Anti-CGRP and Anti-CGRP Receptor Monoclonal Antibodies for Migraine Prophylaxis: Retrospective Observational Study on 209 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Efficacy and Safety Assessment
2.3. Data Collection and Statistical Analysis
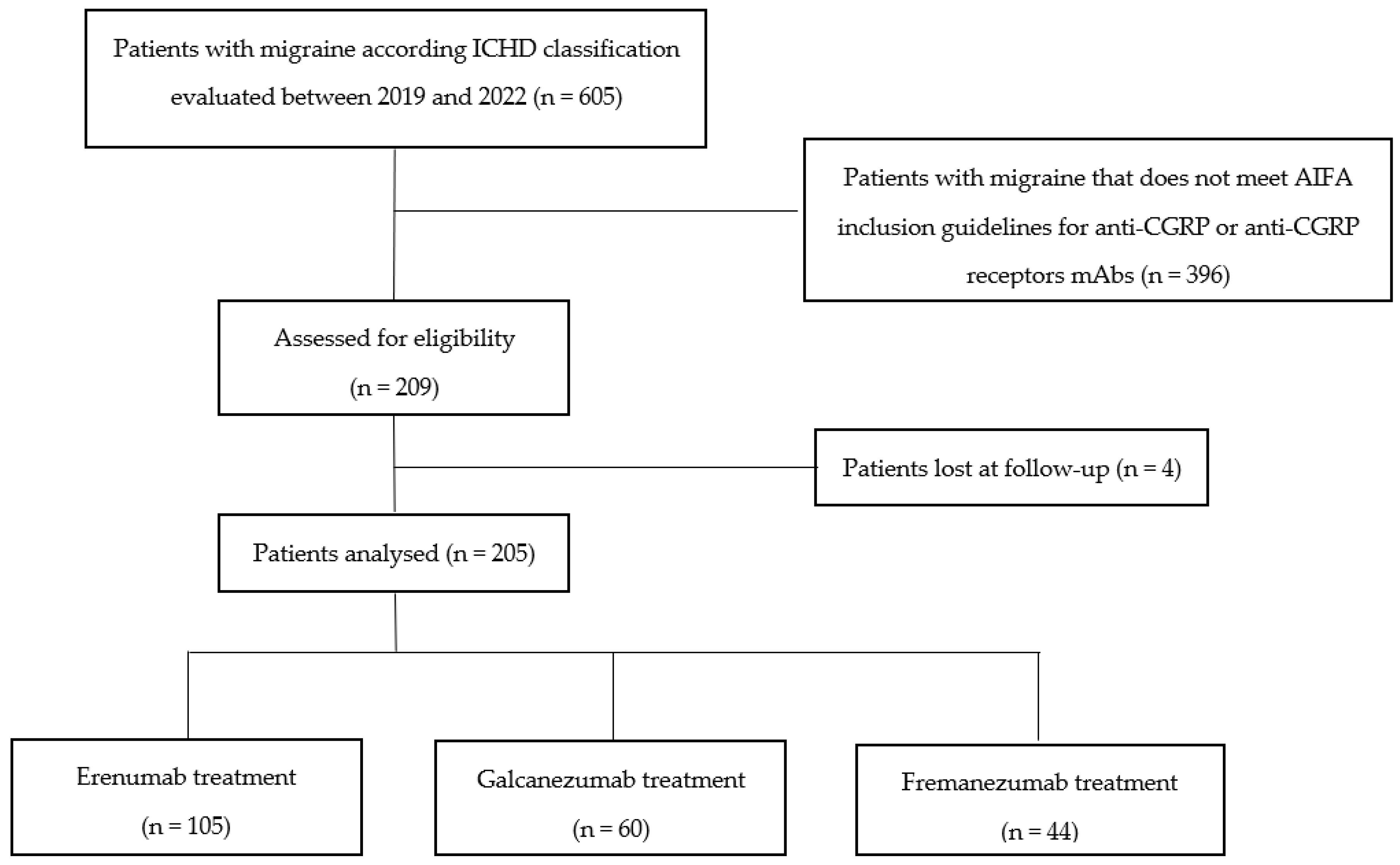
3. Results
3.1. Study Population
3.2. Efficacy
3.3. Treatment Discontinuation
3.4. Safety
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Steiner, T.J.; Stovner, L.J. Global epidemiology of migraine and its implications for public health and health policy. Nat. Rev. Neurol. 2023, 19, 109–117. [Google Scholar] [CrossRef]
- Steiner, T.J.; Stovner, L.J.; Jensen, R.; Uluduz, D.; Katsarava, Z. Migraine remains second among the world’s causes of disability, and first among young women: Findings from GBD2019. J. Headache Pain 2020, 21, 137. [Google Scholar] [CrossRef]
- Thomas, H.; Kothari, S.F.; Husøy, A.; Jensen, R.H.; Katsarava, Z.; Tinelli, M.; Steiner, T.J. The relationship between headache-attributed disability and lost productivity: 2. Empirical evidence from population-based studies in nine disparate countries. J. Headache Pain 2021, 22, 153. [Google Scholar] [CrossRef]
- Eigenbrodt, A.K.; Ashina, H.; Khan, S.; Diener, H.-C.; Mitsikostas, D.D.; Sinclair, A.J.; Pozo-Rosich, P.; Martelletti, P.; Ducros, A.; Lantéri-Minet, M.; et al. Diagnosis and management of migraine in ten steps. Nat. Rev. Neurol. 2021, 17, 501–514. [Google Scholar] [CrossRef]
- Katsarava, Z.; Mania, M.; Lampl, C.; Herberhold, J.; Steiner, T.J. Poor medical care for people with migraine in Europe – evidence from the Eurolight study. J. Headache Pain 2018, 19, 10. [Google Scholar] [CrossRef]
- Edvinsson, L. Role of CGRP in Migraine. Handb. Exp. Pharmacol. 2019, 255, 121–130. [Google Scholar]
- Ray, J.C.; Kapoor, M.; Stark, R.J.; Wang, S.-J.; Bendtsen, L.; Matharu, M.; Hutton, E.J. Calcitonin gene related peptide in migraine: Current therapeutics, future implications and potential off-target effects. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1325–1334. [Google Scholar] [CrossRef]
- AIFA (Agenzia Italiana del Farmaco) Home Page. Available online: https://www.aifa.gov.it/registri-e-piani-terapeutici1 (accessed on 1 January 2019).
