Maximum Pain at Rest in Pediatric Patients Undergoing Elective Thoracic Surgery and the Predictors of Moderate-to-Severe Pain—Secondary Data Analysis
Abstract
1. Introduction
2. Materials and Methods
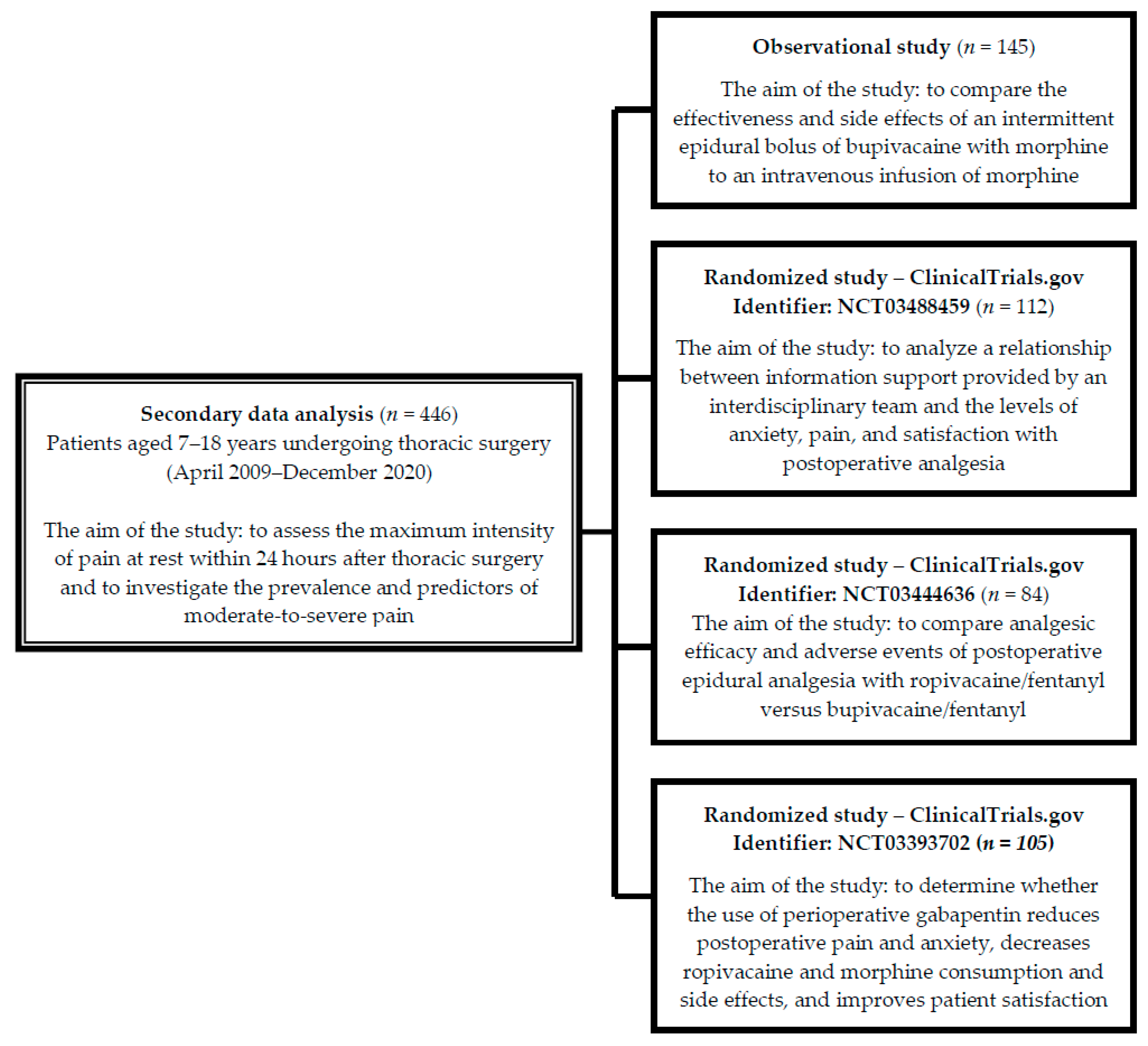
2.1. Study Design, Setting
2.2. Participants
2.3. Postoperative Multimodal Analgesia
2.4. Data Collection
2.5. Outcomes
2.6. Statistical Analysis
2.7. Ethics Consideration
3. Results
