Bridging the Gap: Exploring Bronchopulmonary Dysplasia through the Lens of Biomedical Informatics
Abstract
:1. Introduction
2. Overview of Biomedical Informatics
2.1. Key Concepts and Principles
2.2. Role of Biomedical Informatics in Healthcare and Research
3. Navigating Challenges in BPD Research
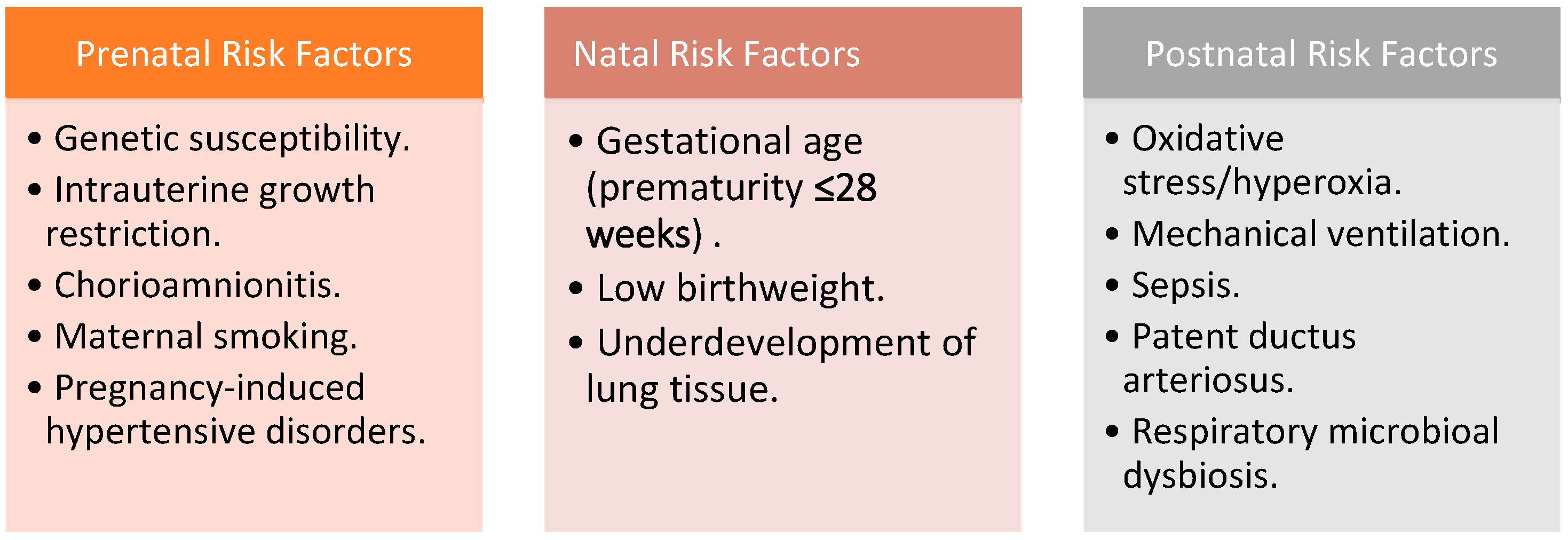
3.1. Unraveling Risk Factors and Causes
- Antenatal factors: These encompass genetic susceptibility, intrauterine growth restriction (IUGR), chorioamnionitis, maternal smoking, and pregnancy-induced hypertensive disorders. While chorioamnionitis itself is not a standalone BPD risk factor, its consequential sequelae, notably sepsis, may elevate the likelihood of BPD development [18]. Pregnancy-induced hypertensive disorders, including gestational hypertension, preeclampsia, and eclampsia, are associated with BPD due to their connection with IUGR [19].
- Natal factors: These chiefly revolve around gestational age and birth weight [17].
- Postnatal factors: BPD’s postnatal milieu includes oxidative stress and hyperoxia, mechanical ventilation, sepsis, patent ductus arteriosus (PDA), and respiratory microbial dysbiosis [17]. Hyperoxic oxidative stress is a well-known risk factor, stemming from the generation of free radicals that culminate in endothelial damage and tissue disruption, ultimately affecting the pulmonary alveolar-capillary membrane [20]. Volume-induced lung injury (VILI) is another significant risk factor, resulting from barotrauma associated with prolonged mechanical ventilation, leading to surfactant dysfunction and regional hypoxia [21]. Hemodynamically significant PDA has also been linked to BPD development, driven by increased pulmonary blood flow, heightened ventilator demands, and exacerbated lung inflammation [22,23].
