Heparin-Mediated Extracorporeal Low-Density Lipoprotein Precipitation Apheresis for Treating Peripheral Arterial Disease in Patients with Chronic Kidney Disease
Abstract
1. Introduction
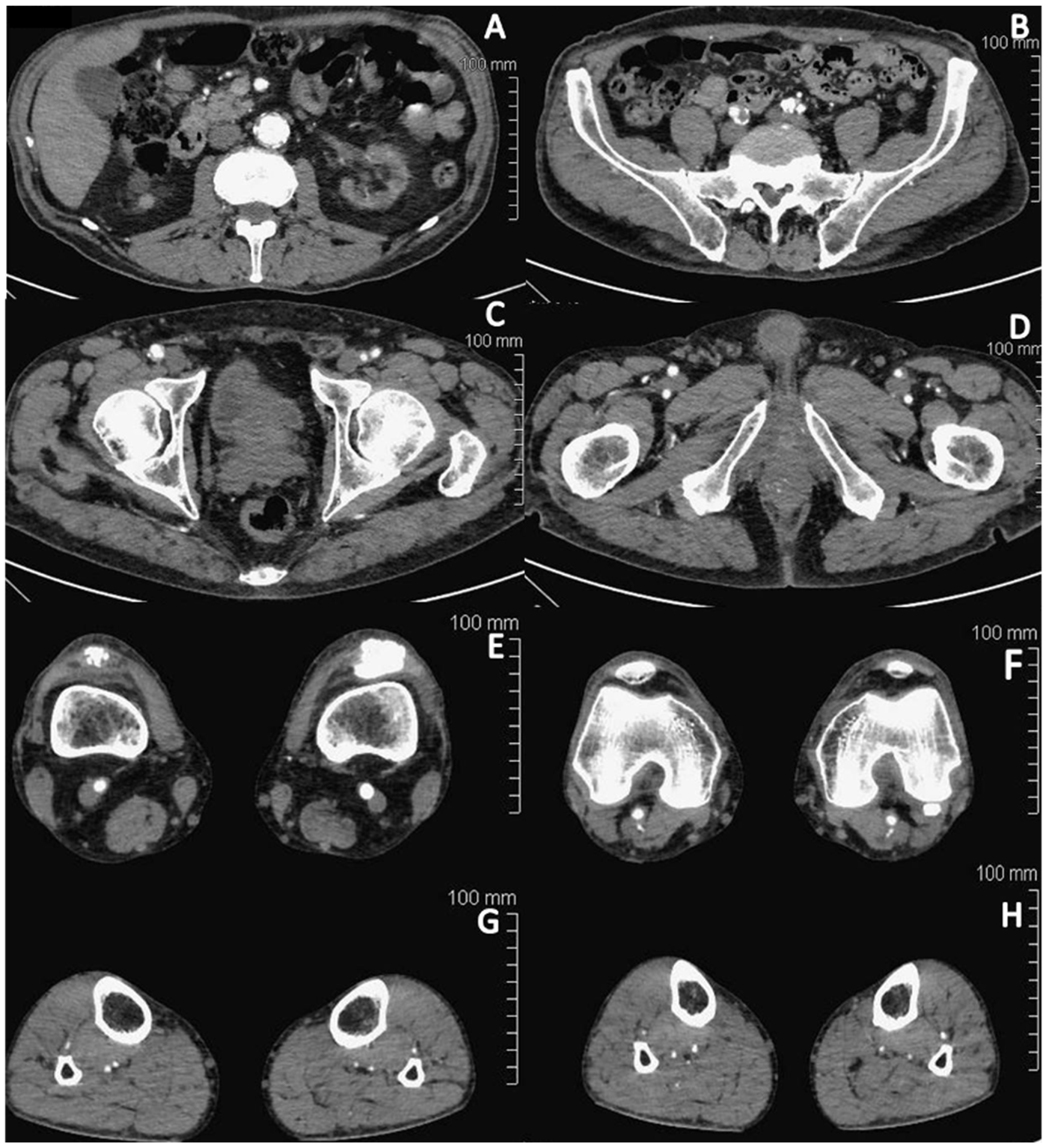
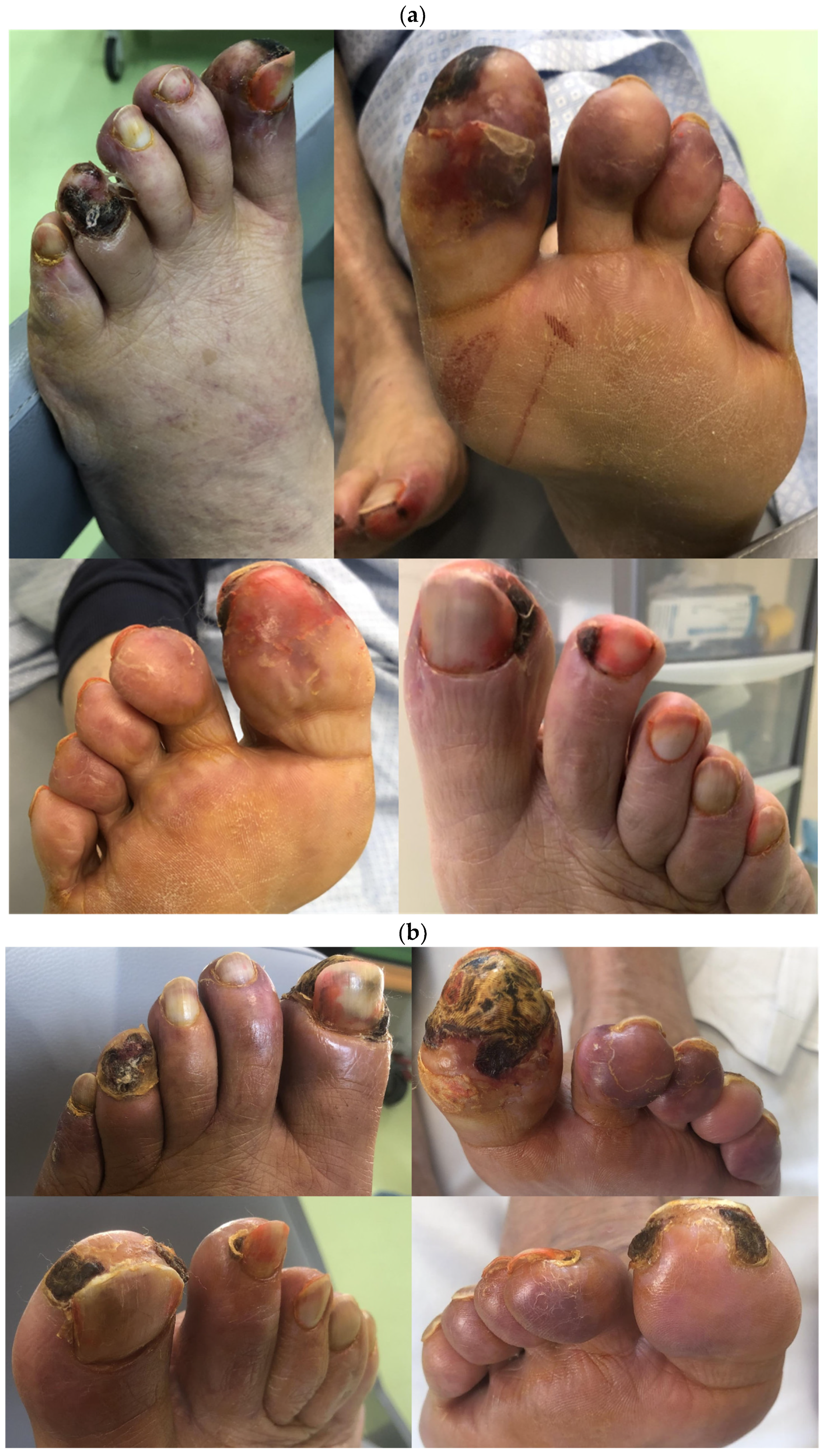
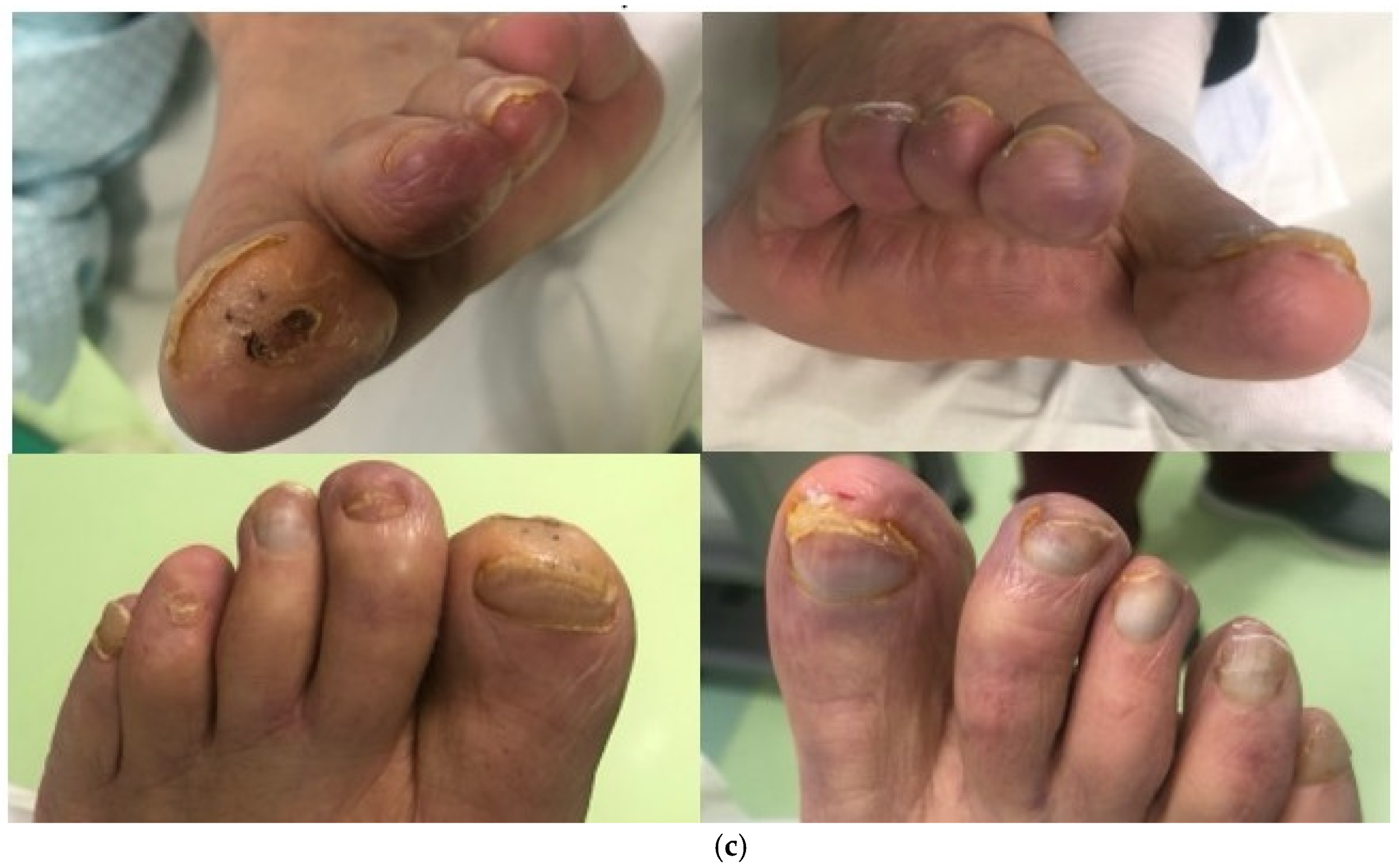
2. Case Report
3. Discussion
3.1. Atherosclerosis and Lipoprotein Apheresis
3.2. Oxidative Stress and LDL Apheresis
3.3. Endothelial Dysfunction and LDL Apheresis
3.4. LDL Apheresis Technique for PAD
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ix, J.H.; Shlipak, M.G.; Liu, H.H.; Schiller, N.B.; Whooley, M.A. Association between renal insufficiency and inducible ischemia in patients with coronary artery disease: The heart and soul study. J. Am. Soc. Nephrol. 2003, 14, 3233–3238. [Google Scholar] [CrossRef]
- Drey, N.; Roderick, P.; Mullee, M.; Rogerson, M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am. J. Kidney Dis. 2003, 42, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Garimella, P.S.; Hart, P.D.; O’Hare, A.; DeLoach, S.; Herzog, C.A.; Hirsch, A.T. Peripheral artery disease and CKD: A focus on peripheral artery disease as a critical component of CKD care. Am. J. Kidney Dis. 2012, 60, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J. Update on the pathophysiology and medical treatment of peripheral artery disease. Nat. Rev. Cardiol. 2022, 19, 456–474. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.T.; Criqui, M.H.; Treat-Jacobson, D.; Regensteiner, J.G.; Creager, M.A.; Olin, J.W.; Krook, S.H.; Hunninghake, D.B.; Comerota, A.J.; Walsh, M.E.; et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001, 286, 1317–1324. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Dellegrottaglie, S.; Furniss, A.L.; Gillespie, B.W.; Satayathum, S.; Lameire, N.; Saito, A.; Akiba, T.; Jadoul, M.; Ginsberg, N.; et al. Peripheral arterial disease in patients with end-stage renal disease: Observations from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Circulation 2006, 114, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Ouriel, K. Peripheral arterial disease. Lancet 2001, 358, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Agishi, T.; Kitano, Y.; Suzuki, T.; Miura, A.; Murakami, J.; Minagawa, H.; Kimio, B. Improvement of peripheral circulation by low density lipoprotein adsorption. ASAIO Trans. 