3.1. Patient Characteristics
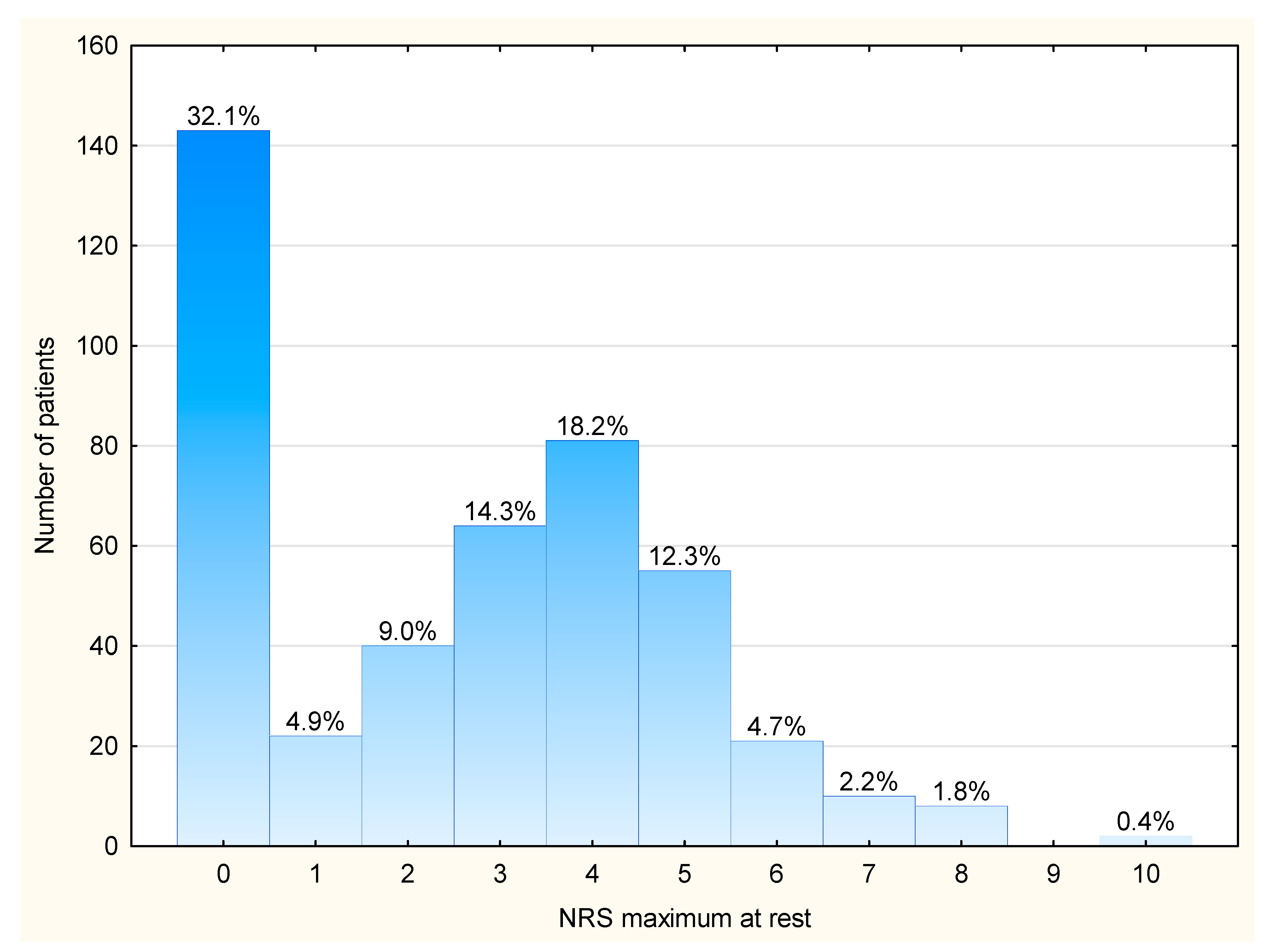
3.2. Maximum Pain Intensity at Rest and the Prevalence of Moderate-to-Severe Pain (NRS > 2/10)
3.2.1. Age
3.2.2. Gender
3.2.3. Type of Surgery
3.2.4. Route of Postoperative Analgesia
3.2.5. Type of Analgesia
- -
- Bupivacaine: 2 vs. 3 vs. 4, Kruskal–Wallis test: H (2, N = 190) = 109.47, p < 0.001; post hoc test results: p < 0.0001 for groups 2 vs. 4, and 3 vs. 4.
