Application of Nuclear Medicine Techniques in Musculoskeletal Infection: Current Trends and Future Prospects
Abstract
:1. Introduction
2. Fundamental Issues and Challenges Related to Conventional NM Techniques in Bone Infections
2.1. Bone Scintigraphy with Technetium-99m
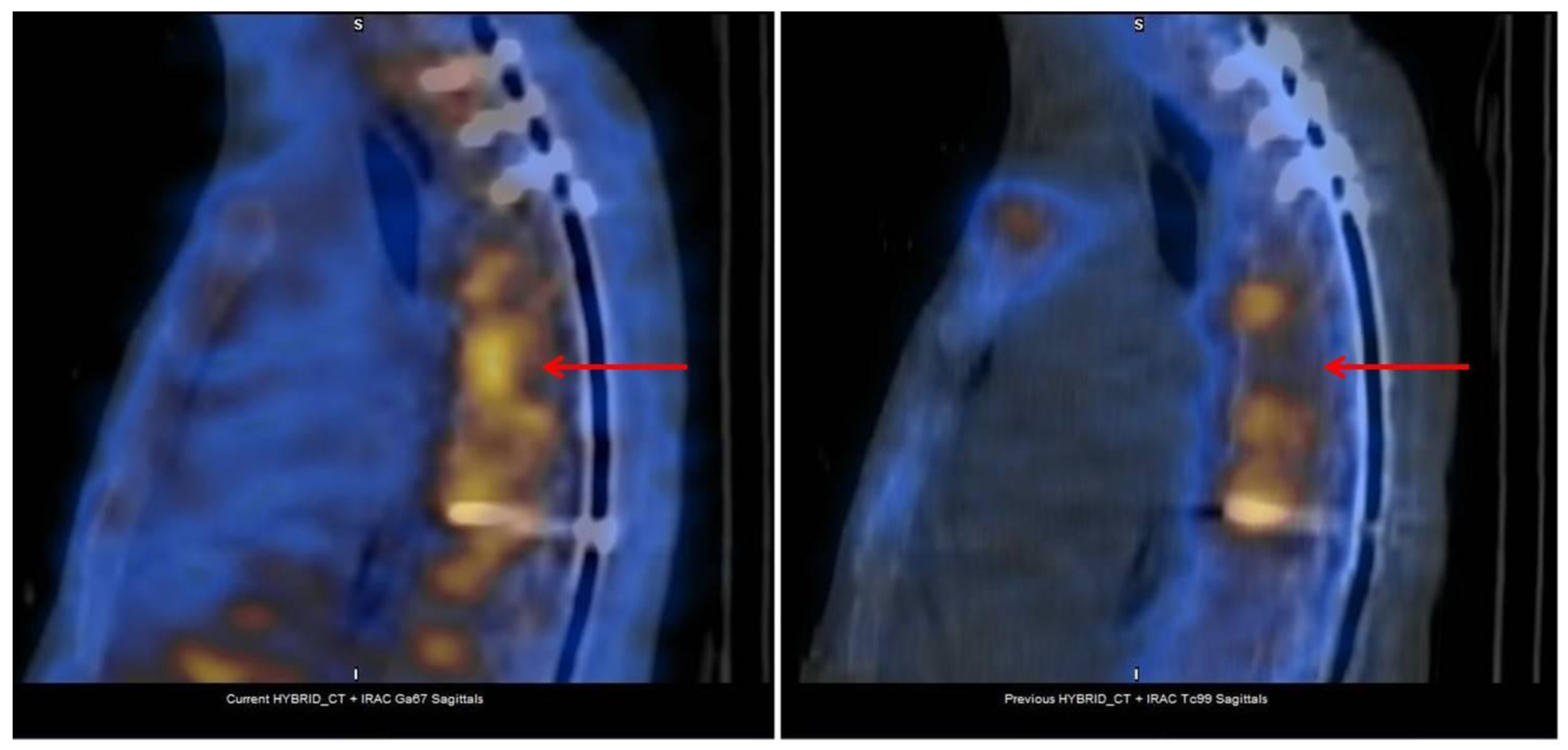
2.2. Bone Scintigraphy with Gallium Citrate (Ga-67)
2.3. In Vitro and In Vivo Labeled Leukocyte Bone Scintigraphy
3. The Introduction of Hybrid NM Techniques in Bone Infections
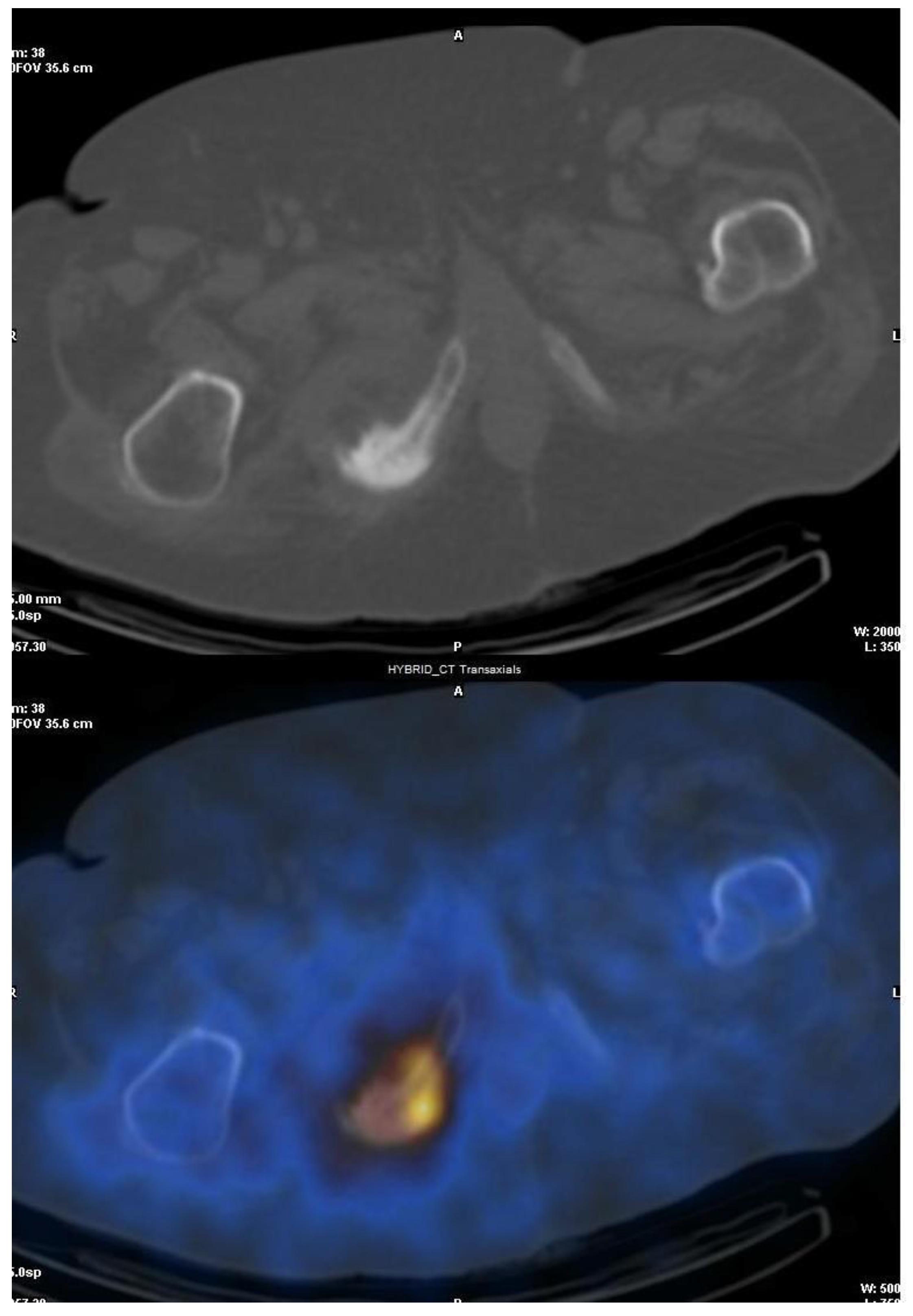
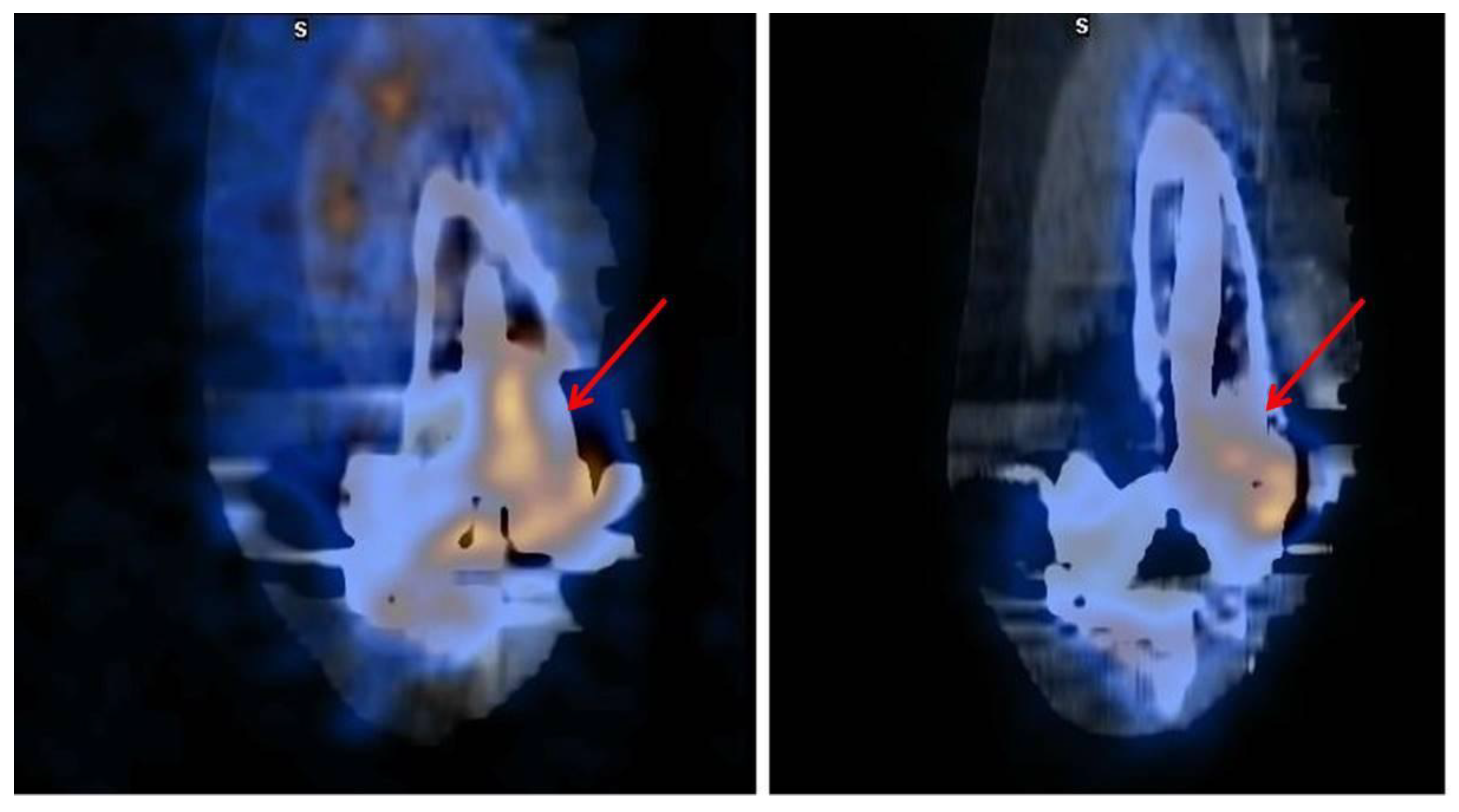
3.1. SPECT/CT plus 99mTc, 67Ga or Labeled Leucocyte Scintigraphy
3.2. Fluorine-18-Fluorodeoxyglucose (18F-FDG) with Positron Emission Tomography (PET/CT)
3.3. Fluorine-18-Fluorodeoxyglucose (18F-FDG) PET/MRI
3.4. Fluorine-18-FDG-Labeled White Blood Cells (18F-FDG-WBC) PET/CT
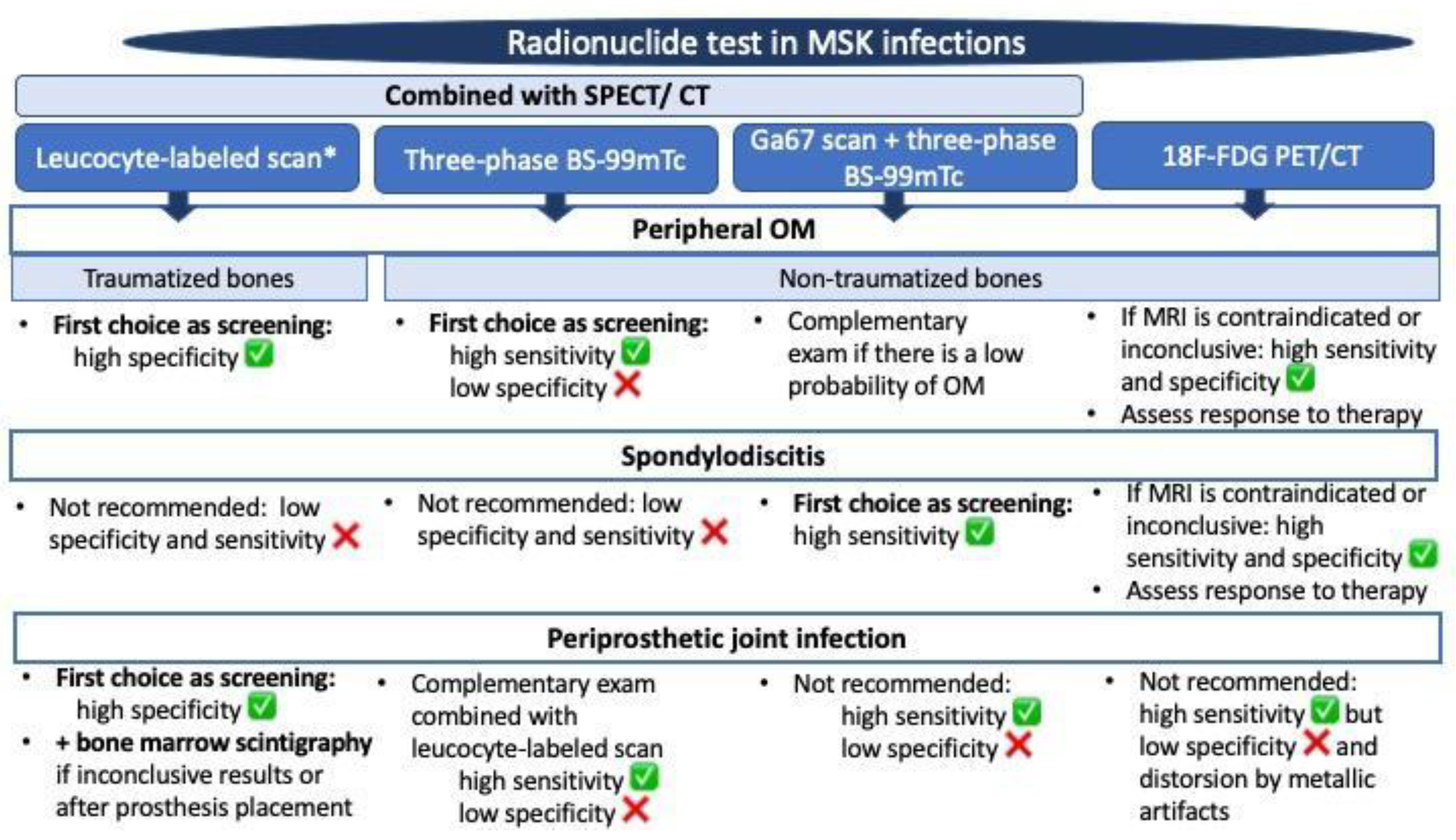
4. Which Is the Most Appropriate Test for the Diagnosis of Bone Infections: Imaging, NM Scans, or Both?
5. Monitoring Musculoskeletal Infections: The Role of NM
6. Potential Developments for NM in Bone Infections: New Radiopharmaceuticals or Techniques
6.1. Gallium-68-Citrate (68Ga)
6.2. Technetium-Labelled Interleukin-8 (IL-8)
6.3. Sodium Fluoride (18F-NaF)
6.4. Radiolabeled Antibiotics
6.5. 99mTc-Ubiquicidin-29-41
6.6. Innovations in PET/CT Utilization
6.6.1. Novel PET Tracers
6.6.2. New PET/CT Applications
7. Machine Learning Tools: A New Era for Nuclear Medicine?
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palestro, C. Radionuclide imaging of osteomyelitis. Semin. Nucl. Med. 2015, 45, 32–46. [Google Scholar] [CrossRef]
- Love, C.; Palestro, C. Nuclear medicine imaging of bone infections. Clin. Radiol. 2016, 71, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Sambri, A.; Spinnato, P.; Tedeschi, S.; Zamparini, E.; Fiore, M.; Zucchini, R.; Giannini, C.; Caldari, E.; Crombé, A.; Viale, P.; et al. Bone and Joint Infections: The Role of Imaging in Tailoring Diagnosis to Improve Patients’ Care. J. Pers. Med. 2021, 11, 1317. [Google Scholar] [CrossRef] [PubMed]
- Sciuk, J. Scintigraphic techniques for the diagnosis of infectious disease of the musculoskeletal system. Semin. Musculoskelet. Radiol. 2004, 8, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Noriega-Álvarez, E.; Domínguez, L.; Orduña, M.P.; Peiró, V.; Sanz, S.; García, R. Role of Nuclear Medicine in the diagnosis of musculoskeletal infection: A review. Rev. Esp. Med. Nucl. Imagen Mol. 2019, 38, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Palestro, C.; Clark, A.; Grady, E.; Heiba, S.; Israel, O.; Klitzke, A.; Love, C.; Sathekge, M.; Treves, S.T.; Yarbrough, T.L. Appropriate Use Criteria for the Use of Nuclear Medicine in Musculoskeletal Infection Imaging. J. Nucl. Med. 2021, 62, 1815–1831. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Sadigh, S.; Mankad, K.; Kapse, N.; Rajeswaran, G. The imaging of osteomyelitis. Quant. Imaging Med. Surg. 2016, 6, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Govaert, G.; IJpma, F.F.; McNally, M.; McNally, E.; Reininga, I.H.; Glaudemans, A.W. Accuracy of diagnostic imaging modalities for peripheral post-traumatic osteomyelitis—A systematic review of the recent literature. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Love, C.; Marwin, S.E.; Palestro, C.J. Nuclear medicine and the infected joint replacement. Semin. Nucl. Med. 2009, 39, 66–78. [Google Scholar] [CrossRef]
- Palestro, C. Nuclear medicine and the failed joint replacement: Past, present, and future. World J. Radiol. 2014, 6, 446–458. [Google Scholar] [CrossRef]
- Auletta, S.; Riolo, D.; Varani, M.; Lauri, C.; Galli, F.; Signore, A. Labelling and Clinical Performance of Human Leukocytes Labelled with 99mTc-HMPAO Using Leukokit® with Gelofusine versus Leukokit® with HES as Sedimentation Agent. Contrast Media Mol. Imaging 2019, 2019, 4368342. [Google Scholar] [CrossRef]
- Reinartz, P.; Mumme, T.; Hermanns, B.; Cremerius, U.; Wirtz, D.C.; Schaefer, W.M.; Niethard, F.; Buell, U. Radionuclide imaging of the painful hip arthroplasty: Positron-emission tomography versus triple-phase bone scanning. J. Bone Jt. Surg. Br. 2005, 87, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Petrosillo, N.; Argento, G.; Sconfienza, L.M.; Treglia, G.; Alavi, A.; Glaudemans, A.W.J.M.; Gheysens, O.; Maes, A.; Lauri, C.; et al. The Role of Imaging Techniques to Define a Peri-Prosthetic Hip and Knee Joint Infection: Multidisciplinary Consensus Statements. J. Clin. Med. 2020, 9, 2548. [Google Scholar] [CrossRef]
- Gratz, S.; Dörner, J.; Oestmann, J.W.; Opitz, M.; Behr, T.; Meller, J.; Grabbe, E.; Becker, W. 67Ga-citrate and 99Tcm-MDP for estimating the severity of vertebral osteomyelitis. Nucl. Med. Commun. 2000, 21, 111–120. [Google Scholar] [CrossRef]
- Love, C.; Patel, M.; Lonner, B.S.; Tomas, M.B.; Palestro, C.J. Diagnosing spinal osteomyelitis: A comparison of bone and Ga-67 scintigraphy and magnetic resonance imaging. Clin. Nucl. Med. 2000, 25, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Schlaeffer, F.; Mikolich, D.J.; Mates, S.M. Technetium Tc 99m diphosphonate bone scan. False-normal findings in elderly patients with hematogenous vertebral osteomyelitis. Arch. Intern. Med. 1987, 147, 2024–2026. [Google Scholar] [CrossRef]
- Gentile, L.; Benazzo, F.; De Rosa, F.; Boriani, S.; Dallagiacoma, G.; Franceschetti, G.; Gaeta, M.; Cuzzocrea, F. A systematic review: Characteristics, complications and treatment of spondylodiscitis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23 (Suppl. S2), 117–128. [Google Scholar] [CrossRef]