1989, 35, 349–351. [Google Scholar]
- Hovland, A.; Lappegard, K.T.; Mollnes, T.E. LDL apheresis and inflammation—Implications for atherosclerosis. Scand. J. Immunol. 2012, 76, 229–236. [Google Scholar] [CrossRef]
- Inoue, I.; Takahashi, K.; Kikuchi, C.; Katayama, S. LDL apheresis reduces the susceptibility of LDL to in vitro oxidation in a diabetic patient with hemodialysis treatment. Diabetes Care 1996, 19, 1103–1107. [Google Scholar] [CrossRef]
- Wang, Y.; Blessing, F.; Walli, A.K.; Uberfuhr, P.; Fraunberger, P.; Seidel, D. Effects of heparin-mediated extracorporeal low-density lipoprotein precipitation beyond lowering proatherogenic lipoproteins–reduction of circulating proinflammatory and procoagulatory markers. Atherosclerosis 2004, 175, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Blessing, F.; Wang, Y.; Nagel, D.; Seidel, D. The efficacy and safety of the new heparin-induced extracorporeal low-density lipoprotein precipitation system (Plasmat Futura) in comparison with the currently used system (Plasmat Secura). Ther. Apher. Dial. 2004, 8, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Cho, L. Peripheral arterial disease: Considerations in risks, diagnosis, and treatment. J. Natl. Med. Assoc. 2009, 101, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Connelly-Smith, L.; Alquist, C.R.; Aqui, N.A.; Hofmann, J.C.; Klingel, R.; Onwuemene, O.A.; Patriquin, C.J.; Pham, H.P.; Sanchez, A.P.; Schneiderman, J.; et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice—Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Ninth Special Issue. J. Clin. Apher. 2023, 38, 77–278. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, P.M.; Gibson, C.A.; Shih, J.; Matias, M.S. C-reactive protein and other markers of inflammation among patients undergoing HELP LDL apheresis. Atherosclerosis 2001, 158, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Schuff-Werner, P.; Schütz, E.; Seyde, W.C.; Eisenhauer, T.; Janning, G.; Armstrong, V.W.; Seidel, D. Improved haemorheology associated with a reduction in plasma fibrinogen and LDL in patients being treated by heparin-induced extracorporeal LDL precipitation (HELP). Eur. J. Clin. Investig. 1989, 19, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Julius, U. Lipoprotein(a) apheresis in patients with peripheral arterial disease: Rationale and clinical results. Clin. Res. Cardiol. Suppl. 2019, 14 (Suppl. S1), 39–44. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Taleb, S.; Mallat, Z.; Tedgui, A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 969–979. [Google Scholar] [CrossRef]
- Hovland, A.; Hardersen, R.; Sexton, J.; Mollnes, T.E.; Lappegård, K.T. Different inflammatory responses induced by three LDL-lowering apheresis columns. J. Clin. Apher. 2009, 24, 247–253. [Google Scholar] [CrossRef]
- Stefanutti, C.; Vivenzio, A.; Di Giacomo, S.; Ferraro, P.M. Cytokines profile in serum of homozygous familial hypercholesterolemia is changed by LDL-apheresis. Cytokine 2011, 55, 245–250. [Google Scholar] [CrossRef]
- Stefanutti, C.; Vivenzio, A.; Ferraro, P.M.; Morozzi, C.; Belotherkovsky, D. Apheresis-inducible cytokine pattern change in severe, genetic dyslipidemias. Cytokine 2011, 56, 835–841. [Google Scholar] [CrossRef]
- Speidl, W.S.; Kastl, S.P.; Hutter, R.; Katsaros, K.M.; Kaun, C.; Bauriedel, G.; Maurer, G.; Huber, K.; Badimon, J.J.; Wojta, J. The complement component C5a is present in human coronary lesions in vivo and induces the expression of MMP-1 and MMP-9 in human macrophages in vitro. Faseb J. 2011, 25, 35–44. [Google Scholar] [CrossRef]
- Kobayashi, A.; Nakatani, M.; Furuyoshi, S.; Tani, N. In vitro evaluation of dextran sulfate cellulose beads for whole blood infusion low-density lipoprotein-hemoperfusion. Ther. Apher. 2002, 6, 365–371. [Google Scholar] [CrossRef]
