; Zhang, X.; Yang, J.; He, T.; Xing, Y. Use of optical coherence tomography angiography for assessment of microvascular changes in the macula and optic nerve head in hypertensive patients without hypertensive retinopathy. Microvasc. Res. 2020, 129, 103969. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Garg, I.; Bannai, D.; Kasetty, M.; Katz, R.; Park, J.Y.; Lizano, P.; Miller, J.B. Retinal Microvasculature and vasoreactivity changes in hypertension using optical coherence tomography-angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 3503–3515. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Hu, Y.; Huang, M.; Wu, Y.; Zhong, P.; Dong, X.; Wu, Q.; Liu, B.; Li, C.; Xie, J.; et al. Retinal neurovascular impairment in patients with essential hypertension: An optical coherence tomography angiography study. Investig. Ophthalmol. Vis. Sci. 2020, 61, 42. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Chin, C.W.L.; Hong, J.; Chee, M.L.; Le, T.T.; Ting, D.S.W.; Wong, T.Y.; Schmetterer, L. Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. J. Hypertens. 2019, 37, 572–580. [Google Scholar] [CrossRef]
- Donati, S.; Maresca, A.M.; Cattaneo, J.; Grossi, A.; Mazzola, M.; Caprani, S.M.; Premoli, L.; Docchio, F.; Rizzoni, D.; Guasti, L.; et al. Optical coherence tomography angiography and arterial hypertension: A role in identifying subclinical microvascular damage? Eur. J. Ophthalmol. 2021, 31, 158–165. [Google Scholar] [CrossRef]
- Remolí Sargues, L.; Monferrer Adsuara, C.; Castro Navarro, V.; Navarro Palop, C.; Montero Hernández, J.; Cervera Taulet, E. Swept-source optical coherence tomography angiography automatic analysis of microvascular changes secondary to systemic hypertension. Eur. J. Ophthalmol. 2022, 33, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Sun, H.; Huang, X.; Qu, Y. Retinal microvascular metrics in untreated essential hypertensives using optical coherence tomography angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Ladores, C.; Hong, J.; Nguyen, D.Q.; Chua, J.; Ting, D.; Schmetterer, L.; Wong, T.Y.; Cheng, C.Y.; Tan, A.C.S. Systemic hypertension associated retinal microvascular changes can be detected with optical coherence tomography angiography. Sci. Rep. 2020, 10, 9580. [Google Scholar] [CrossRef]
- Pascual-Prieto, J.; Burgos-Blasco, B.; Ávila Sánchez-Torija, M.; Fernández-Vigo, J.I.; Arriola-Villalobos, P.; Barbero Pedraz, M.A.; García-Feijoo, J.; Martínez-de-la-Casa, J.M. Utility of optical coherence tomography angiography in detecting vascular retinal damage caused by arterial hypertension. Eur. J. Ophthalmol. 2020, 30, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Terheyden, J.H.; Wintergerst, M.W.M.; Pizarro, C.; Pfau, M.; Turski, G.N.; Holz, F.G.; Finger, R.P. Retinal and Choroidal Capillary Perfusion Are Reduced in Hypertensive Crisis Irrespective of Retinopathy. Transl. Vis Sci Technol. 2020, 9, 42. [Google Scholar] [CrossRef]
- Lim, H.B.; Lee, M.W.; Park, J.H.; Kim, K.; Jo, Y.J.; Kim, J.Y. Changes in ganglion cell-inner plexiform layer thickness and retinal microvasculature in hypertension: An optical coherence tomography angiography study. Am. J. Ophthalmol. 2019, 199, 167–176. [Google Scholar] [CrossRef]
- Shin, Y.I.; Nam, K.Y.; Lee, W.H.; Ryu, C.K.; Lim, H.B.; Jo, Y.J.; Kim, J.Y. Peripapillary microvascular changes in patients with systemic hypertension: An optical coherence tomography angiography study. Sci. Rep. 2020, 10, 6541. [Google Scholar] [CrossRef]