- Gemmel, F.; Dumarey, N.; Palestro, C. Radionuclide imaging of spinal infections. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Adatepe, M.H.; Powell, O.M.; Isaacs, G.H.; Nichols, K.; Cefola, R. Hematogenous pyogenic vertebral osteomyelitis: Diagnostic value of radionuclide bone imaging. J. Nucl. Med. 1986, 27, 1680–1685. [Google Scholar]
- Maamari, J.; Tande, A.J.; Diehn, F.; Tai, D.B.G.; Berbari, E.F. Diagnosis of vertebral osteomyelitis. J. Bone Jt. Infect. 2022, 27, 23–32. [Google Scholar] [CrossRef]
- Termaat, M.F.; Raijmakers, P.G.; Scholten, H.J.; Bakker, F.C.; Patka, P.; Haarman, H.J. The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: A systematic review and meta-analysis. J. Bone Jt. Surg. Am. 2005, 87, 2464–2471. [Google Scholar] [CrossRef]
- Glaudemans, A.W.J.M.; Jutte, P.C.; Cataldo, M.A.; Cassar-Pullicino, V.; Gheysens, O.; Borens, O.; Trampuz, A.; Wörtler, K.; Petrosillo, N.; Winkler, H.; et al. Consensus document for the diagnosis of peripheral bone infection in adults: A joint paper by the EANM, EBJIS, and ESR (with ESCMID endorsement). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 957–970. [Google Scholar] [CrossRef]
- Verberne, S.J.; Raijmakers, P.G.; Temmerman, O.P. The Accuracy of Imaging Techniques in the Assessment of Periprosthetic Hip Infection: A Systematic Review and Meta-Analysis. J. Bone Jt. Surg. Am. 2016, 98, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Palestro, C.; Love, C.; Bhargava, K.K. Labeled leukocyte imaging: Current status and future directions. Q. J. Nucl. Med. Mol. Imaging 2009, 53, 105–123. [Google Scholar] [PubMed]
- Signore, A.; Jamar, F.; Israel, O.; Buscombe, J.; Martin-Comin, J.; Lazzeri, E. Clinical indications, image acquisition and data interpretation for white blood cellsand anti-granulocyte monoclonal antibody scintigraphy: An EANM procedural guideline. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1816–1831. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Zhao, K.; Liu, Z.F.; Dong, M.J.; Yang, S.Y. A meta-analysis of fluorodeoxyglucose-positron emission tomography versus scintigraphy in the evaluation of suspected osteomyelitis. Nucl. Med. Commun. 2011, 32, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Palestro, C.; Love, C.; Tronco, G.G.; Tomas, M.B.; Rini, J.N. Combined labeled leukocyte and technetium-99m sulphur colloid marrow imaging for diagnosing musculoskeletal infection: Principles, technique, interpretation, indications and limitations. RadioGraphics 2006, 26, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Love, C.; Marwin, S.E.; Tomas, M.B.; Krauss, E.S.; Tronco, G.G.; Bhargava, K.K.; Nichols, K.J.; Palestro, C.J. Diagnosing infection in the failed joint replacement: A comparison of coincidence detection 18F-FDG and 111In-labeled leukocyte/99mTc-sulfur colloid marrow imaging. J. Nucl. Med. 2004, 45, 1864–1871. [Google Scholar] [PubMed]
- El Espera, I.; Blondet, C.; Moullart, V.; Saïdi, L.; Havet, E.; Mertl, P.; Canarelli, B.; Schmit, J.L.; Meyer, M.E. The usefulness of 99mTc sulfur colloid bone marrow scintigraphy combined with 111In leucocyte scintigraphy in prosthetic joint infection. Nucl. Med. Commun. 2004, 25, 171–175. [Google Scholar] [CrossRef]
- Gemmel, F.; Van den Wyngaert, H.; Love, C.; Welling, M.M.; Gemmel, P.; Palestro, C. Prosthetic joint infections: Radionuclide state-of-the-art imaging. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 892–909. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Signore, A.; Cassar-Pullicino, V.; Cataldo, M.A.; Gheysens, O.; Borens, O.; Trampuz, A.; Wörtler, K.; Petrosillo, N.; Winkler, H.; et al. Diagnosis of peripheral bone and prosthetic joint infections: Overview on the consensus documents by the EANM, EBJIS, and ESR (with ESCMID endorsement). Eur. Radiol. 2019, 29, 6425–6438. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, M.; Lazzeri, E.; Palestro, C. Imaging of Spondylodiscitis. Semin. Nucl. Med. 2018, 48, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Ma, X.; Ma, J.; Wang, J.; Chen, Y.; Yang, Y. Use of anti-granulocyte scintigraphy with 99mTc-labeled monoclonal antibodies for the diagnosis of periprosthetic infection in patients after total joint arthroplasty: A diagnostic meta-analysis. PLoS ONE 2013, 8, e69857. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Na, S.J.; Oh, S.J.; Jung, B.S.; Lee, S.H.; Chang, J.S.; Bin, S.I.; Ryu, J.S. Usefulness of adding SPECT/CT to 99mTc-hexamethylpropylene amine oxime (HMPAO)-labeled leukocyte imaging for diagnosing prosthetic joint infections. J. Comput. Assist. Tomogr. 2014, 38, 313–319. [Google Scholar] [CrossRef]
- Gratz, S.; Höffken, H.; Kaiser, J.W.; Behr, T.M.; Strosche, H.; Reize, P. Nuclear medical imaging in case of painful knee arthroplasty. Radiologe 2009, 49, 59–67. [Google Scholar] [CrossRef]
- Rubello, D.; Rampin, L.; Banti, E.; Massaro, A.; Cittadin, S.; Cattelan, A.M.; Al-Nahhas, A. Diagnosis of infected total knee arthroplasty with anti-granulocyte scintigraphy: The importance of a dual-time acquisition protocol. Nucl. Med. Commun. 2008, 29, 331–335. [Google Scholar] [CrossRef]
- Gratz, S.; Behr, T.M.; Reize, P.; Pfestroff, A.; Kampen, W.U.; Höffken, H. (99m)Tc-Fab’ fragments (sulesomab) for imaging septically loosened total knee arthroplasty. J. Int. Med. Res. 2009, 37, 54–67. [Google Scholar] [CrossRef]
- Sousa, R.; Massada, M.; Pereira, A.; Fontes, F.; Amorim, I.; Oliveira, A. Diagnostic accuracy of combined 99mTc-sulesomab and 99mTc-nanocolloid bone marrow imaging in detecting prosthetic joint infection. Nucl. Med. Commun. 2011, 32, 834–839. [Google Scholar] [CrossRef]