3.2. Current Diagnostic and Treatment Approaches
3.3. Unveiling Research Frontiers in BPD
- Categorization and diagnosis: A profound need persists for refined methods of categorizing and diagnosing BPD. This entails delving deeper into its subtypes and associated clinical markers.
- Risk factors: A thorough investigation of contributing risk factors is essential. This encompasses a multifaceted analysis, spanning antenatal, natal, and postnatal factors, to decipher the intricate interplay leading to BPD.
- Delivery room interventions: Unanswered questions abound in the domain of delivery room interventions. Innovative approaches such as noninvasive surfactant administration techniques, cord clamping vs. cord milking, and the complexities surrounding antenatal consent warrant rigorous examination.
- NICU management: The neonatal intensive care unit (NICU) presents a rich landscape for exploration. The role of novel therapies, the identification of BPD susceptibility in the postnatal period, and the establishment of optimal fluid and electrolyte targets demand meticulous investigation.
- Pathological lung samples: An in-depth analysis of pathological lung samples offers invaluable insights into the underlying mechanisms of BPD. This avenue calls for comprehensive studies to unravel the cellular and molecular intricacies within afflicted lungs.
- Outpatient follow-up: Given the chronic complications faced by BPD patients, the optimization of treatment strategies in the outpatient follow-up setting is imperative. Long-term care protocols should be meticulously crafted to enhance patient outcomes and quality of life.
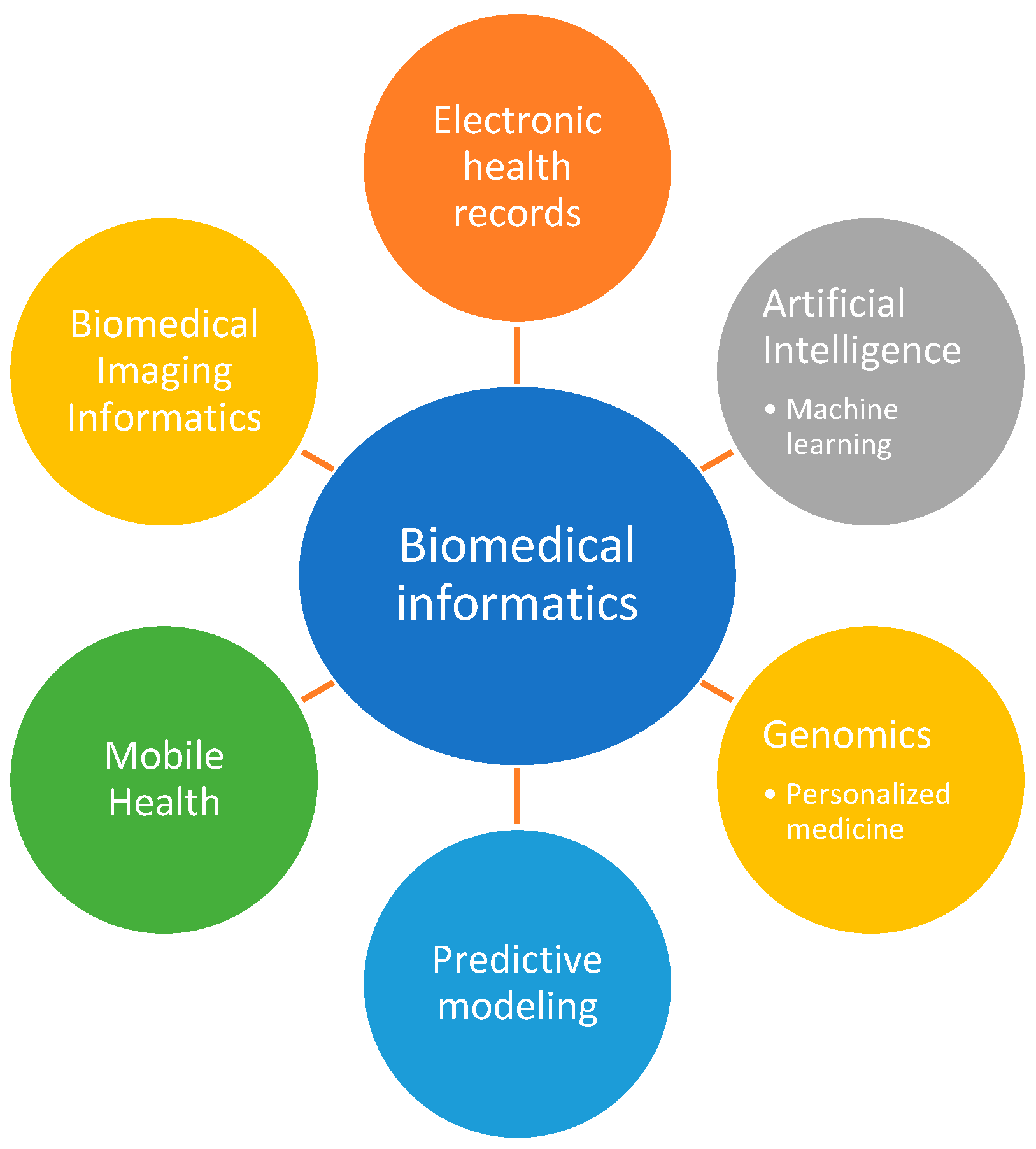
4. Biomedical Informatics Approaches for BPD Research
4.1. Data Collection and Management from Electronic Health Records
4.2. Machine Learning and Artificial Intelligence: Transforming Medicine
4.3. Genomics and Personalized Medicine
4.4. Predictive Modeling and Simulation
4.5. Mobile Health
4.6. Biomedical Imaging Informatics
5. Clinical Tangibles of Biomedical Informatics
- Early diagnosis and risk stratification: Biomedical informatics enables the development of predictive models that can identify infants at the highest risk of developing BPD. By leveraging clinical data, genetic markers, and imaging, these models can provide early warnings to healthcare providers, allowing for targeted monitoring and interventions.
- Severity prediction: One of the primary objectives is to predict the severity of BPD in affected infants accurately. Biomedical informatics can aid in assessing the extent of lung damage, the need for respiratory support, and the duration of oxygen therapy. These predictions are invaluable in tailoring treatment strategies and resource allocation in neonatal intensive care units.
- Personalized treatment plans: Biomedical informatics can assist in tailoring treatment plans for individual patients based on their unique clinical profiles and genetic markers. Personalized interventions can help optimize outcomes and minimize potential complications associated with standard treatments.