- Takayama, K.; Kaneko, H.; Ito, Y.; Kataoka, K.; Iwase, T.; Yasuma, T.; Matsuura, T.; Tsunekawa, T.; Shimizu, H.; Suzumura, A.; et al. Novel classification of early-stage systemic hypertensive changes in human retina based on OCTA measurement of choriocapillaris. Sci. Rep. 2018, 8, 15163. [Google Scholar] [CrossRef]
- Chua, J.; Le, T.T.; Sim, Y.C.; Chye, H.Y.; Tan, B.; Yao, X.; Wong, D.; Ang, B.W.Y.; Toh, D.F.; Lim, H.; et al. Relationship of quantitative retinal capillary network and myocardial remodeling in systemic hypertension. J. Am. Heart. Assoc. 2022, 11, e024226. [Google Scholar] [CrossRef]
- Tabatabaee, A.; Asharin, M.R.; Dehghan, M.H.; Pourbehi, M.R.; Nasiri-Ahmadabadi, M.; Assadi, M. Retinal vessel abnormalities predict coronary artery diseases. Perfusion 2013, 28, 232–237. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Sharrett, A.R.; Duncan, B.B.; Couper, D.J.; Tielsch, J.M.; Klein, B.E.; Hubbard, L.D. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA 2002, 287, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, J.; Zhang, Y.; Qian, Y.W.; Zhang, J.F.; Wang, Z.L. Retinal and choroidal vascular changes in coronary heart disease: An optical coherence tomography angiography study. Biomed. Opt. Express 2019, 10, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Aschauer, J.; Aschauer, S.; Pollreisz, A.; Datlinger, F.; Gatterer, C.; Mylonas, G.; Egner, B.; Hofer, D.; Steiner, I.; Hengstenberg, C.; et al. Identification of subclinical microvascular biomarkers in coronary heart disease in retinal imaging. Transl. Vis. Sci. Technol. 2021, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Matulevičiūtė, I.; Sidaraitė, A.; Tatarūnas, V.; Veikutienė, A.; Dobilienė, O.; Žaliūnienė, D. Retinal and Choroidal Thinning-A Predictor of Coronary Artery Occlusion? Diagnostics 2022, 12, 2016. [Google Scholar] [CrossRef] [PubMed]
- Sideri, A.M.; Kanakis, M.; Katsimpris, A.; Karamaounas, A.; Brouzas, D.; Petrou, P.; Papakonstaninou, E.; Droutsas, K.; Kandarakis, S.; Giannopoulos, G.; et al. Correlation between coronary and retinal microangiopathy in patients with STEMI. Transl. Vis. Sci. Technol. 2023, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Arnould, L.; Guenancia, C.; Azemar, A.; Alan, G.; Pitois, S.; Bichat, F.; Zeller, M.; Gabrielle, P.H.; Bron, A.M.; Creuzot-Garcher, C.; et al. The EYE-MI Pilot Study: A Prospective Acute Coronary Syndrome Cohort Evaluated With Retinal Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4299–4306. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Hu, Y.; Jiang, L.; Peng, Q.; Huang, M.; Li, C.; Kuang, Y.; Tan, N.; Yu, H.; Yang, X. Retinal microvasculature changes in patients with coronary total occlusion on optical coherence tomography angiography. Front. Med. 2021, 8, 708491. [Google Scholar] [CrossRef]
- Ren, Y.; Hu, Y.; Li, C.; Zhong, P.; Liu, H.; Wang, H.; Kuang, Y.; Fu, B.; Wang, Y.; Zhao, H.; et al. Impaired retinal microcirculation in patients with non-obstructive coronary artery disease. Microvasc. Res. 2023, 148, 104533. [Google Scholar] [CrossRef]
- Arnould, L.; Guenancia, C.; Gabrielle, P.H.; Pitois, S.; Baudin, F.; Pommier, T.; Zeller, M.; Bron, A.M.; Creuzot-Garcher, C.; Cottin, Y. Influence of cardiac hemodynamic variables on retinal vessel density measurement on optical coherence tomography angiography in patients with myocardial infarction. J. Fr. Ophtalmol. 2020, 43, 216–221. [Google Scholar] [CrossRef]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef]