- -
- Fentanyl: 2 vs. 3 vs. 4 vs. 5, Kruskal–Wallis test: H (2, N = 197) = 22.45, p < 0.0001; post hoc test results: 3 vs. 4, p = 0.0016; 4 vs. 5, p < 0.0001.
- -
- Non-opioid: 1 vs. 2 vs. 3 vs. 4 vs. 5, Kruskal–Wallis test: H (4, N = 446) = 220.87, p < 0.001; post hoc test results: p < 0.0001 for groups 1 vs. 2, 1 vs. 3, 1 vs. 4, 1 vs. 5, 2 vs. 3, 2 vs. 5, 3 vs. 4, and 4 vs. 5.
- -
- Itching, p = 0.0153.
- -
- Oxygen saturation < 94%, p < 0.00001.
3.3. Predictors of Moderate-to-Severe Pain (Maximum NRS > 2/10)
4. Discussion
4.1. Postoperative Pain and Route of Analgesia
4.2. Postoperative Pain and Type of Analgesia
4.3. Postoperative Pain and Age
4.4. Strengths of the Current Study
4.5. Limitations of the Current Study
5. Conclusions
Implications for Clinical Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Semmelmann, A.; Kaltofen, H.; Loop, T. Anesthesia of thoracic surgery in children. Pediatr. Anesth. 2018, 28, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Gebreselassie, H.A.; Tadesse, M.M.; Woldeselassie, H.G. Thoracotomy in Children: Review from a Low-Income Country. Pediatr. Health Med. Ther. 2023, 14, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, J.; Ligarski, D.; Polewczyk, T. Long-term results after the modified Ravitch procedure performed in children and adolescents—A one-time procedure without the need to use additional support of the sternum. A retrospective study. Kardiochirurgia I Torakochirurgia Pol. Pol. J. Cardio-Thorac. Surg. 2020, 17, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.H.; Peter, S.D.S.; Fallat, M.E.; Saito, J.M.; Burns, C.R.; Deans, K.J.; Fraser, J.D.; Gadepalli, S.K.; Helmrath, M.A.; Hirschl, R.B.; et al. Midwest Pediatric Surgery Consortium. Thoracoscopic versus open lobectomy in infants with congenital lung malformations: A multi-institutional propensity score analysis. J. Pediatr. Surg. 2021, 56, 2148–2156. [Google Scholar] [CrossRef]
- Mao, Y.Z.; Tan, S.; Li, S. Comparison of the Nuss versus Ravitch procedure for pectus excavatum repair: An updated meta-analysis. J. Pediatr. Surg. 2017, 52, 1545–1552. [Google Scholar] [CrossRef]
- Gad-Allah, A.; Abdelfattah, K.; Badawy, F.; Abd El-Rahman, A.; Mohamed, S. Post-Thoracotomy Pain: Review Article. Egypt. J. Hosp. Med. 2021, 85, 3519–3523. [Google Scholar] [CrossRef]
- Schnabel, A.; Thyssen, N.M.; Goeters, C.; Zheng, H.; Zahn, P.K.; Van Aken, H.; Pogatzki-Zahn, E.M. Age- and procedure-specific differences of epidural analgesia in children—A database analysis. Pain Med. 2015, 16, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Batoz, H.; Semjen, F.; Bordes-Demolis, M.; Bénard, A.; Nouette-Gaulain, K. Chronic postsurgical pain in children: Prevalence and risk factors. A prospective observational study. Br. J. Anaesth. 2016, 117, 489–496. [Google Scholar] [CrossRef]
- Cettler, M.; Zielińska, M.; Rosada-Kurasińska, J.; Kubica-Cielińska, A.; Jarosz, K.; Bartkowska-Śniatkowska, A. Guidelines for treatment of acute pain in children—The consensus statement of the Section of Paediatric Anaesthesiology and Intensive Therapy of the Polish Society of Anaesthesiology and Intensive Therapy. Anaesthesiol. Intensive Ther. 2022, 54, 197–218. [Google Scholar] [CrossRef]
- Friedrichsdorf, S.J.; Goubert, L. Pediatric pain treatment and prevention for hospitalized children. Pain Rep. 2019, 5, e804. [Google Scholar] [CrossRef]
- Heo, M.H.; Kim, J.Y.; Kim, J.H.; Kim, K.W.; Lee, S.I.; Kim, K.T.; Park, J.S.; Choe, W.J.; Kim, J.H. Epidural analgesia versus intravenous analgesia after minimally invasive repair of pectus excavatum in pediatric patients: A systematic review and meta-analysis. Korean J. Anesthesiol. 2021, 74, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, G.; Vittori, A.; Ferrari, F.; Francia, E.; Mascilini, I.; Petrucci, E.; Piga, S.; Pardi, V.; Cascella, M.; Contini, G.; et al. Incidence of Acute and Chronic Post-Thoracotomy Pain in Pediatric Patients. Children 2021, 8, 642. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, F.; Templeton, T.V.; Morris, B.; Valenza, F. Pediatric thoracic anesthesia: Airway management for lung isolation and postoperative analgesia. Pediatr. Med. 2019, 2, 23. [Google Scholar] [CrossRef]