- Long-term outcome prediction: Beyond the neonatal period, biomedical informatics can also contribute to predicting long-term outcomes in infants with BPD. This includes assessing the risk of chronic respiratory conditions, neurodevelopmental disorders, and cardiovascular complications and providing families and clinicians with valuable information for ongoing care.
- Identification of therapeutic targets: Biomedical informatics can aid in identifying potential therapeutic targets for BPD. By analyzing molecular pathways and genetic factors associated with the condition, researchers can pinpoint novel interventions and drug candidates that have the potential to mitigate lung damage and improve outcomes.
- Mobile health integration: Biomedical informatics aims to seamlessly integrate mobile health technologies and data into the management of BPD. This includes the development of smartphone applications, wearable devices, and remote monitoring systems that allow real-time tracking of vital signs, respiratory patterns, and other relevant health metrics in infants with BPD. The aim would be to provide both healthcare providers and caregivers with accessible, real-time data, enabling early detection of deteriorating health and facilitating timely interventions.
- Enhanced clinical decision support: Biomedical informatics seeks to improve the accuracy and effectiveness of clinical decisions related to BPD through the integration of advanced decision support systems within EHRs. These systems utilize patient-specific data, including medical history, diagnostic results, and treatment plans, to provide evidence-based recommendations to healthcare providers. The purpose would be to reduce diagnostic errors and optimize treatment strategies.
6. Successful Applications of Bioinformatics in BPD Research
6.1. Benefits and Limitations of Biomedical Informatics
6.2. Potential for Future Research and Development
7. Discussion
7.1. Ethical and Legal Issues Related to Biomedical Informatics in BPD Research
7.2. Future Directions for BPD Research Using Biomedical Informatics
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Poets, C.F.; Lorenz, L. Prevention of bronchopulmonary dysplasia in extremely low gestational age neonates: Current evidence. Arch. Dis. Child.—Fetal Neonatal Ed. 2018, 103, F285–F291. [Google Scholar] [CrossRef]
- Jensen, E.A.; Schmidt, B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res. Part A Clin. Mol. Teratol. 2014, 100, 145–157. [Google Scholar] [CrossRef]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef]
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef]
- Botet, F.; Figueras-Aloy, J.; Miracle-Echegoyen, X.; Rodríguez-Miguélez, J.M.; Salvia-Roiges, M.; Carbonell-Estrany, X. Trends in survival among extremely-low-birth-weight infants (less than 1000 g) without significant bronchopulmonary dysplasia. BMC Pediatr. 2012, 12, 63. [Google Scholar] [CrossRef]
- Zhou, W.; Shao, F.; Li, J. Bioinformatic analysis of the molecular mechanism underlying bronchial pulmonary dysplasia using a text mining approach. Medicine 2019, 98, e18493. [Google Scholar] [CrossRef] [PubMed]
- AHIMA Facts. 2007. Available online: http://www.ahima.org/about/about.asp (accessed on 17 December 2023).