- Klett, R.; Kordelle, J.; Stahl, U.; Khalisi, A.; Puille, M.; Steiner, D.; Bauer, R. Immunoscintigraphy of septic loosening of knee endoprosthesis: A retrospective evaluation of the antigranulocyte antibody BW 250/183. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1463–1466. [Google Scholar] [CrossRef]
- Verberne, S.J.; Sonnega, R.J.; Temmerman, O.P.; Raijmakers, P.G. What is the Accuracy of Nuclear Imaging in the Assessment of Periprosthetic Knee Infection? A Meta-analysis. Clin. Orthop. Relat. Res. 2017, 475, 1395–1410. [Google Scholar] [CrossRef]
- Richter, W.S.; Ivancevic, V.; Meller, J.; Lang, O.; Le Guludec, D.; Szilvazi, I.; Amthauer, H.; Chossat, F.; Dahmane, A.; Schwenke, C.; et al. 99mTc-besilesomab (Scintimun) in peripheral osteomyelitis: Comparison with 99mTc-labelled white blood cells. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 899–910. [Google Scholar] [CrossRef]
- Domínguez, M.L.; Lorente, R.; Rayo, J.I.; Serrano, J.; Sánchez, R.; Infante, J.R.; García, L.; Durán, C. SPECT-CT with 67Ga-citrate in the management of spondylodiscitis. Rev. Esp. Med. Nucl. Imagen Mol. 2012, 31, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Fuster, D.; Solà, O.; Soriano, A.; Monegal, A.; Setoain, X.; Tomás, X.; Garcia, S.; Mensa, J.; Rubello, D.; Pons, F. A prospective study comparing whole-body FDG PET/CT to combined planar bone scan with 67Ga SPECT/CT in the Diagnosis of Spondylodiskitis. Clin. Nucl. Med. 2012, 37, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Liévano, P.; De la Cueva, L.; Navarro, P.; Arroyo, E.; Añaños, M.; Abós, M.D. SPECT-TAC de baja dosis con 67Ga en un caso de espondilodiscitis y hernia de Schmorl. Rev. Esp. Med. Nucl. 2009, 28, 288–290. [Google Scholar] [CrossRef]
- Thang, S.P.; Tong, A.K.; Lam, W.W.; Ng, D.C. SPECT/CT in musculoskeletal infections. Semin. Musculoskelet. Radiol. 2014, 18, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Sanlı, Y.; Ozkan, Z.G.; Unal, S.N.; Türkmen, C.; Kılıçoğlu, O. The Additional Value of Tc 99m HMPAO White Blood Cell SPECT in the Evaluation of Bone and Soft Tissue Infections. Mol. Imaging Radionucl. Ther. 2011, 20, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O. Hybrid imaging systems in the diagnosis of osteomyelitis and prosthetic joint infection. Q. J. Nucl. Med. Mol. Imaging 2009, 53, 95–104. [Google Scholar]
- Filippi, L.; Schillaci, O. Usefulness of hybrid SPECT/CT in 99mTc-HMPAO-labeled leukocyte scintigraphy for bone and joint infections. J. Nucl. Med. 2006, 47, 1908–1913. [Google Scholar]
- Bar-Shalom, R.; Yefremov, N.; Guralnik, L.; Keidar, Z.; Engel, A.; Nitecki, S.; Israel, O. SPECT/CT using 67Ga and 111In-labeled leukocyte scintigraphy for diagnosis of infection. J. Nucl. Med. 2006, 47, 587–594. [Google Scholar]
- Glaudemans, A.W.; Israel, O.; Slart, R.H. Pitfalls and limitations of radionuclide and hybrid imaging in infection and inflammation. Semin. Nucl. Med. 2015, 45, 500–512. [Google Scholar] [CrossRef]
- Linke, R.; Kuwert, T.; Uder, M.; Forst, R.; Wuest, W. Skeletal SPECT/CT of the peripheral extremities. Am. J. Roentgenol. 2010, 194, W329–W335. [Google Scholar] [CrossRef] [PubMed]
- Tamm, A.S.; Abele, J.T. Bone and Gallium Single-Photon Emission Computed Tomography-Computed Tomography is Equivalent to Magnetic Resonance Imaging in the Diagnosis of Infectious Spondylodiscitis: A Retrospective Study. Can. Assoc. Radiol. J. 2017, 68, 41–46. [Google Scholar] [CrossRef]
- Graute, V.; Feist, M.; Lehner, S.; Haug, A.; Müller, P.E.; Bartenstein, P.; Hacker, M. Detection of low-grade prosthetic joint infections using 99mTc-antigranulocyte SPECT/CT: Initial clinical results. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Pawaskar, A.; Basu, S.; Jahangiri, P.; Alavi, A. In Vivo Molecular Imaging of Musculoskeletal Inflammation and Infection. PET Clin. 2019, 14, 43–59. [Google Scholar] [CrossRef]
- Casali, M.; Lauri, C.; Altini, C.; Bertagna, F.; Cassarino, G.; Cistaro, A.; Erba, A.P.; Ferrari, C.; Mainolfi, C.G.; Palucci, A.; et al. State of the art of 18F-FDG PET/CT application in inflammation and infection: A guide for image acquisition and interpretation. Clin. Transl. Imaging 2021, 9, 299–339. [Google Scholar] [CrossRef]
- Palestro, C. FDG-PET in musculoskeletal infections. Sem. Nucl. Med. 2013, 43, 367–376. [Google Scholar] [CrossRef]
- Demirev, A.; Weijers, R.; Geurts, J.; Mottaghy, F.; Walenkamp, G.; Brans, B. Comparison of [18 F] FDG PET/CT and MRI in the diagnosis of active osteomyelitis. Skelet. Radiol. 2014, 43, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Glaudemans, A.W.; Signore, A. FDG-PET/CT in infections: The imaging method of choice? Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1986–1991. [Google Scholar] [CrossRef]
- Pijl, J.P.; Nienhuis, P.H.; Kwee, T.C.; Glaudemans, A.W.J.M.; Slart, R.H.J.A.; Gormsen, L.C. Limitations and Pitfalls of FDG-PET/CT in Infection and Inflammation. Semin. Nucl. Med. 2021, 51, 633–645. [Google Scholar] [CrossRef]
- Prodromou, M.L.; Ziakas, P.D.; Poulou, L.S.; Karsaliakos, P.; Thanos, L.; Mylonakis, E. FDG PET is a robust tool for the diagnosis of spondylodiscitis: A meta-analysis of diagnostic data. Clin. Nucl. Med. 2014, 39, 330–335. [Google Scholar] [CrossRef]
- Gratz, S.; Dörner, J.; Fischer, U.; Behr, T.M.; Béhé, M.; Altenvoerde, G.; Meller, J.; Grabbe, E.; Becker, W. 18F-FDG hybrid PET in patients with suspected spondylitis. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 516–524. [Google Scholar] [CrossRef]