- Dihazi, H.; Koziolek, M.J.; Söllner, T.; Kahler, E.; Klingel, R.; Neuhoff, R.; Strutz, F.; Mueller, G.A. Protein adsorption during LDL-apheresis: Proteomic analysis. Nephrol. Dial. Transplant. 2008, 23, 2925–2935. [Google Scholar] [CrossRef]
- Hovland, A.; Hardersen, R.; Nielsen, E.W.; Enebakk, T.; Christiansen, D.; Ludviksen, J.K.; Mollnes, T.E.; Lappegård, K.T. Complement profile and activation mechanisms by different LDL apheresis systems. Acta Biomater. 2012, 8, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Cottone, S.; Mulè, G.; Nardi, E.; Vadalà, A.; Guarneri, M.; Briolotta, C.; Arsena, R.; Palermo, A.; Riccobene, R.; Cerasola, G. Relation of C-Reactive Protein to Oxidative Stress and to Endothelial Activation in Essential Hypertension. Am. J. Hypertens. 2006, 19, 313–318. [Google Scholar] [CrossRef]
- Hershcovici, T.; Schechner, V.; Orlin, J.; Harell, D.; Beigel, Y. Effect of different LDL-apheresis methods on parameters involved in atherosclerosis. J. Clin. Apher. 2004, 19, 90–97. [Google Scholar] [CrossRef]
- Kobayashi, S.; Moriya, H.; Maesato, K.; Okamoto, K.; Ohtake, T. LDL-apheresis improves peripheral arterial occlusive disease with an implication for anti-inflammatory effects. J. Clin. Apher. 2005, 20, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Oka, M.; Moriya, H.; Maesato, K.; Okamoto, K.; Ohtake, T. LDL-apheresis reduces P-Selectin, CRP and fibrinogen—Possible important implications for improving atherosclerosis. Ther. Apher. Dial. 2006, 10, 219–223. [Google Scholar] [CrossRef]
- Lee, D.M.; Jackson, K.W.; Knowlton, N.; Wages, J.; Alaupovic, P.; Samuelsson, O.; Saeed, A.; Centola, M.; Attman, P.-O. Oxidative stress and inflammation in renal patients and healthy subjects. PLoS ONE 2011, 6, e22360. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Frolich, J.C.; Haller, H.; Fliser, D. ADMA (asymmetric dimethylarginine): An atherosclerotic disease mediating agent in patients with renal disease? Nephrol. Dial. Transplant. 2001, 16, 1742–1745. [Google Scholar] [CrossRef]