- Hersh, W.R. Chapter 1: Introduction to Biomedical and Health Informatics. In Health Informatics: Practical Guide, 8th ed.; Lulu Press, Inc.: Morrisville, NC, USA, 2022. [Google Scholar]
- Bernstam, E.V.; Smith, J.W.; Johnson, T.R. What is biomedical informatics? J. Biomed. Inform. 2010, 43, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Payne, P.R.O.; Johnson, S.B.; Starren, J.B.; Tilson, H.H.; Dowdy, D. Breaking the Translational Barriers: The Value of Integrating Biomedical Informatics and Translational Research. J. Investig. Med. 2005, 53, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Embi, P.J.; Kaufman, S.E.; Payne, P.R.O. Biomedical Informatics and Outcomes Research: Enabling Knowledge-driven Healthcare. Circulation 2009, 120, 2393–2399. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Aschner, J.L. The Natural History of Bronchopulmonary Dysplasia (BPD): The Case for Primary Prevention. Clin. Perinatol. 2015, 42, 911–931. [Google Scholar] [CrossRef] [PubMed]
- Jobe, A.H. The new bronchopulmonary dysplasia. Curr. Opin. Pediatr. 2011, 23, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Schittny, J.C. Development of the lung. Cell Tissue Res. 2017, 367, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Morrow, L.A.; Wagner, B.D.; Ingram, D.A.; Poindexter, B.B.; Schibler, K.; Cotten, C.M.; Dagle, J.; Sontag, M.K.; Mourani, P.M.; Abman, S.H. Antenatal Determinants of Bronchopulmonary Dysplasia and Late Respiratory Disease in Preterm Infants. Am. J. Respir. Crit. Care Med. 2017, 196, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Thekkeveedu, R.K.; Guaman, M.C.; Shivanna, B. Bronchopulmonary dysplasia: A review of pathogenesis and pathophysiology. Respir. Med. 2017, 132, 170–177. [Google Scholar] [CrossRef]
- Ballard, A.R.; Mallett, L.H.; Pruszynski, J.E.; Cantey, J.B. Chorioamnionitis and subsequent bronchopulmonary dysplasia in very-low-birth weight infants: A 25-year cohort. J. Perinatol. 2016, 36, 1045–1048. [Google Scholar] [CrossRef]
- Torchin, H.; Ancel, P.-Y.; Goffinet, F.; Hascoët, J.-M.; Truffert, P.; Tran, D.; Lebeaux, C.; Jarreau, P.-H. Placental complications and bronchopulmonary dysplasia: Epipage-2 cohort study. Pediatrics 2016, 137, e20152163. [Google Scholar] [CrossRef]
- Kulkarni, A.C.; Kuppusamy, P.; Parinandi, N. Oxygen, the lead actor in the pathophysiologic drama: Enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxidants Redox Signal. 2007, 9, 1717–1730. [Google Scholar] [CrossRef]
- Bouhuys, A. Physiology and musical instruments. Nature 1969, 221, 1199–1204. [Google Scholar] [CrossRef]
- Slaughter, J.L.; Reagan, P.B.; Newman, T.B.; Klebanoff, M.A. Comparative effectiveness of nonsteroidal anti-inflammatory drug treatment vs no treatment for patent ductus arteriosus in preterm infants. JAMA Pediatr. 2017, 171, e164354. [Google Scholar] [CrossRef]
- Gerhardt, T.; Bancalari, E. Lung Compliance in newborns with patent ductus arteriosus before and after surgical ligation. Neonatology 1980, 38, 96–105. [Google Scholar] [CrossRef]
- Álvarez-Fuente, M.; Moreno, L.; Mitchell, J.A.; Reiss, I.K.; Lopez, P.; Elorza, D.; Duijts, L.; Avila-Alvarez, A.; Arruza, L.; Orellana, M.R.; et al. Preventing bronchopulmonary dysplasia: New tools for an old challenge. Pediatr. Res. 2018, 85, 432–441. [Google Scholar] [CrossRef]
- Xie, F.; Yuan, H.; Ning, Y.; Ong, M.E.H.; Feng, M.; Hsu, W.; Chakraborty, B.; Liu, N. Deep learning for temporal data representation in electronic health records: A systematic review of challenges and methodologies. J. Biomed. Inform. 2022, 126, 103980. [Google Scholar] [CrossRef]
- Fennelly, O.; Grogan, L.; Reed, A.; Hardiker, N.R. Use of standardized terminologies in clinical practice: A scoping review. Int. J. Med. Inform. 2021, 149, 104431. [Google Scholar] [CrossRef]
- Tapuria, A.; Porat, T.; Kalra, D.; Dsouza, G.; Xiaohui, S.; Curcin, V. Impact of patient access to their electronic health record: Systematic review. Inform. Health Soc. Care 2021, 46, 192–204. [Google Scholar] [CrossRef]