- Zafar, S.; McCormick, J.; Giancardo, L.; Saidha, S.; Abraham, A.; Channa, R. Retinal imaging for neurological diseases: “A window into the brain”. Int. Ophthalmol. Clin. 2019, 59, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Yang, X.; Jiang, B.; Li, H.; Wang, Y. Multimodal Retinal Imaging for Detection of Ischemic Stroke. Front. Aging Neurosci. 2021, 13, 615813. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Klein, R.; Couper, D.J.; Cooper, L.S.; Shahar, E.; Hubbard, L.D.; Wofford, M.R.; Sharrett, A.R. Retinal microvascular abnormalities and incident stroke: The atherosclerosis risk in communities study. Lancet 2001, 358, 1134–1140. [Google Scholar] [CrossRef]
- Mitchell, P.; Wang, J.J.; Wong, T.Y.; Smith, W.; Klein, R.; Leeder, S.R. Retinal microvascular signs and risk of stroke and stroke mortality. Neurology 2005, 65, 1005–1009. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Bravo, P.E.; Gupta, A.; Farhad, H.; Klein, B.E.; Klein, R.; Di Carli, M.; Solomon, S.D. Retinal Vessel Calibers in Predicting Long-Term Cardiovascular Outcomes: The Atherosclerosis Risk in Communities Study. Circulation 2016, 134, 1328–1338. [Google Scholar] [CrossRef]
- McGrory, S.; Ballerini, L.; Doubal, F.N.; Staals, J.; Allerhand, M.; Valdes-Hernandez, M.D.C.; Wang, X.; MacGillivray, T.; Doney, A.S.F.; Dhillon, B.; et al. Retinal microvasculature and cerebral small vessel disease in the Lothian Birth Cohort 1936 and Mild Stroke Study. Sci. Rep. 2019, 9, 6320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jiang, B.; Li, H.; Yang, X.; Cheng, X.; Hong, H.; Wang, Y. Risk Stratification Tool for Ischemic Stroke: A Risk Assessment Model Based on Traditional Risk Factors Combined With White Matter Lesions and Retinal Vascular Caliber. Front. Neurol. 2021, 12, 696986. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, C.; Chen, Y.; Wang, W.; Huang, S.; Han, Z.; Lin, X.; Lu, F.; Shen, M. Retinal Structural and Microvascular Alterations in Different Acute Ischemic Stroke Subtypes. J. Ophthalmol. 2020, 2020, 8850309. [Google Scholar] [CrossRef]
- Duan, H.; Xie, J.; Zhou, Y.; Zhang, H.; Liu, Y.; Tang, C.; Zhao, Y.; Qi, H. Characterization of the Retinal Microvasculature and FAZ Changes in Ischemic Stroke and Its Different Types. Transl. Vis. Sci. Technol. 2022, 11, 21. [Google Scholar] [CrossRef]
- Cao, Y.; Yan, J.; Zhan, Z.; Liang, Y.; Han, Z. Macula structure and microvascular changes in recent small subcortical infarct patients. Front. Neurol. 2021, 11, 615252. [Google Scholar] [CrossRef]
- Ye, C.; Kwapong, W.R.; Tao, W.; Lu, K.; Pan, R.; Wang, A.; Liu, J.; Liu, M.; Wu, B. Characterization of macular structural and microvascular changes in thalamic infarction patients: A swept-source optical coherence tomography-angiography study. Brain Sci. 2022, 12, 518. [Google Scholar] [CrossRef]
- Molero-Senosiain, M.; Vidal-Villegas, B.; Pascual-Prieto, J.; Valor-Suarez, C.; Saenz-Frances, F.; Santos-Bueso, E. Correlation between retrograde trans-synaptic degeneration of ganglion cells and optical coherence tomography angiography following ischemic stroke. Cureus 2021, 13, e19788. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, B.; Xiao, Y.; Zeng, X.; Wu, G.; Du, Z.; Fang, Y.; Hu, Y.; Yang, X.; Yu, H. Retinal neurovascular changes in patients with ischemic stroke investigated by optical coherence tomography angiography. Front. Aging Neurosci. 2022, 14, 834560. [Google Scholar] [CrossRef]