- Alshahawey, M.; Shaheen, S.M.; Elsaid, T.; Sabri, N.A. Effect of febuxostat on oxidative stress in hemodialysis patients with endothelial dysfunction: A randomized, placebo-controlled, double-blinded study. Int. Urol. Nephrol. 2019, 51, 1649–1657. [Google Scholar] [CrossRef]
- Sibal, L.; Agarwal, S.C.; Home, P.D.; Boger, R.H. The Role of Asymmetric Dimethylarginine (ADMA) in Endothelial Dysfunction and Cardiovascular Disease. Curr. Cardiol. Rev. 2010, 6, 82–90. [Google Scholar] [CrossRef]
- Mittermayer, F.; Krzyzanowska, K.; Exner, M.; Mlekusch, W.; Amighi, J.; Sabeti, S.; Minar, E.; Müller, M.; Wolzt, M.; Schillinger, M.; et al. Asymmetric dimethylarginine predicts major adverse cardiovascular events in patients with advanced peripheral artery disease. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2536–2540. [Google Scholar] [CrossRef] [PubMed]
- Stefanutti, C.; Di Giacomo, S.; Vivenzio, A.; Isacchi, G.C.; Masella, R.; Caprari, P.; Varì, R.; Tarzia, A.; Mosiello, A.; Cantafora, A. Acute and long-term effects of low-density lipoprotein (LDL)-apheresis on oxidative damage to LDL and reducing capacity of erythrocytes in patients with severe familial hypercholesterolaemia. Clin. Sci. 2001, 100, 191–198. [Google Scholar] [CrossRef]
- Tsurumi-Ikeya, Y.; Tamura, K.; Azuma, K.; Mitsuhashi, H.; Wakui, H.; Nakazawa, I.; Sugano, T.; Mochida, Y.; Ebina, T.; Hirawa, N.; et al. Sustained inhibition of oxidized low-density lipoprotein is involved in the long-term therapeutic effects of apheresis in dialysis patients. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Kiyomoto, H.; Hitomi, H.; Moriwaki, K.; Ihara, G.; Kaifu, K.; Fujita, Y.; Higashiyama, C.; Nishiyama, A.; Kohno, M. Low-density lipoprotein apheresis for haemodialysis patients with peripheral arterial disease reduces reactive oxygen species production via suppression of NADPH oxidase gene expression in leucocytes. Nephrol. Dial. Transplant. 2009, 24, 3818–3825. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trimm, E.; Red-Horse, K. Vascular endothelial cell development and diversity. Nat. Rev. Cardiol. 