- Uslu, A.; Stausberg, J. Value of the Electronic Medical Record for Hospital Care: Update from the Literature. J. Med. Internet Res. 2021, 23, e26323. [Google Scholar] [CrossRef]
- Haenlein, M.; Kaplan, A. A Brief History of Artificial Intelligence: On the Past, Present, and Future of Artificial Intelligence. Calif. Manag. Rev. 2019, 61, 5–14. [Google Scholar] [CrossRef]
- Dwivedi, K.; Sharkey, M.; Condliffe, R.; Uthoff, J.M.; Alabed, S.; Metherall, P.; Lu, H.; Wild, J.M.; Hoffman, E.A.; Swift, A.J.; et al. Pulmonary Hypertension in Association with Lung Disease: Quantitative CT and Artificial Intelligence to the Rescue? State-of-the-Art Review. Diagnostics 2021, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Verder, H.; Heiring, C.; Ramanathan, R.; Scoutaris, N.; Verder, P.; Jessen, T.E.; Höskuldsson, A.; Bender, L.; Dahl, M.; Eschen, C.; et al. Bronchopulmonary dysplasia predicted at birth by artificial intelligence. Acta Paediatr. 2021, 110, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, T.; Jiang, Y.; Wu, P.; Fu, J.; Zhang, T.; McGrath, E. Risk Identification of Bronchopulmonary Dysplasia in Premature Infants Based on Machine Learning. Front. Pediatr. 2021, 9, 719352. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Chen, H.; Dong, X.; Chen, J.; Mei, M.; Lu, Y.; Yang, L.; Wu, B.; Cao, Y.; Wang, J.; et al. Bronchopulmonary Dysplasia Predicted by Developing a Machine Learning Model of Genetic and Clinical Information. Front. Genet. 2021, 12, 689071. [Google Scholar] [CrossRef]
- Morag, I.; Barkai, E.; Wazana, Y.; Elizur, A.; Stern, O.L.; Staretz-Chacham, O.; Pinchevski-Kadir, S.; Shlomai, N.O. Predictors of Developmental and Respiratory Outcomes Among Preterm Infants with Bronchopulmonary Dysplasia. Front. Pediatr. 2021, 9, 780518. [Google Scholar] [CrossRef]
- Ochab, M.; Wajs, W. Expert system supporting an early prediction of the bronchopulmonary dysplasia. Comput. Biol. Med. 2016, 69, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Julien, K.R.S.; Stevenson, D.K.; Hoffmann, T.J.; Witte, J.S.; Lazzeroni, L.C.; Krasnow, M.A.; Quaintance, C.C.; Oehlert, J.W.; Jelliffe-Pawlowski, L.L.; et al. A genome-wide association study (GWAS) for bronchopulmonary dysplasia. Pediatrics 2013, 132, 290–297. [Google Scholar] [CrossRef]
- Gentle, S.J.; Lal, C.V. Predicting BPD: Lessons Learned from the Airway Microbiome of Preterm Infants. Front. Pediatr. 2020, 7, 564. [Google Scholar] [CrossRef]
- Pais, R.J. Predictive modelling in clinical bioinformatics: Key concepts for startups. BioTech 2022, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Somvanshi, P.R.; Venkatesh, K.V. A conceptual review on Systems Biology in health and diseases: From biological networks to Modern Therapeutics. Syst. Synth. Biol. 2013, 8, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Le Novère, N. Quantitative and logic modelling of Molecular and Gene Networks. Nat. Rev. Genet. 2015, 16, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.J.; Taveira, N. Predicting the evolution and control of the COVID-19 pandemic in Portugal. F1000Research 2020, 9, 283. [Google Scholar] [CrossRef]
- Murray, C.J. Forecasting COVID-19 impact on hospital bed-days, ICU-days, ventilator-days and deaths by US state in the next 4 months. medRxiv 2020. [Google Scholar] [CrossRef]
- Kucharski, A.J.; Russell, T.W.; Diamond, C.; Liu, Y.; Edmunds, J.; Funk, S.; Eggo, R.M.; Sun, F.; Jit, M.; Munday, J.D.; et al. Early dynamics of transmission and control of COVID-19: A mathematical modelling study. Lancet Infect. Dis. 2020, 20, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Tovar, M.; Smith, A.M.; Lee, G.C.; Meunier, J.A.; Cheema, Z.; Moreira, A.; Winter, C.; Mustafa, S.B.; Seidner, S.; et al. Development of a peripheral blood transcriptomic gene signature to predict bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 324, L76–L87. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.G.; Arora, T.; Arya, S.; Winter, C.; Valadie, C.T.; Kwinta, P. Leveraging transcriptomics to develop bronchopulmonary dysplasia endotypes: A concept paper. Respir. Res. 2023, 24, 284. [Google Scholar] [CrossRef] [PubMed]