- Schmitz, A.; Risse, J.H.; Grünwald, F.; Gassel, F.; Biersack, H.J.; Schmitt, O. Fluorine-18 fluorodeoxyglucose positron emission tomography findings in spondylodiscitis: Preliminary results. Eur. Spine J. 2001, 10, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Smids, C.; Kouijzer, I.J.; Vos, F.J.; Sprong, T.; Hosman, A.J.; de Rooy, J.W.; Aarntzen, E.H.; de Geus-Oei, L.F.; Oyen, W.J.; Bleeker-Rovers, C.P. A comparison of the diagnostic value of MRI and 18F-FDG-PET/CT in suspected spondylodiscitis. Infection 2017, 45, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kouijzer, I.J.; Scheper, H.; de Rooy, J.W.; Bloem, J.L.; Janssen, M.J.; van den Hoven, L.; Hosman, A.J.; Visser, L.G.; Oyen, W.J.; Bleeker-Rovers, C.P.; et al. The diagnostic value of 18F-FDG-PET/CT and MRI in suspected vertebral osteomyelitis—A prospective study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Kälicke, T.; Schmitz, A.; Risse, J.H.; Arens, S.; Keller, E.; Hansis, M.; Schmitt, O.; Biersack, H.J.; Grünwald, F. Fluorine-18 fluorodeoxyglucose PET in infectious bone diseases: Results of histologically confirmed cases. Eur. J. Nucl. Med. 2000, 27, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Eid, K.; Dora, C.; Trentz, O.; von Schulthess, G.K.; Stumpe, K.D. Diagnostic value of 18F-FDG PET/CT in trauma patients with suspected chronic osteomyelitis. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Meller, J.; Köster, G.; Liersch, T.; Siefker, U.; Lehmann, K.; Meyer, I.; Schreiber, K.; Altenvoerde, G.; Becker, W. Chronic bacterial osteomyelitis: Prospective comparison of (18)F-FDG imaging with a dual-head coincidence camera and (111)In-labelled autologous leucocyte scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Pascale, M.; Lazzeri, E.; van der Bruggen, W.; Delgado Bolton, R.C.; Glaudemans, A.W. Diagnostic performance of 18F-FDG PET/CT in patients with spinal infection: A systematic review and a bivariate meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Suzuki, M.; Koshi, T.; Yamashita, M.; Yamauchi, K.; Inoue, G.; Orita, S.; Eguchi, Y.; Kuniyoshi, K.; Ochiai, N.; et al. 18F-fluorodeoxyglucose-PET for patients with suspected spondylitis showing Modic change. Spine 2010, 15, E1599–E1603. [Google Scholar] [CrossRef]
- Stumpe, K.D.; Zanetti, M.; Weishaupt, D.; Hodler, J.; Boos, N.; Von Schulthess, G.K. FDG positron emission tomography for differentiation of degenerative and infectious endplate abnormalities in the lumbar spine detected on MR imaging. AJR Am. J. Roentgenol. 2002, 179, 1151–1157. [Google Scholar] [CrossRef]
- Guhlmann, A.; Brecht-Krauss, D.; Suger, G.; Glatting, G.; Kotzerke, J.; Kinzl, L.; Reske, S.N. Fluorine-18-FDG PET and technetium-99m antigranulocyte antibody scintigraphy in chronic osteomyelitis. J. Nucl. Med. 1998, 39, 2145–2152. [Google Scholar]
- Wenter, V.; Albert, N.L.; Brendel, M.; Fendler, W.P.; Cyran, C.C.; Bartenstein, P.; Friederichs, J.; Müller, J.P.; Militz, M.; Hacker, M.; et al. [18F]FDG PET accurately differentiates infected and non-infected non-unions after fracture fixation. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 432–440. [Google Scholar] [CrossRef]
- Volpe, L.; Indelli, P.F.; Latella, L.; Poli, P.; Yakupoglu, J.; Marcucci, M. Periprosthetic joint infections: A clinical practice algorithm. Joints 2015, 2, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Pill, S.G.; Parvizi, J.; Tang, P.H.; Garino, J.P.; Nelson, C.; Zhuang, H.; Alavi, A. Comparison of fluorodeoxyglucose positron emission tomography and (111)indium-white blood cell imaging in the diagnosis of periprosthetic infection of the hip. J. Arthroplast. 2006, 21, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, F.; Nuyts, J.; Maes, A.; Vanquickenborne, B.; Stuyck, J.; Bellemans, J.; Vleugels, S.; Bormans, G.; Mortelmans, L. FDG-PET, 99mtc-HMPAO white blood cell SPET and bone scintigraphy in the evaluation of painful total knee arthroplasties. Eur. J. Nucl. Med. 2001, 28, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Vanquickenborne, B.; Maes, A.; Nuyts, J.; Van Acker, F.; Stuyck, J.; Mulier, M.; Verbruggen, A.; Mortelmans, L. The value of (18)FDG-PET for the detection of infected hip prosthesis. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Kwee, T.C.; Saboury, B.; Garino, J.P.; Nelson, C.L.; Zhuang, H.; Parsons, M.; Chen, W.; Kumar, R.; Salavati, A.; et al. FDG PET for diagnosing infection in hip and knee prostheses: Prospective study in 221 prostheses and subgroup comparison with combined (111)In-labeled leukocyte/(99m)Tc-sulfur colloid bone marrow imaging in 88 prostheses. Clin. Nucl. Med. 2014, 39, 609–615. [Google Scholar] [CrossRef]
- Zhuang, H.; Duarte, P.S.; Pourdehnad, M.; Maes, A.; Van Acker, F.; ShnieR, D.; Garino, J.P.; Fitzgerald, R.H.; Alavi, A. The promising role of 18F-FDG PET in detecting infected lower limb prosthesis implants. J. Nucl. Med. 2001, 42, 44–48. [Google Scholar] [PubMed]
- Chacko, T.K.; Zhuang, H.; Nakhoda, K.Z.; Moussavian, B.; Alavi, A. Applications of fluorodeoxyglucose positron emission tomography in the diagnosis of infection. Nucl. Med. Commun. 2003, 24, 615–624. [Google Scholar] [CrossRef]
- Kiran, M.; Donnelly, T.D.; Armstrong, C.; Kapoor, B.; Kumar, G.; Peter, V. Diagnostic utility of fluorodeoxyglucose positron emission tomography in prosthetic joint infection based on MSIS criteria. Bone Jt. J. 2019, 101-B, 910–914. [Google Scholar] [CrossRef]