- Le, S.; Lo, C.; Costandi, A.; Kim, E. Bilateral erector spinae plane (ESP) catheters for Ravitch procedure in a pediatric patient with Harrington rods. J. Clin. Anesth. 2020, 66, 109925. [Google Scholar] [CrossRef] [PubMed]
- Lucente, M.; Ragonesi, G.; Sanguigni, M.; Sbaraglia, F.; Vergari, A.; Lamacchia, R.; Del Prete, D.; Rossi, M. Erector spinae plane block in children: A narrative review. Korean J. Anesthesiol. 2022, 75, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Fenikowski, D.; Tomaszek, L.; Mazurek, H.; Gawron, D.; Maciejewski, P. The Effects of Gabapentin on Post-Operative Pain and Anxiety, Morphine Consumption and Patient Satisfaction in Paediatric Patients Following the Ravitch Procedure-A Randomised, Double-Blind, Placebo-Controlled, Phase 4 Trial. J. Clin. Med. 2022, 11, 4695. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.A.; Sommerfield, D.; Drake-Brockman, T.F.E.; Lagrange, C.; Ramgolam, A.; von Ungern-Sternberg, B.S. A prospective audit of pain profiles following general and urological surgery in children. Paediatr. Anaesth. 2017, 27, 1155–1164. [Google Scholar] [CrossRef]
- Tiruneh, A.; Tamire, T.; Kibret, S. The magnitude and associated factors of post-operative pain at Debre Tabor compressive specialized hospital, Debre Tabor Ethiopia, 2018. SAGE Open Med. 2021, 9, 20503121211014730. [Google Scholar] [CrossRef]
- Belachew, D.W.; Mekdelawit, M.T. Practice of Postoperative Pain Management in Under-Five Children in A Tertiary Hospital: A Prospective Crossectional Study. Ethiop. J. Health Sci. 2022, 32, 1117. [Google Scholar]
- Mekonnen, Z.A.; Melesse, D.Y.; Kassahun, H.G.; Flatie, T.D.; Workie MMChekol, W.B. Prevalence and contributing factors associated with postoperative pain in pediatric patients: A cross-sectional follow-up study. Perioper. Care Oper. Room Manag. 2021, 23, 100159. [Google Scholar] [CrossRef]
- Karišik, M.; Gligorović Barhanović, N.; Vulović, T.; Simić, D. Postoperative pain and stress response: Does child’s gender have an influence? Acta Clin. Croat. 2019, 58, 274–280. [Google Scholar] [CrossRef]
- Rawal, N. Epidural analgesia for postoperative pain: Improving outcomes or adding risks? Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 53–65. [Google Scholar] [CrossRef]
- Tomaszek, L.; Tomalak, W.; Gajdosz, R.; Buchwald, J. Intermittent thoracic epidural administration of bupivacaine-morphine versus intravenous infusion of morphine after thoracic surgery in children and adolescents. Anestezjol. I Ratow. 2015, 9, 260–268. [Google Scholar]
- Tomaszek, L.; Fenikowski, D.; Komotajtys, H.; Gawron, D. Ropivacaine/Fentanyl vs. Bupivacaine/Fentanyl for Pain Control in Children after Thoracic Surgery: A Randomized Study. Pain Manag. Nurs. Off. J. Am. Soc. Pain Manag. Nurses 2019, 20, 390–397. [Google Scholar] [CrossRef]
- Tomaszek, L.; Fenikowski, D.; Maciejewski, P.; Komotajtys, H.; Gawron, D. Perioperative Gabapentin in Pediatric Thoracic Surgery Patients-Randomized, Placebo-Controlled, Phase 4 Trial. Pain Med. 2020, 21, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Tomaszek, L. Evaluation of the Efficacy and Safety of Thoracic Epidural Analgesia in Children after Thoracic Surgery. Ph.D. Thesis, Jagiellonian University Medical College, Krakow, Poland, 2012. [Google Scholar]
- Birnie, K.A.; Hundert, A.S.; Lalloo, C.; Nguyen, C.; Stinson, J.N. Recommendations for selection of self-report pain intensity measures in children and adolescents: A systematic review and quality assessment of measurement properties. Pain 2019, 160, 5–18. [Google Scholar] [CrossRef]
- Lenhard, W.; Lenhard, A. Computation of Effect Sizes. Available online: https://www.psychometrica.de/effect_size.html (accessed on 14 August 2022).