- Istepanian, R.S.H. Mobile Health (m-Health) in retrospect: The known unknowns. Int. J. Environ. Res. Public Health 2022, 19, 3747. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S. Book Review: mHealth: New Horizons for Health through Mobile Technologies: Based on the Findings of the Second Global Survey on eHealth (Global Observatory for eHealth Series, Volume 3). Health Inform. Res. 2012, 18, 231. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Global Health Matters Newsletter—Fogarty International Center @ NIH. Fogarty International Center. 2023. Available online: https://www.fic.nih.gov/News/GlobalHealthMatters (accessed on 17 April 2023).
- Xing, W.; He, W.; Li, X.; Chen, J.; Cao, Y.; Zhou, W.; Shen, Q.; Zhang, X.; Ta, D. Early severity prediction of BPD for premature infants from chest X-ray images using deep learning: A study at the 28th day of oxygen inhalation. Comput. Methods Programs Biomed. 2022, 221, 106869. [Google Scholar] [CrossRef]
- Chen, J.; Huang, S.; Zhang, Y.; Chang, Q.; Zhang, Y.; Li, D.; Qiu, J.; Hu, L.; Peng, X.; Du, Y.; et al. Congenital heart disease detection by pediatric electrocardiogram based deep learning integrated with human concepts. Nat. Commun. 2024, 15, 976. [Google Scholar] [CrossRef]
- Lavilla, O.C.; Aziz, K.B.; Lure, A.C.; Gipson, D.; de la Cruz, D.; Wynn, J.L. Hourly Kinetics of Critical Organ Dysfunction in Extremely Preterm Infants. Am. J. Respir. Crit. Care Med. 2022, 205, 75–87. [Google Scholar] [CrossRef]
- Hum, R.S.; Cato, K.; Sheehan, B.; Patel, S.; Duchon, J.; DeLaMora, P.; Ferng, Y.; Graham, P.; Vawdrey, D.; Perlman, J.; et al. Developing clinical decision support within a commercial electronic health record system to improve antimicrobial prescribing in the neonatal ICU. Appl. Clin. Inform. 2014, 05, 368–387. [Google Scholar] [CrossRef]
- Campbell, J.P.; Chiang, M.F.; Chen, J.S.; Moshfeghi, D.M.; Nudleman, E.; Ruambivoonsuk, P.; Cherwek, H.; Cheung, C.Y.; Singh, P.; Kalpathy-Cramer, J.; et al. Artificial Intelligence for Retinopathy of Prematurity: Validation of a Vascular Severity Scale against International Expert Diagnosis. Ophthalmology 2022, 129, e69–e76. [Google Scholar] [CrossRef]
- Das, A.; Ariyakumar, G.; Gupta, N.; Kamdar, S.; Barugahare, A.; Deveson-Lucas, D.; Gee, S.; Costeloe, K.; Davey, M.S.; Fleming, P.; et al. Identifying immune signatures of sepsis to increase diagnostic accuracy in very preterm babies. Nat. Commun. 2024, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.G.; McDonald, S.A.; Laughon, M.M.; Tanaka, D.; Jensen, E.; Van Meurs, K.; Eichenwald, E.; Brumbaugh, J.E.; Duncan, A.; Walsh, M.; et al. Online clinical tool to estimate risk of bronchopulmonary dysplasia in extremely preterm infants. Arch. Dis. Child.—Fetal Neonatal Ed. 2022, 107, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, K.-H.; Oehlert, J.; Jeliffe-Pawlowski, L.L.; Gould, J.B.; Stevenson, D.K.; Snyder, M.; Shaw, G.M.; O’brodovich, H.M. Exome sequencing of neonatal blood spots and the identification of genes implicated in bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2015, 192, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Cuna, A.; Halloran, B.; Faye-Petersen, O.; Kelly, D.; Crossman, D.; Cui, X.; Pandit, K.; Kaminski, N.; Bhattacharya, S.; Ahmad, A.; et al. Alterations in gene expression and DNA methylation during murine and human lung alveolar septation. Pediatrics 2016, 137 (Suppl. 3), 434. [Google Scholar] [CrossRef]
- Bammler, T.; Beyer, R.P.; Bhattacharya, S.; Boorman G., A.; Boyles, A.; Bradford, B.U.; Bumgarner, R.E.; Bushel, P.R.; Chaturvedi, K.; Choi, D.; et al. Standardizing global gene expression analysis between laboratories and across platforms. Nat. Methods 2005, 2, 351–356, Correction in Nat. Methods 2005, 2, 477. [Google Scholar] [CrossRef] [PubMed]