- Lu, K.; Kwapong, W.R.; Jiang, S.; Zhang, X.; Xie, J.; Ye, C.; Yan, Y.; Cao, L.; Zhao, Y.; Wu, B. Differences in retinal microvasculature between large artery atherosclerosis and small artery disease: An optical coherence tomography angiography study. Front. Aging Neurosci. 2022, 14, 1053638. [Google Scholar] [CrossRef]
- Jessup, M.; Brozena, S. Medical progress: Heart failure. N. Engl. J. Med. 2003, 348, 2007–2018. [Google Scholar] [CrossRef]
- Gavin, J.B.; Maxwell, L.; Edgar, S.G. Microvascular involvement in cardiac pathology. J. Mol. Cell Cardiol. 1998, 30, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.P.; Mak, S.; Stewart, D.J. Potential role of the microvasculature in progression of heart failure. Am. J. Cardiol. 1999, 84, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P.; Flicker, L. The mind of a failing heart: A systematic review of the association between congestive heart failure and cognitive functioning. Intern Med. J. 2001, 31, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Zelis, R.; Sinoway, L.I.; Musch, T.I.; Davis, D.; Just, H. Regional blood flow in congestive heart failure:concept of compensatory mechanisms with short and long time constants. Am. J. Cardiol. 1988, 62, 2–8. [Google Scholar] [CrossRef]
- Saha, M.; Muppala, M.R.; Castaldo, J.E.; Gee, W.; Reed, J.F., 3rd; Morris, D.L. The impact of cardiac index on cerebral hemodynamics. Stroke 1993, 24, 1686–1690. [Google Scholar] [CrossRef]
- Choi, B.R.; Kim, J.S.; Yang, Y.J.; Park, K.M.; Lee, C.W.; Kim, Y.H.; Hong, M.K.; Song, J.K.; Park, S.W.; Park, S.J.; et al. Factors associated with decreased cerebral blood flow in congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2006, 97, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Meira-Freitas, D.; Melo, L.A., Jr.; Almeida-Freitas, D.B.; Paranhos, A., Jr. Glaucomatous optic nerve head alterations in patients with chronic heart failure. Clin. Ophthalmol. 2012, 6, 623. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Altinkaynak, H.; Kara, N.; Sayın, N.; Güneş, H.; Avşar, S.; Yazıcı, A.T. Subfoveal choroidal thickness in patients with chronic heart failure analyzed by spectral-domain optical coherence tomography. Curr. Eye Res. 2014, 39, 1123–1128. [Google Scholar] [CrossRef]
- Topaloglu, C.; Bekmez, S. Retinal vascular density change in patients with heart failure. Photodiagnosis Photodyn Ther. 2023, 42, 103621. [Google Scholar] [CrossRef]
- Alnawaiseh, M.; Eckardt, F.; Mihailovic, N.; Frommeyer, G.; Diener, R.; Rosenberger, F.; Eckardt, L.; Eter, N.; Lahme, L.; Lange, P.S. Ocular perfusion in patients with reduced left ventricular ejection fraction measured by optical coherence tomography angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 3605–3611. [Google Scholar] [CrossRef]
- Rakusiewicz, K.; Kanigowska, K.; Hautz, W.; Ziółkowska, L. The Impact of Chronic Heart Failure on Retinal Vessel Density Assessed by Optical Coherence Tomography Angiography in Children with Dilated Cardiomyopathy. J. Clin. Med. 2021, 10, 2659. [Google Scholar] [CrossRef] [PubMed]
- Chaikijurajai, T.; Ehlers, J.P.; Tang, W.H. Retinal microvasculature: A potential window into heart failure prevention. JACC Heart Fail. 2022, 10, 785–791. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).