- Kirienko, M.; Erba, P.A.; Chiti, A.; Sollini, M. Hybrid PET/MRI in Infection and Inflammation: An Update About the Latest Available Literature Evidence. Semin. Nucl. Med. 2023, 53, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Hulsen, D.J.W.; Mitea, C.; Arts, J.J.; Loeffen, D.; Geurts, J. Diagnostic value of hybrid FDG-PET/MR imaging of chronic osteomyelitis. Eur. J. Hybrid. Imaging 2022, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Fahnert, J.; Purz, S.; Jarvers, J.S.; Heyde, C.E.; Barthel, H.; Stumpp, P.; Kahn, T.; Sabri, O.; Friedrich, B. Use of Simultaneous 18F-FDG PET/MRI for the Detection of Spondylodiskitis. J. Nucl. Med. 2016, 57, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.; Kong, E.; Yu, D.; Hong, C.P. Clinical and Radiological Analysis of Pyogenic Vertebral Osteomyelitis Immediately after Successful Antimicrobial Therapy: Considerations for Assessing Therapeutic Response. Diagnostics 2020, 10, 861. [Google Scholar] [CrossRef] [PubMed]
- Seong, I.; Kong, E.; Jeon, I. Clinical and Radiological Features Predicting Intervertebral Autofusion after Successful Antibiotic Therapy in Pyogenic Vertebral Osteomyelitis. Diagnostics 2021, 11, 1349. [Google Scholar] [CrossRef] [PubMed]
- Henkelmann, J.; Henkelmann, R.; Denecke, T.; Zajonz, D.; Roth, A.; Sabri, O.; Purz, S. Simultaneous 18F-FDG-PET/MRI for the detection of periprosthetic joint infections after knee or hip arthroplasty: A prospective feasibility study. Int. Orthop. 2022, 46, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraju, V.S.; Singh, H.; Kumar, R.; Sharma, S.; Mittal, B.R.; Bhattacharya, A. Infection imaging using [18F]FDG-labelled white blood cell positron emission tomography-computed tomography. Br. J. Radiol. 2021, 94, 20201204. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Testart, N.; Jreige, M.; Kamani, C.; Moshebah, M.; Muoio, B.; Nicod-Lalonde, M.; Schaefer, N.; Giovanella, L.; Prior, J.O.; et al. Diagnostic Performance of PET or PET/CT Using 18F-FDG Labeled White Blood Cells in Infectious Diseases: A Systematic Review and a Bivariate Meta-Analysis. Diagnostics 2019, 9, 60. [Google Scholar] [CrossRef]
- Manda, D.; Thakral, P.; Sen, I.; Das, S.S.; Cb, V.; Malik, D. Incremental Value of 18 F-FDG-Labeled Leukocytes PET/CT Over 18 F-FDG PET/CT Scan in the Detection of Occult Infection. Clin. Nucl. Med. 2022, 47, e574–e581. [Google Scholar] [CrossRef]
- Aksoy, S.Y.; Asa, S.; Ozhan, M.; Ocak, M.; Sager, M.S.; Erkan, M.E.; Halac, M.; Kabasakal, L.; Sönmezoglu, K.; Kanmaz, B. FDG and FDG-labelled leucocyte PET/CT in the imaging of prosthetic joint infection. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 556–564. [Google Scholar] [CrossRef]
- Rastogi, A.; Bhattacharya, A.; Prakash, M.; Sharma, S.; Mittal, B.R.; Khandelwal, N.; Bhansali, A. Utility of PET/CT with fluorine-18-fluorodeoxyglucose-labeled autologous leukocytes for diagnosing diabetic foot osteomyelitis in patients with Charcot’s neuroarthropathy. Nucl. Med. Commun. 2016, 37, 1253–1259. [Google Scholar] [CrossRef]
- Simpfendorfer, C.S. Radiologic Approach to Musculoskeletal Infections. Infect. Dis. Clin. N. Am. 2017, 31, 299–324. [Google Scholar] [CrossRef]
- Pierce, J.L.; Perry, M.T.; Wessell, D.E.; Lenchik, L.; Ahlawat, S.; Baker, J.C.; Banks, J.; Caracciolo, J.T.; DeGeorge, K.C.; Demertzis, J.; et al. ACR Appropriateness Criteria® Suspected Osteomyelitis, Septic Arthritis, or Soft Tissue Infection (Excluding Spine and Diabetic Foot): 2022 Update. J. Am. Coll. Radiol. 2022, 19, S473–S487. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, D.L.; Katzap, E.; Horowitz, S.; Barilla-LaBarca, M.L. Approach to septic arthritis. Am. Fam. Physician 2011, 84, 653–660. [Google Scholar] [PubMed]
- García-Arias, M.; Balsa, A.; Mola, E.M. Septic arthritis. Best. Pract. Res. Clin. Rheumatol. 2011, 25, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Taljanovic, M.S.; Gimber, L.H.; Omar, I.M.; Klauser, A.S.; Miller, M.D.; Wild, J.R.; Chadaz, T.S. Imaging of Postoperative Infection at the Knee Joint. Semin. Musculoskelet. Radiol. 2018, 22, 464–480. [Google Scholar] [CrossRef]
- Morales, H. Infectious spondylitis mimics: Mechanisms of disease and imaging findings. Semin. Ultrasound CT MR 2018, 39, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Fayad, L.M.; Carrino, J.A.; Fishman, E.K. Musculoskeletal infection: Role of CT in the emergency department. Radiographics 2007, 27, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Modic, M.T.; Feiglin, D.H.; Piraino, D.W.; Boumphrey, F.; Weinstein, M.A.; Duchesneau, P.M.; Rehm, S. Vertebral osteomyelitis: Assessment using MR. Radiology 1985, 157, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Hadjipavlou, A.G.; Cesani-Vazquez, F.; Villaneuva-Meyer, J.; Mader, J.T.; Necessary, J.T.; Crow, W.; Jensen, R.E.; Chaljub, G. The effectiveness of gallium citrate Ga 67 radionuclide imaging in vertebral osteomyelitis revisited. Am. J. Orthop. 1998, 27, 179–183. [Google Scholar]
- Kim, S.J.; Pak, K.; Kim, K.; Lee, J.S. Comparing the Diagnostic Accuracies of F-18 Fluorodeoxyglucose Positron Emission Tomography and Magnetic Resonance Imaging for the Detection of Spondylodiscitis: A Meta-analysis. Spine 2019, 44, E414–E422. [Google Scholar] [CrossRef]
- Fuster, D.; Tomás, X.; Mayoral, M.; Soriano, A.; Manchón, F.; Cardenal, C.; Monegal, A.; Granados, U.; Garcia, S.; Pons, F. Prospective comparison of whole-body (18)F-FDG PET/CT and MRI of the spine in the diagnosis of haematogenous spondylodiscitis. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 264–271. [Google Scholar] [CrossRef]