- Moreau, Y.; Aerts, S.; De Moor, B.; De Strooper, B.; Dabrowski, M. Comparison and meta-analysis of microarray data: From the bench to the computer desk. Trends Genet. 2003, 19, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Floridi, L. The method of levels of abstraction. In The Philosophy of Information; Oxford Academic: Oxford, UK, 2011; pp. 46–79. [Google Scholar] [CrossRef]
- Johnson, T.R.; Bernstam, E.V. Why is biomedical informatics hard? A fundamental framework. J. Biomed. Inform. 2023, 140, 104327. [Google Scholar] [CrossRef] [PubMed]
- Shortliffe, E.H.; Cimino, J.J. (Eds.) The Future of Biomedical Informatics: Bottlenecks and Opportunities. In Biomedical Informatics: Computer Applications in Health Care and Biomedicine; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Goodman, K.W. Ethics in Health Informatics. Yearb. Med. Inform. 2020, 29, 26–31. [Google Scholar] [CrossRef]
- Manca, D.P. Do electronic medical records improve quality of care? Yes. Can. Fam. Physician 2015, 61, 846–851. [Google Scholar]
- Phillips, W. Ethical controversies about proper health informatics practices. Mo. Med. 2015, 112, 53–57. [Google Scholar]
- Payne, P.R.O.; Bernstam, E.V.; Starren, J.B. Biomedical informatics meets data science: Current state and future directions for interaction. JAMIA Open 2018, 1, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Wiens, J.; Guttag, J.; Horvitz, E. A study in Transfer learning: Leveraging data from multiple hospitals to enhance hospital-specific predictions. J. Am. Med. Inform. Assoc. 2014, 21, 699–706. [Google Scholar] [CrossRef] [PubMed]

| Author(s) | Geographic Location | Year of Publication | No. of Neonates Involved in the Study | Inclusion Criteria | Artificial Intelligence Model Used | Overall Outcome |
|---|---|---|---|---|---|---|
| Verder et al. [31] | Denmark | 2021 | 61 premature infants: 26 with BPD 35 without BPD | Infants born prematurely who were between 24 and 31 gestational weeks (previously enrolled in a past observational study by authors). | Support Vector Machine (SVM) | Authors predicted BPD in premature infants using surfactant treatment, spectral data from samples of gastric aspirate, birth weight, and gestational age. The specificity and sensitivity of the test were 91% and 88%, respectively. Compared to other diagnostic tests, the test developed by the authors provides results more quickly, allowing for treatment to begin as soon as possible. |
| Lei et al. [32] | Western China | 2021 | 648 premature infants: 149 with BPD 499 without BPD | Infants with congenital anomalies, premature infants that died, and premature infants with incomplete data before diagnosis of BPD were excluded from the study. | Random Forest | The authors formulated a model using six variables to predict BPD (area under the receiver operating curve [AUC] of 0.929). The variables used included total oxygen inhalation time, first PCO2, first MAP (mean airway pressure), gestational age, gross birth weight, and first FIO2. |
| Dai et al. [33] | Shanghai, China | 2021 | 245 premature infants: 131 with BPD 114 without BPD | Premature infants (<32 weeks gestational age) who required supplemental oxygen on their first day of post-natal life and were admitted to the NICU. Those with significant diseases, those that died, and those that refused to participate/undergo sequencing were excluded from the study. | Unsupervised Machine Learning and Least Absolute Shrinkage and Selection Operator | Using machine learning, the authors built predictive models that incorporated clinical and genetic data (from identified risk genes). They were successful in predicting both BPD and severe forms of BPD (AUC 0.915 and AUC 0.907, respectively). The accuracy of these models exceeded that of models only incorporating clinical data. |