2023, 20, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.I.; Saglam, M.; Caglar, K.; Cakir, E.; Sonmez, A.; Ozgurtas, T.; Aydin, A.; Eyileten, T.; Ozcan, O.; Acikel, C.; et al. The determinants of endothelial dysfunction in CKD: Oxidative stress and asymmetric dimethylarginine. Am. J. Kidney Dis. 2006, 47, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kizaki, Y.; Ueki, Y.; Yoshida, K.; Yano, M.; Matsumoto, K.; Miyake, S.; Tominaga, Y.; Eguchi, K.; Yano, K. Does the production of nitric oxide contribute to the early improvement after a single low-density lipoprotein apheresis in patients with peripheral arterial obstructive disease? Blood Coagul. Fibrinolysis 1999, 10, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Sampietro, T.; Tuoni, M.; Ferdeghini, M.; Ciardi, A.; Marraccini, P.; Prontera, C.; Sassi, G.; Taddei, M.; Bionda, A. Plasma cholesterol regulates soluble cell adhesion molecule expression in familial hypercholesterolemia. Circulation 1997, 96, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Empen, K.; Otto, C.; Brödl, U.C.; Parhofer, K.G. The effects of three different LDL-apheresis methods on the plasma concentrations of E-selectin, VCAM-1, and ICAM-1. J. Clin. Apher. 2002, 17, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, K.; Kawabe, M.; Hirama, A.; Ueda, K.; Kamada, Y.; Arii, K.; Komaba, Y.; Katsura, K.-I.; Iino, Y.; Katayama, Y. Effects of selective LDL apheresis on plasma concentrations of ICAM-1, VCAM-1 and P-selectin in diabetic patients with arteriosclerosis obliterans and receiving maintenance hemodialysis. Clin. Chim. Acta 2007, 377, 198–200. [Google Scholar] [CrossRef]
- Ramunni, A.; Giancipoli, G.; Saracino, A.; Guerriero, S.; Saliani, M.; Gentile, M.; Sborgia, C.; Coratelli, P. LDL-apheresis in acute anterior ischemic optic neuropathy. Int. J. Artif. Organs 2004, 27, 337–341. [Google Scholar] [CrossRef]
- Poller, W.C.; Dreger, H.; Morgera, S.; Berger, A.; Flessenkämper, I.; Enke-Melzer, K. Lipoprotein apheresis in patients with peripheral artery disease and hyperlipoproteinemia(a). Atheroscler. Suppl. 2015, 18, 187–193. [Google Scholar] [CrossRef]