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Mansfield, S.A.; Woodroof, J.; Murphy, A.J.; Davidoff, A.M.; Morgan, K.J. Does epidural analgesia really enhance recovery in pediatric surgery patients? Pediatr. Surg. Int. 2021, 37, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Ocay, D.D.; Li, M.; Ingelmo, P.; Ouellet, J.A.; Pag’e, M.G.; Ferland, C.E. Predicting acute postoperative pain trajectories and long-term outcomes of adolescents after spinal fusion surgery. Pain Res. Manag. 2020, 20, 9874739. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, K.W.; Dalton, B.G.; Millspaugh, D.L.; Thomas, P.G.; Peter, S.D.S. Epidural versus patient-controlled analgesia after pediatric thoracotomy for malignancy: A preliminary review. Eur. J. Pediatr. Surg. 2016, 26, 340–343. [Google Scholar]
- Grap, S.M.; Lehman, E.B.; Heasley, V.; Dalal, P.G.; Parekh, U.R. Addition of Local Anesthetic Epidural Infusion Catheter to Intravenous Opioid Analgesia for Postoperative Pain Control in Children Undergoing Video Assisted Thoracoscopic Surgery (VATS). J. Perianesthesia Nurs. Off. J. Am. Soc. PeriAnesthesia Nurses 2022, 37, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Sujka, J.A.; Dekonenko, C.; Millspaugh, D.L.; Doyle, N.M.; Walker, B.J.; Leys, C.M.; Ostlie, D.J.; Aguayo, P.; Fraser, J.D.; Alemayehu, H.; et al. Epidural versus PCA Pain Management after Pectus Excavatum Repair: A Multi-Institutional Prospective Randomized Trial. Eur. J. Pediatr. Surg. Off. J. Austrian Assoc. Pediatr. Surgery. Z. Fur Kinderchir. 2020, 30, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Cassady, J.F., Jr.; Lederhaas, G.; Cancel, D.D.; Cummings, R.J.; Loveless, E.A. A randomized comparison of the effects of continuous thoracic epidural analgesia and intravenous patient-controlled analgesia after posterior spinal fusion in adolescents. Reg. Anesth. Pain Med. 2000, 25, 246–253. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, J.F., Jr.; Cywinski, J.B.; Tetzlaff, J.E.; Xu, M.; Gurd, A.R.; Andrish, J.T. The effect of epidural vs intravenous analgesia for posterior spinal fusion surgery. Paediatr. Anaesth. 2004, 14, 1009–1015. [Google Scholar] [CrossRef]
- Winnie, L.; Kao, Y.H.; Liao, C.C.; Tamura, T.; Chang, M.L.; Hsieh, K.Y. Comparative Analgesic Efficacies of Ropivacaine and Bupivacaine for Postoperative Rectus Sheath Block in Paediatric Abdominal Surgery: A Meta-Analysis of Randomized Controlled Trial and Retrospective Cohort Studies. Pain Res. Manag. 2021, 2021, 5535730. [Google Scholar] [CrossRef]
- Chow, C.H.T.; Yu, C.; Yu, W.; Yeung, K.; Schmidt, L.A.; Buckley, N. Risk and protective factors in predicting pediatric acute postsurgical pain: A systematic review and meta-analysis. Health Psychol. 2023, 42, 723–734. [Google Scholar] [CrossRef]