| Morag et al. [34] | Israel | 2021 | 208 premature infants: 40 with mild BPD, 16 with moderate-severe 152 without BPD | Infants born prematurely between January 2012 and August 2015 and completed a follow-up exam. Those with major congenital anomalies were excluded from the study. | Random forest, logistic regression classifier, gradient boosting classifier, XGBoost, and ExtraTree classifier | The authors used machine learning to assess the impact of several environmental factors on inhaler use in children who were born with BPD. Frequency of inhaler use served as a measure of long-term respiratory outcomes for these individuals. Inhaler use was more significant (p= 0.0014) in those with a greater number of risk factors and moderate-severe BPD. The identification of these factors (cigarette smoking, allergies, etc.) could allow for early intervention by the doctor and parents to limit exposure. |
| Ochab and Wajs [35] | Poland | 2016 | 109 premature infants: 46 diagnosed with BPD after the fourth week of life | Infants born prematurely that weighed less than or equal to 1500 g and were admitted into the NICU by their second day of life. | Support Vector Machine | The authors developed an expert system that allowed for the sequential inclusion of clinical parameters (presence of patent ductus arteriosus, birth weight, etc.) to yield a model capable of producing the most accurate prediction of BPD. They compared both logistic regression and SVM models and found that models that incorporated more than 7 parameters had more accuracy using an SVM approach (accuracy of 83.29%) |
| Author | Publication Year | PMID | Objective | Theme | Outcome |
|---|---|---|---|---|---|
| Lavilla et al. [51] | 2021 | 34550843 | Measure organ dysfunction changes by gestational age and among extremely preterm infants | Descriptive | Neonatal sequential organ failure assessment scores, calculated hourly, effectively discriminated between survival and non-survival from the first day of life. |
| Hum et al. [52] | 2014 | 25024755 | Develop and implement a clinical decision support (CDS) tool aimed at enhancing antibiotic prescribing in the NICU | CDS | The CDS tool was activated for 22% of patients prescribed antibiotics. Summarized culture results and antibiotic recommendations were described as the most useful features of the tool. Widespread use was hindered by multiple systemic changes (e.g., new EHR, changes to antimicrobial testing). |
| Campbell et al. [53] | 2022 | 35157950 | Validate an AI software to detect retinopathy of prematurity (ROP) | Digital imaging | Strong correlation between the ROP vascular severity score and diagnosis of stage among ophthalmologists. |
| Das et al. [54] | 2024 | 38195661 | Profile the blood of very preterm babies during episodes of sepsis and identify immune signatures | Bioinformatics | Amphiregulin (AREG), gene involved in tissue repair, was identified as a gene that becomes dysregulated in neonates with bacterial sepsis. |
| Greenberg et al. [55] | 2022 | 35728925 | Create an accurate online estimator for predicting bronchopulmonary dysplasia BPD or death | Predictive | Authors developed multinomial regression models and translated them into a web-based tool for estimating BPD risk in extremely preterm infants. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Villarreal, M.; Arya, S.; Hernandez, A.; Moreira, A. Bridging the Gap: Exploring Bronchopulmonary Dysplasia through the Lens of Biomedical Informatics. J. Clin. Med. 2024, 13, 1077. https://doi.org/10.3390/jcm13041077
Kim J, Villarreal M, Arya S, Hernandez A, Moreira A. Bridging the Gap: Exploring Bronchopulmonary Dysplasia through the Lens of Biomedical Informatics. Journal of Clinical Medicine. 2024; 13(4):1077. https://doi.org/10.3390/jcm13041077
Chicago/Turabian StyleKim, Jennifer, Mariela Villarreal, Shreyas Arya, Antonio Hernandez, and Alvaro Moreira. 2024. "Bridging the Gap: Exploring Bronchopulmonary Dysplasia through the Lens of Biomedical Informatics" Journal of Clinical Medicine 13, no. 4: 1077. https://doi.org/10.3390/jcm13041077