- Seifen, T.; Rettenbacher, L.; Thaler, C.; Holzmannhofer, J.; Mc Coy, M.; Pirich, C. Prolonged back pain attributed to suspected spondylodiscitis. The value of ¹⁸F-FDG PET/CT imaging in the diagnostic work-up of patients. Nuklearmedizin 2012, 51, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, E.; Bozzao, A.; Cataldo, M.A.; Petrosillo, N.; Manfrè, L.; Trampuz, A.; Signore, A.; Muto, M. Joint EANM/ESNR and ESCMID-endorsed consensus document for the diagnosis of spine infection (spondylodiscitis) in adults. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2464–2487. [Google Scholar] [CrossRef] [PubMed]
- van der Bruggen, W.; Bleeker-Rovers, C.P.; Boerman, O.C.; Gotthardt, M.; Oyen, W.J. PET and SPECT in osteomyelitis and prosthetic bone and joint infections: A systematic review. Semin. Nucl. Med. 2010, 40, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Warmann, S.W.; Dittmann, H.; Seitz, G.; Bares, R.; Fuchs, J.; Schäfer, J.F. Follow-up of acute osteomyelitis in children: The possible role of PET/CT in selected cases. J. Pediatr. Surg. 2011, 46, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Riccio, S.A.; Chu, A.K.; Rabin, H.R.; Kloiber, R. Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Interpretation Criteria for Assessment of Antibiotic Treatment Response in Pyogenic Spine Infection. Can. Assoc. Radiol. J. 2015, 66, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, I.J.; Suh, K.T.; Kim, Y.K.; Lee, J.S. Prediction of residual disease of spine infection using F-18 FDG PET/CT. Spine 2009, 34, 2424–2430. [Google Scholar] [CrossRef]
- Dauchy, F.A.; Dutertre, A.; Lawson-Ayayi, S.; de Clermont-Gallerande, H.; Fournier, C.; Zanotti-Fregonara, P.; Dutronc, H.; Vital, J.M.; Dupon, M.; Fernandez, P. Interest of [(18)F]fluorodeoxyglucose positron emission tomography/computed tomography for the diagnosis of relapse in patients with spinal infection: A prospective study. Clin. Microbiol. Infect. 2016, 22, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Nanni, C.; Boriani, L.; Salvadori, C.; Zamparini, E.; Rorato, G.; Ambrosini, V.; Gasbarrini, A.; Tumietto, F.; Cristini, F.; Scudeller, L.; et al. FDG PET/CT is useful for the interim evaluation of response to therapy in patients affected by haematogenous spondylodiscitis. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1538–1544. [Google Scholar] [CrossRef]
- Russo, A.; Graziano, E.; Carnelutti, A.; Sponza, M.; Cadeo, B.; Sartor, A.; Righi, E.; Bassetti, M. Management of vertebral osteomyelitis over an eight-year period: The UDIPROVE (UDIne PROtocol on VErtebral osteomyelitis). Int. J. Infect. Dis. 2019, 89, 116–121. [Google Scholar] [CrossRef]
- Righi, E.; Carnelutti, A.; Muser, D.; Di Gregorio, F.; Cadeo, B.; Melchioretto, G.; Merelli, M.; Alavi, A.; Bassetti, M. Incremental value of FDG-PET/CT to monitor treatment response in infectious spondylodiscitis. Skeletal Radiol. 2020, 49, 903–912. [Google Scholar] [CrossRef]
- Nanni, C.; Errani, C.; Boriani, L.; Fantini, L.; Ambrosini, V.; Boschi, S.; Rubello, D.; Pettinato, C.; Mercuri, M.; Gasbarrini, A.; et al. 68Ga-citrate PET/CT for evaluating patients with infections of the bone: Preliminary results. J. Nucl. Med. 2010, 51, 1932–1936. [Google Scholar] [CrossRef]
- Tseng, J.R.; Chang, Y.H.; Yang, L.Y.; Wu, C.T.; Chen, S.Y.; Wan, C.H.; Hsiao, I.T.; Yen, T.C. Potential usefulness of 68Ga-citrate PET/CT in detecting infected lower limb prostheses. EJNMMI Res. 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Jødal, L.; Afzelius, P.; Alstrup, A.K.O.; Jensen, S.B. Radiotracers for Bone Marrow Infection Imaging. Molecules 2021, 26, 3159. [Google Scholar] [CrossRef] [PubMed]
- Bleeker-Rovers, C.P.; Rennen, H.J.; Boerman, O.C.; Wymenga, A.B.; Visser, E.P.; Bakker, J.H.; van der Meer, J.W.; Corstens, F.H.; Oyen, W.J. 99mTc-labeled interleukin 8 for the scintigraphic detection of infection and inflammation: First clinical evaluation. J. Nucl. Med. 2007, 48, 337–343. [Google Scholar] [PubMed]
- Afzelius, P.; Heegaard, P.M.H.; Jensen, S.B.; Alstrup, A.K.O.; Schønheyder, H.C.; Eek, A.; Boerman, O.; Nielsen, O.L. [99mTc]-labelled interleukin-8 as a diagnostic tool compared to [18F]FDG and CT in an experimental porcine osteomyelitis model. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 32–46. [Google Scholar] [PubMed]
- Adams, C.; Banks, K.P. Bone Scan; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Beheshti, M.; Mottaghy, F.M.; Paycha, F.; Behrendt, F.F.F.; Van den Wyngaert, T.; Fogelman, I.; Strobel, K.; Celli, M.; Fanti, S.; Giammarile, F.; et al. (18)F-NaF PET/CT: EANM procedure guidelines for bone imaging. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Yu, S.N.; Yoo, I.D.; Jeon, M.H.; Hong, C.H.; Shim, J.J.; Chang, S.H.; Lee, S.M. Clinical application of dual-phase F-18 sodium-fluoride bone PET/CT for diagnosing surgical site infection following orthopedic surgery. Medicine 2019, 98, e14770. [Google Scholar] [CrossRef]
- Adesanya, O.; Sprowson, A.; Masters, J.; Hutchinson, C. Review of the role of dynamic 18F-NaF PET in diagnosing and distinguishing between septic and aseptic loosening in hip prosthesis. J. Orthop. Surg. Res. 2015, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.A.R. 99m Tc-labeled antibiotics for infection diagnosis: Mechanism, action, and progress. Chem. Biol. Drug Des. 2022, 99, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Malamitsi, J.; Giamarellou, H.; Kanellakopoulou, K.; Dounis, E.; Grecka, V.; Christakopoulos, J.; Koratzanis, G.; Antoniadou, A.; Panoutsopoulos, G.; Batsakis, C.; et al. Infecton: A 99mTc-ciprofloxacin radiopharmaceutical for the detection of bone infection. Clin. Microbiol. Infect. 2003, 9, 101–109. [Google Scholar] [CrossRef]