| Variables | n (%) | Me (Q25; Q 75) | M (SD) |
|---|---|---|---|
| Age (years) | 14 [12; 16] | 13.7 ± 2.6 | |
| 175 (39.2) | 2 [0; 4] | 2.5 ± 2.3 |
| 271 (60.8) | 3 [0; 4] | 2.7 ± 2.2 |
| Gender and maximum pain | |||
| 105 (23.5) | 3 [0; 5] | 3.0 ± 2.4 |
| 341 (76.4) | 3 [0; 4] | 2.5 ± 2.2 |
| Body weight (kg) | 52 [43; 59] | 51 ± 13 | |
| Body height (cm) | 168 [157; 176] | 165 ± 16 | |
| ASA | |||
| 392 (87.9) | ||
| 51 (11.4) | ||
| 3 (0.7) | ||
| Type of surgery and maximum pain | |||
| 116 (26.0) | 3 [0; 5] | 2.9 ± 2.4 |
| 330 (74.0) | 3 [0; 4] | 2.5 ± 2.2 |
| Chest drain and maximum pain | |||
| 405 (90.8) | ||
| 41 (9.2) | ||
| Duration of surgery (min) | 140 [117; 169] | 148 ± 48 | |
| Duration of anesthesia (min) | 190 [165; 220] | 198 ± 50 | |
| Route of analgesic administration and maximum pain | |||
| 174 (39) | 3 [0; 4] | 2.9 ± 2.3 |
| 272 (61) | 3 [0; 4] | 2.4 ± 2.2 |
| Type of postoperative multimodal analgesia and maximum pain | |||
| 174 (39.0) | 3 [0; 4] | 2.9 ± 2.3 |
| 75 (16.8) | 1 [0; 3] | 1.8 ± 1.8 |
| 54 (12.1) | 4 [0; 5] | 3.1 ± 2.6 |
| 61 (13.7) | 0 [0; 3] | 1.4 ± 1.9 |
| 82 (18.4) | 4 [2; 5] | 3.4 2.1 |
| Gabapentin | 54 (12.1) | ||
| Average pain intensity (for the whole cohort) | 0.4 [0; 0.8] | 0.7 ± 0.8 | |
| Maximum pain intensity (for the whole cohort) | 3 [0; 4] | 2.6 ± 2.3 | |
| Maximum NRS > 2/10 (for the whole cohort) | 241 (54.0) |
| Type of Analgesia | |||||
|---|---|---|---|---|---|
| Variables | Intravenous M (1) | 0.25% Bupivacaine + M (2) | 0.125% Bupivacaine + F (3) | 0.25% Bupivacaine + F (4) | 0.2% Ropivacaine + F (5) |
| n = 174 | n = 75 | n = 54 | n = 61 | n = 82 | |
| NRS > 2/10 (%) | 104 (59.8) | 27 (36.0) | 33 (61.1) | 17 (27.9) | 60 (73.2) |
| LA (mg/kg) | – | 2.2 [2.1; 2.4] | 2.4 [1.9; 2.9] | 6.1 [4.6; 7.2] | 3.8 [2.9; 4.5] |
| Opioid (µg/kg) | 725 [568; 921] | 89 [85; 96] | 9.7 [7.6; 11.1] | 12.3 [9.2; 13.8] | 9.4 [7.2; 11.3] |
| Non-opioid (number of doses) | 4 [2; 7] | 1 [1; 2] | 7 [7; 8] | 2 [1; 3] | 7.5 [7; 8] |
| Side effects (%) | |||||
| Nausea/vomiting | 61 (35.1) | 29 (38.7) | 25 (46.3) | 26 (42.6) | 36 (43.9) |
| Itching | 13 (7.5) | 13 (17.3) | 7 (13) | 10 (16.3) | 3 (3.7) |
| Urine retention | 7 (4.0) | 6 (8.0) | 1 (1.8) | 2 (3.3) | 0 (0.0) |
| Oxygen saturation < 94% | 60 (34.5) | 6 (8.0) | 32 (59.3) | 31 (50.8) | 34 (41.5) |
| Anisocoria/Horner | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 8 (9.7%) |
| Paresthesia | 0 (0%) | 6 (8.0) | 0 (0%) | 0 (0%) | 7 (8.5%) |
| Muscle trembling | 0 (0%) | 3 (4.0) | 0 (0%) | 0 (0%) | 0 (0%) |
| Variables | B | SE (B) | Wald Test | p Value | OR (Cl 95%) |
|---|---|---|---|---|---|