- Poller, W.C.; Berger, A.; Dreger, H.; Morgera, S.; Enke-Melzer, K. Lipoprotein apheresis in patients with peripheral artery disease and lipoprotein(a)-hyperlipoproteinemia: 2-year follow-up of a prospective single center study. Atheroscler. Suppl. 2017, 30, 174–179. [Google Scholar] [CrossRef]
- Kroon, A.A.; van Asten, W.N.; Stalenhoef, A.F. Effect of apheresis of low-density lipoprotein on peripheral vascular disease in hypercholesterolemic patients with coronary artery disease. Ann. Intern. Med. 1996, 125, 945–954. [Google Scholar] [CrossRef]
- Ohtake, T.; Mochida, Y.; Matsumi, J.; Tobita, K.; Ishioka, K.; Oka, M.; Maesato, K.; Moriya, H.; Hidaka, S.; Saito, S.; et al. Beneficial Effect of Endovascular Therapy and Low-Density Lipoprotein Apheresis Combined Treatment in Hemodialysis Patients With Critical Limb Ischemia due to Below-Knee Arterial Lesions. Ther. Apher. Dial. 2016, 20, 661–667. [Google Scholar] [CrossRef]
- Tsuchida, H.; Shigematsu, H.; Ishimaru, S.; Iwai, T.; Akaba, N.; Umezu, S. Effect of low-density lipoprotein apheresis on patients with peripheral arterial disease. Peripheral Arterial Disease LDL Apheresis Multicenter Study (P-LAS). Int. Angiol. 2006, 25, 287–292. [Google Scholar] [PubMed]
- Rietzsch, H.; Panzner, I.; Selisko, T.; Julius, U.; Jabs, N.; Reimann, M.; Bonifacio, E.; Bornhäuser, M.; Bornstein, S.R. Heparin-induced Extracorporal LDL precipitation (H.E.L.P) in diabetic foot syndrome—Preventive and regenerative potential? Horm. Metab. Res. 2008, 40, 487–490. [Google Scholar] [CrossRef] [PubMed]
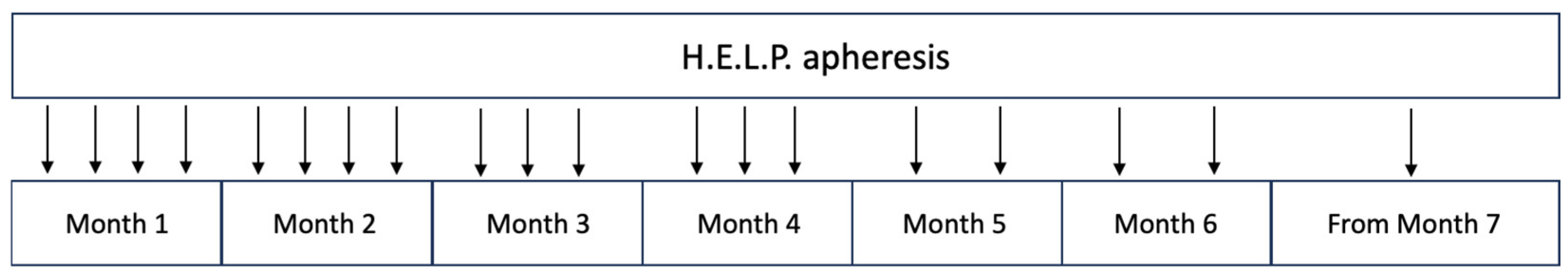

| Intensive H.E.L.P. Treatment (Months 1–2) | Low-Intensity H.E.L.P. Treatment (Months 3–6) | Maintenance H.E.L.P. Treatment (from Month 7) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PRE | POST | REDUCTION | PRE | POST | REDUCTION | PRE | POST | REDUCTION | |
| Total cholesterol (mg/dL) | 134.2 ± 12.1 | 90.12 ± 7.1 | 44.2 ± 7.1 | 147.5 ± 9.6 | 100.7 ± 10.5 | 46.7 ± 10.6 | 144.6 ± 11.5 | 907.3 ± 9.7 | 47.2 ± 8.9 |