- Sarda, L.; Crémieux, A.C.; Lebellec, Y.; Meulemans, A.; Lebtahi, R.; Hayem, G.; Génin, R.; Delahaye, N.; Huten, D.; Le Guludec, D. Inability of 99mTc-ciprofloxacin scintigraphy to discriminate between septic and sterile osteoarticular diseases. J. Nucl. Med. 2003, 44, 920–926. [Google Scholar] [PubMed]
- Yapar, Z.; Kibar, M.; Yapar, A.F.; Toğrul, E.; Kayaselçuk, U.; Sarpel, Y. The efficacy of technetium-99m ciprofloxacin (Infecton) imaging in suspected orthopaedic infection: A comparison with sequential bone/gallium imaging. Eur. J. Nucl. Med. 2001, 28, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Sonmezoglu, K.; Sonmezoglu, M.; Halac, M.; Akgün, I.; Türkmen, C.; Onsel, C.; Kanmaz, B.; Solanki, K.; Britton, K.E.; Uslu, I. Usefulness of 99mTc-ciprofloxacin (infecton) scan in diagnosis of chronic orthopedic infections: Comparative study with 99mTc-HMPAO leukocyte scintigraphy. J. Nucl. Med. 2001, 42, 567–574. [Google Scholar]
- Singh, B.; Mittal, B.R.; Bhattacharya, A.; Aggarwal, A.; Nagi, O.N.; Singh, A.K. Technetium-99m ciprofloxacin imaging in the diagnosis of postsurgical bony infection and evaluation of the response to antibiotic therapy: A case report. J. Orthop. Surg. 2005, 13, 190–194. [Google Scholar] [CrossRef]
- Dadachova, E.; Rangel, D.E.N. Highlights of the Latest Developments in Radiopharmaceuticals for Infection Imaging and Future Perspectives. Front. Med. 2022, 9, 819702. [Google Scholar] [CrossRef]
- Naqvi, S.A.R.; Drlica, K. Fluoroquinolones as imaging agents for bacterial infection. Dalton Trans. 2017, 46, 14452–14460. [Google Scholar] [CrossRef]
- Lee, I.K.; Jacome, D.A.; Cho, J.K.; Tu, V.; Young, A.J.; Dominguez, T.; Northrup, J.D.; Etersque, J.M.; Lee, H.S.; Ruff, A.; et al. Imaging sensitive and drug-resistant bacterial infection with [11C]-trimethoprim. J. Clin. Investig. 2022, 132, e156679. [Google Scholar] [CrossRef] [PubMed]
- Sellmyer, M.A.; Lee, I.; Hou, C.; Weng, C.C.; Li, S.; Lieberman, B.P.; Zeng, C.; Mankoff, D.A.; Mach, R.H. Bacterial infection imaging with [18F]fluoropropyl-trimethoprim. Proc. Natl. Acad. Sci. USA 2017, 114, 8372–8377. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.; Garcia-Perez, O.; Paez, D.; El-Haj, N.; Kain-Godoy, T.; Lawal, I.; Estrada-Lobato, E. Molecular imaging in musculoskeletal infections with 99mTc-UBI 29-41 SPECT/CT. Ann. Nucl. Med. 2018, 32, 54–59. [Google Scholar] [CrossRef]
- Paez, D.; Sathekge, M.M.; Douis, H.; Giammarile, F.; Fatima, S.; Dhal, A.; Puri, S.K.; Erba, P.A.; Lazzeri, E.; Ferrando, R.; et al. Comparison of MRI, [18F]FDG PET/CT, and 99mTc-UBI 29-41 scintigraphy for postoperative spondylodiscitis-a prospective multicenter study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1864–1875. [Google Scholar] [CrossRef]
- Mokoala, K.M.G.; Ndlovu, H.; Lawal, I.; Sathekge, M.M. PET/CT and SPECT/CT for Infection in Joints and Bones: An Overview and Future Directions. Semin. Nucl. Med. 2023, in press. [Google Scholar] [CrossRef]
- Pickett, J.E.; Thompson, J.M.; Sadowska, A.; Tkaczyk, C.; Sellman, B.R.; Minola, A.; Corti, D.; Lanzavecchia, A.; Miller, L.S.; Thorek, D.L. Molecularly specific detection of bacterial lipoteichoic acid for diagnosis of prosthetic joint infection of the bone. Bone Res. 2018, 6, 13. [Google Scholar] [CrossRef]
- Holt, D.P.; Kalinda, A.S.; Bambarger, L.E.; Jain, S.K.; Dannals, R.F. Radiosynthesis and validation of [Carboxy-11 C]4-Aminobenzoic acid ([11C]PABA), a PET radiotracer for imaging bacterial infections. J. Labelled Comp. Radiopharm. 2019, 62, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Xing, H.; Zhu, W.; Wu, Z.; Zhang, Y.; Ma, Y.; Liu, Y.; Huo, L.; Zhu, Z.; Li, Z.; et al. Infection Imaging With (18)F-FDS and First-in-Human Evaluation. Nucl. Med. Biol. 2016, 43, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Han, L.; Wang, J.; Zhang, C.; Qi, E.; Zhang, D.; Zhang, X.; Huan, Y.; Tian, J. 18F-FDG and 68 Ga-FAPI PET/CT for the evaluation of periprosthetic joint infection and aseptic loosening in rabbit models. BMC Musculoskelet. Disord. 2022, 23, 592. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ding, H.; Fan, D.; Shu, Q.; Liu, G.; Zhang, S.; Chen, Y. Prospective Comparison of the Imaging Value of 99m Tc-MDP Bone Scan and 68 Ga-FAPI-04 PET/CT in Synovitis, Acne, Pustulosis, Hyperostosis, and Osteitis Syndrome. Clin. Nucl. Med. 2023, 48, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Yang, H.; Zhou, Z.; Li, M.; Jiang, X.; Li, F.; Luo, Y.; Li, M. [68Ga]Ga-FAPI-04 PET/CT may be a predictor for early treatment response in rheumatoid arthritis. EJNMMI Res. 2024, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Song, Y.; Liu, X.; Gai, Y.; Liu, Q.; Ruan, W.; Liu, F.; Hu, F.; Lan, X. Increased uptake of 68Ga-DOTA-FAPI-04 in bones and joints: Metastases and beyond. Eur. J. Nucl. Med. Mol. Imaging. 2022, 49, 709–720. [Google Scholar] [CrossRef]
- Diaz, L.A.; Foss, C.A.; Thornton, K.; Nimmagadda, S.; Endres, C.J.; Uzuner, O.; Seyler, T.M.; Ulrich, S.D.; Conway, J.; Bettegowda, C.; et al. Imaging of musculoskeletal bacterial infections by [I-124] FIAU-PET/CT. PLoS ONE 2007, 2, e1007. [Google Scholar] [CrossRef]
- [I-124] FIAU-PET/CT Scanning in Patients with Pain in a Prosthetic Knee or Hip Joint (PJI). Clinical- Trials.gov Website. 2012. Updated 8 February 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT01705496 (accessed on 31 January 2024).
- FIAU-PET/CT Scanning in Diagnosing Osteomyelitis in Patients with Diabetic Foot Infection. 2013. Updated 6 April 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT01764919 (accessed on 31 January 2024).