| Simple logistic regression | |||||
| Aged 14–18 reference category: Aged 7–13 | 0.20 | 0.10 | 4.21 | 0.0402 | 1.22 (1.01–1.48) |
| Male | −0.13 | 0.11 | 1.38 | 0.2393 | 0.87 (0.70–1.09) |
| Ravitch procedure reference category: Thoracotomy | −0.10 | 0.11 | 0.87 | 0.3500 | 0.90 (0.73–1.12) |
| Gabapentin reference category: No | 0.40 | 0.16 | 6.36 | 0.0117 | 1.49 (1.09–2.02) |
| Intravenous route reference category: Epidural | 0.19 | 0.10 | 3.76 | 0.0524 | 1.21 (0.998–1.47) |
| Type of analgesia reference category: Intravenous MF | |||||
| Epidural 0.25% bupivacaine + MF | −0.64 | 0.22 | 8.75 | 0.0031 | 0.53 (0.34–0.81) |
| Epidural 0.125% bupivacaine + FNT | 0.39 | 0.24 | 2.54 | 0.1107 | 1.47 (0.91–2.37) |
| Epidural 0.25% bupivacaine + FNT | −1.02 | 0.25 | 16.90 | 0.0000 | 0.36 (0.22–0.59) |
| Epidural 0.2% ropivacaine + FNT | 0.94 | 0.22 | 17.82 | 0.0000 | 2.55 (1.65–3.95) |
| Multiple logistic regression model | |||||
| Aged 14–18 reference category: Aged 7–13 | 0.27 | 0.10 | 6.64 | 0.010 | 1.31 (1.07–1.60) |
| Intravenous route reference category: Epidural | −2.40 | 0.56 | 18.35 | 0.000 | 0.09 (0.03–0.27) |
| Type of analgesia reference category: Intravenous MF | |||||
| Epidural 0.25% bupivacaine + MF | −1.60 | 0.35 | 20.95 | 0.000 | 0.20 (0.10–0.40) |
| Epidural 0.25% bupivacaine + FNT | −2.04 | 0.38 | 28.30 | 0.000 | 0.13 (0.06–0.27) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaszek, L.; Fenikowski, D.; Cież-Piekarczyk, N.; Mędrzycka-Dąbrowska, W. Maximum Pain at Rest in Pediatric Patients Undergoing Elective Thoracic Surgery and the Predictors of Moderate-to-Severe Pain—Secondary Data Analysis. J. Clin. Med. 2024, 13, 844. https://doi.org/10.3390/jcm13030844
Tomaszek L, Fenikowski D, Cież-Piekarczyk N, Mędrzycka-Dąbrowska W. Maximum Pain at Rest in Pediatric Patients Undergoing Elective Thoracic Surgery and the Predictors of Moderate-to-Severe Pain—Secondary Data Analysis. Journal of Clinical Medicine. 2024; 13(3):844. https://doi.org/10.3390/jcm13030844
Chicago/Turabian StyleTomaszek, Lucyna, Dariusz Fenikowski, Nina Cież-Piekarczyk, and Wioletta Mędrzycka-Dąbrowska. 2024. "Maximum Pain at Rest in Pediatric Patients Undergoing Elective Thoracic Surgery and the Predictors of Moderate-to-Severe Pain—Secondary Data Analysis" Journal of Clinical Medicine 13, no. 3: 844. https://doi.org/10.3390/jcm13030844
APA StyleTomaszek, L., Fenikowski, D., Cież-Piekarczyk, N., & Mędrzycka-Dąbrowska, W. (2024). Maximum Pain at Rest in Pediatric Patients Undergoing Elective Thoracic Surgery and the Predictors of Moderate-to-Severe Pain—Secondary Data Analysis. Journal of Clinical Medicine, 13(3), 844. https://doi.org/10.3390/jcm13030844