| LDL cholesterol (mg/dL) | 82.7 ± 12.3 | 45.2 ± 6.03 | 37.5 ± 7.9 | 93.8 ± 9.2 | 58 ± 7.5 | 35.8 ± 3.6 | 94.5 ± 8.6 | 50.3 ± 6.7 | 44.1 ± 7.4 |
| HDL cholesterol (mg/dL) | 37.5 ± 2.6 | 33.8 ± 1.9 | 3.66 ± 1.65 | 33.6 ± 2.72 | 29.7 ± 4.77 | 3.8 ± 2.3 | 41.3 ± 4.3 | 36.1 ± 3.6 | 5.2 ± 1.1 |
| Triglycerides (mg/dL) | 102.6 ± 26.8 | 55.1 ± 10.4 | 47.5 ± 19.5 | 163.6 ± 58.8 | 92 ± 47.8 | 70.3 ± 18.9 | 110.3 ± 47.6 | 67.8 ± 31.5 | 42.5 ± 19.9 |
| Lp(a) (mg/dL) | 23.5 ± 2.9 | 11 ± 1.5 | 12 ± 2.3 | 24.7 ± 3 | 11.78 ± 1.6 | 12.4 ± 2.9 | 21.4 ± 1.1 | 10 | 11.4 ± 1.1 |
| Fibrinogen (mg/dL) | 431 ± 50.1 | 231.6 ± 19.1 | 199.3 ± 40.5 | 510 ± 56.2 | 282.6 ± 25.7 | 228 ± 68.6 | 508.7 ± 63.4 | 254.8 ± 36.7 | 253.8 ± 65.9 |
| CRP (mg/L) | 11 ± 5 | 5.5 ± 1.5 | 5.5 ± 3.5 | 12.2 ± 3.5 | 5.7 ± 1.7 | 6.4 ± 1.7 | 19.8 ± 2 | 9.75 ± 0.8 | 10.1 ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotella, S.; Gesualdo, L.; Fiorentino, M. Heparin-Mediated Extracorporeal Low-Density Lipoprotein Precipitation Apheresis for Treating Peripheral Arterial Disease in Patients with Chronic Kidney Disease. J. Clin. Med. 2024, 13, 1121. https://doi.org/10.3390/jcm13041121
Rotella S, Gesualdo L, Fiorentino M. Heparin-Mediated Extracorporeal Low-Density Lipoprotein Precipitation Apheresis for Treating Peripheral Arterial Disease in Patients with Chronic Kidney Disease. Journal of Clinical Medicine. 2024; 13(4):1121. https://doi.org/10.3390/jcm13041121
Chicago/Turabian StyleRotella, Stefania, Loreto Gesualdo, and Marco Fiorentino. 2024. "Heparin-Mediated Extracorporeal Low-Density Lipoprotein Precipitation Apheresis for Treating Peripheral Arterial Disease in Patients with Chronic Kidney Disease" Journal of Clinical Medicine 13, no. 4: 1121. https://doi.org/10.3390/jcm13041121
APA StyleRotella, S., Gesualdo, L., & Fiorentino, M. (2024). Heparin-Mediated Extracorporeal Low-Density Lipoprotein Precipitation Apheresis for Treating Peripheral Arterial Disease in Patients with Chronic Kidney Disease. Journal of Clinical Medicine, 13(4), 1121. https://doi.org/10.3390/jcm13041121