- Stewart, M.N.; Parker, M.F.L.; Jivan, S.; Luu, J.M.; Huynh, T.L.; Schulte, B.; Seo, Y.; Blecha, J.E.; Villanueva-Meyer, J.E.; Flavell, R.R.; et al. High Enantiomeric Excess In-Loop Synthesis of d-[methyl-11C]Methionine for Use as a Diagnostic Positron Emission Tomography Radiotracer in Bacterial Infection. ACS Infect. Dis. 2020, 6, 43–49. [Google Scholar] [CrossRef]
- Neumann, K.D.; Villanueva-Meyer, J.E.; Mutch, C.A.; Flavell, R.R.; Blecha, J.E.; Kwak, T.; Sriram, R.; VanBrocklin, H.F.; Rosenberg, O.S.; Ohliger, M.A.; et al. Imaging Active Infection in vivo Using D-Amino Acid Derived PET Radiotracers. Sci. Rep. 2017, 7, 7903. [Google Scholar] [CrossRef]
- Polvoy, I.; Seo, Y.; Parker, M.; Stewart, M.; Siddiqua, K.; Manacsa, H.S.; Ravanfar, V.; Blecha, J.; Hope, T.A.; Vanbrocklin, H.; et al. Imaging joint infections using D-methyl-11C-methionine PET/MRI: Initial experience in humans. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3761–3771. [Google Scholar] [CrossRef]
- Rahmim, A.; Lodge, M.A.; Karakatsanis, N.A.; Panin, V.Y.; Zhou, Y.; McMillan, A.; Cho, S.; Zaidi, H.; Casey, M.E.; Wahl, R.L. Dynamic whole-body PET imaging: Principles, potentials and applications. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 501–518. [Google Scholar] [CrossRef]
- Kaji, T.; Osanai, K.; Takahashi, A.; Kinoshita, A.; Satoh, D.; Nakata, T.; Tamaki, N. Improvement of motion artifacts using dynamic whole-body 18F-FDG PET/CT imaging. Jpn. J. Radiol. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.; Lee, D.E.; Liu, B.; Langevin, B.; Ordonez, A.A.; Dikeman, D.A.; Shafiq, B.; Thompson, J.M.; Sponseller, P.D.; Flavahan, K.; et al. Dynamic PET-facilitated modeling and high-dose rifampin regimens for Staphylococcus aureus orthopedic implant-associated infections. Sci. Transl. Med. 2021, 13, eabl6851. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.L.; Spencer, H.J.; Beenken, K.E.; Alpe, T.L.; Bartel, T.B.; Bellamy, W.; Gruenwald, J.M.; Skinner, R.A.; McLaren, S.G.; Smeltzer, M.S. Evaluation of dynamic [18F]-FDG-PET imaging for the detection of acute post-surgical bone infection. PLoS ONE 2012, 7, e41863. [Google Scholar] [CrossRef]
- Stecker, F.F.; Schierz, J.H.; Opfermann, T.; Driesch, D.; Hofmann, G.O.; Winkens, T.; Freesmeyer, M. Early dynamic 18F-FDG PET/CT to diagnose chronic osteomyelitis following lower extremity fractures. A pilot study. Nuklearmedizin 2014, 53, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Freesmeyer, M.; Stecker, F.F.; Schierz, J.H.; Hofmann, G.O.; Winkens, T. First experience with early dynamic (18)F-NaF-PET/CT in patients with chronic osteomyelitis. Ann. Nucl. Med. 2014, 28, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Cherry, S.R.; Jones, T.; Karp, J.S.; Qi, J.; Moses, W.W.; Badawi, R.D. Total-Body PET: Maximizing Sensitivity to Create New Opportunities for Clinical Research and Patient Care. J. Nucl. Med. 2018, 59, 3–12. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, P.; Pi, Y.; Jiang, L.; Zhong, X.; Cheng, J.; Xiang, Y.; Wei, J.; Li, L.; Yi, Z.; et al. Automatic identification of suspicious bone metastatic lesions in bone scintigraphy using convolutional neural network. BMC Med. Imaging 2021, 21, 131. [Google Scholar] [CrossRef]
- Cheng, D.C.; Hsieh, T.C.; Yen, K.Y.; Kao, C.H. Lesion-Based Bone Metastasis Detection in Chest Bone Scintigraphy Images of Prostate Cancer Patients Using Pre-Train, Negative Mining, and Deep Learning. Diagnostics 2021, 11, 518. [Google Scholar] [CrossRef]
- Liu, S.; Feng, M.; Qiao, T.; Cai, H.; Xu, K.; Yu, X.; Jiang, W.; Lv, Z.; Wang, Y.; Li, D. Deep Learning for the Automatic Diagnosis and Analysis of Bone Metastasis on Bone Scintigrams. Cancer Manag. Res. 2022, 14, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Y.; Wernick, M.N.; Pretorius, P.H.; King, M.A. Deep learning with noise-to-noise training for denoising in SPECT myocardial perfusion imaging. Med. Phys. 2021, 48, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Shiri, I.; AmirMozafari Sabet, K.; Arabi, H.; Pourkeshavarz, M.; Teimourian, B.; Ay, M.R.; Zaidi, H. Standard SPECT myocardial perfusion estimation from half-time acquisitions using deep convolutional residual neural networks. J. Nucl. Cardiol. 2021, 28, 2761–2779. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Qi, N.; Meng, Q.; Wang, J.; Peng, S.; Qi, C.; Gong, N.J.; Zhao, J. Ultra high speed SPECT bone imaging enabled by a deep learning enhancement method: A proof of concept. EJNMMI Phys. 2022, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Moon, J.B.; Cho, S.G.; Kim, J.; Song, H.C. Applying Pix2pix to Translate Hyperemia in Blood Pool Image into Corresponding Increased Bone Uptake in Delayed Image in Three-Phase Bone Scintigraphy. Nucl. Med. Mol. Imaging 2023, 57, 103–109. [Google Scholar] [CrossRef]
- Huang, K.; Huang, S.; Chen, G.; Li, X.; Li, S.; Liang, Y.; Gao, Y. An end-to-end multi-task system of automatic lesion detection and anatomical localization in whole-body bone scintigraphy by deep learning. Bioinformatics 2023, 39, btac753. [Google Scholar] [CrossRef] [PubMed]
- Hajianfar, G.; Sabouri, M.; Salimi, Y.; Amini, M.; Bagheri, S.; Jenabi, E.; Hekmat, S.; Maghsudi, M.; Mansouri, Z.; Khateri, M.; et al. Artificial intelligence-based analysis of whole-body bone scintigraphy: The quest for the optimal deep learning algorithm and comparison with human observer performance. Z. Med. Phys. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Petibon, Y.; Fahey, F.; Cao, X.; Levin, Z.; Sexton-Stallone, B.; Falone, A.; Zukotynski, K.; Kwatra, N.; Lim, R.; Bar-Sever, Z.; et al. Detecting lumbar lesions in 99m Tc-MDP SPECT by deep learning: Comparison with physicians. Med. Phys. 2021, 48, 4249–4261. [Google Scholar] [CrossRef]
- Shin, H.; Kong, E.; Yu, D.; Choi, G.S.; Jeon, I. Assessment of Therapeutic Responses Using a Deep Neural Network Based on 18F-FDG PET and Blood Inflammatory Markers in Pyogenic Vertebral Osteomyelitis. Medicina 2022, 58, 1693. [Google Scholar] [CrossRef]
- Mukaihata, T.; Maki, S.; Eguchi, Y.; Geundong, K.; Shoda, J.; Yokota, H.; Orita, S.; Shiga, Y.; Inage, K.; Furuya, T.; et al. Differentiating Magnetic Resonance Images of Pyogenic Spondylitis and Spinal Modic Change Using a Convolutional Neural Network. Spine 2023, 48, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, S.; Lee, Y.H.; Lee, S.H.; Lee, H.S.; Kim, S. Performance of the deep convolutional neural network based magnetic resonance image scoring algorithm for differentiating between tuberculous and pyogenic spondylitis. Sci. Rep. 2018, 8, 13124. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Chen, X.; Man, F. Imaging Manifestations and Evaluation of Postoperative Complications of Bone and Joint Infections under Deep Learning. J. Healthc. Eng. 2021, 2021, 6112671. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valero-Martínez, C.; Castillo-Morales, V.; Gómez-León, N.; Hernández-Pérez, I.; Vicente-Rabaneda, E.F.; Uriarte, M.; Castañeda, S. Application of Nuclear Medicine Techniques in Musculoskeletal Infection: Current Trends and Future Prospects. J. Clin. Med. 2024, 13, 1058. https://doi.org/10.3390/jcm13041058
Valero-Martínez C, Castillo-Morales V, Gómez-León N, Hernández-Pérez I, Vicente-Rabaneda EF, Uriarte M, Castañeda S. Application of Nuclear Medicine Techniques in Musculoskeletal Infection: Current Trends and Future Prospects. Journal of Clinical Medicine. 2024; 13(4):1058. https://doi.org/10.3390/jcm13041058
Chicago/Turabian StyleValero-Martínez, Cristina, Valentina Castillo-Morales, Nieves Gómez-León, Isabel Hernández-Pérez, Esther F. Vicente-Rabaneda, Miren Uriarte, and Santos Castañeda. 2024. "Application of Nuclear Medicine Techniques in Musculoskeletal Infection: Current Trends and Future Prospects" Journal of Clinical Medicine 13, no. 4: 1058. https://doi.org/10.3390/jcm13041058
APA StyleValero-Martínez, C., Castillo-Morales, V., Gómez-León, N., Hernández-Pérez, I., Vicente-Rabaneda, E. F., Uriarte, M., & Castañeda, S. (2024). Application of Nuclear Medicine Techniques in Musculoskeletal Infection: Current Trends and Future Prospects. Journal of Clinical Medicine, 13(4), 1058. https://doi.org/10.3390/jcm13041058