- Stewart, W.F.; Lipton, R.B.; Kolodner, K.B.; Sawyer, J.; Lee, C.; Liberman, J.N. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain 2000, 88, 41–52. [Google Scholar] [CrossRef]
- D’Amico, D.; Mosconi, P.; Genco, S.; Usai, S.; Prudenzano, A.; Grazzi, L.; Leone, M.; Puca, F.; Bussone, G. The Migraine Disability Assessment (MIDAS) questionnaire: Translation and reliability of the Italian version. Cephalalgia 2001, 21, 947–952. [Google Scholar] [CrossRef]
- Shin, H.E.; Park, J.W.; Kim, Y.I.; Lee, K.S. Headache Impact Test-6 (HIT-6) scores for migraine patients: Their relation to disability as measured from a headache diary. J. Clin. Neurol. 2008, 4, 158–163. [Google Scholar] [CrossRef]
- HIT-6 Scoring Interpretation Italy (Italian) Version 1.1© 2001 QualityMetric, Inc. and GlaxoSmithKline Group of Companies. Available online: http://www.centrocefaleeroma.it/ (accessed on 1 September 2018).
- Di Tanna, G.L.; Porter, J.K.; Lipton, R.B.; Brennan, A.; Palmer, S.; Hatswell, A.J.; Sapra, S.; Villa, G. Migraine day frequency in migraine prevention: Longitudinal modelling approaches. BMC Med Res. Methodol. 2019, 19, 20. [Google Scholar] [CrossRef]
- Pradeep, R.; Nemichandra, S.C.; Harsha, S.; Radhika, K. Migraine disability, quality of life, and its predictors. Ann. Neurosci. 2020, 27, 18–23. [Google Scholar]
- Tzankova, V.; Becker, W.J.; Chan, T.L. Pharmacologic prevention of migraine. Can. Med Assoc. J. 2023, 195, E187–E192. [Google Scholar] [CrossRef]
- Ha, H.; Gonzalez, A. Migraine Headache Prophylaxis. Am. Fam. Physician 2019, 99, 17–24. [Google Scholar]
- Agostoni, E.C.; Barbanti, P.; Calabresi, P.; Colombo, B.; Cortelli, P.; Frediani, F.; Geppetti, P.; Grazzi, L.; Leone, M.; Martelletti, P.; et al. Current and emerging evidence-based treatment options in chronic migraine: A narrative review. J. Headache Pain 2019, 20, 92. [Google Scholar] [CrossRef]
- Vanya, M.; Desai, P.; Clifford, S.; Howard, K.; Lisle, T.C.; Sapra, S. Understanding patient adherence to prophylactic migraine medications. Neurology 2016, 86 (Suppl. 16), P1.164. [Google Scholar] [CrossRef]
- Durham, P.L. Calcitonin gene-related peptide (CGRP) and migraine. Headache 2006, 46, S3–S8. [Google Scholar] [CrossRef]
- Lampl, C.; Maassen Van Den Brink, A.; Deligianni, C.I.; Gil-Gouveia, R.; Jassal, T.; Sanchez-Del-Rio, M.; Reuter, U.; Uluduz, D.; Versijpt, J.; Zeraatkar, D.; et al. The comparative effectiveness of migraine preventive drugs: A systematic review and network me-ta-analysis. J. Headache Pain 2023, 24, 56. [Google Scholar] [CrossRef]
- Sauro, K.M.; Rose, M.S.; Becker, W.J.; Christie, S.N.; Giammarco, R.; Mackie, G.F.; Eloff, A.G.; Gawel, M.J. HIT-6 and MIDAS as measures of headache disability in a headache referral population. Headache J. Head Face Pain 2010, 50, 383–395. [Google Scholar] [CrossRef]
- Houts, C.R.; Wirth, R.J.; McGinley, J.S.; Cady, R.; Lipton, R.B. Determining thresholds for meaningful change for the Headache Impact Test (HIT-6) total and item-specific scores in chronic migraine. Headache J. Head Face Pain 2020, 60, 2003–2013. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Silberstein, S.D.; Yeung, P.P.; Cohen, J.M.; Ning, X.; Yang, R.; Dodick, D.W. Long-term safety, tolerability, and efficacy of fremanezumab in migraine: A randomized study. Neurology 2020, 95, e2487–e2499. [Google Scholar] [CrossRef]
- Lambru, G.; Hill, B.; Murphy, M.; Tylova, I.; Andreou, A.P. A prospective real-world analysis of erenumab in refractory chronic migraine. J. Headache Pain 2020, 21, 61. [Google Scholar] [CrossRef]
- Torres-Ferrús, M.; Gallardo, V.J.; Alpuente, A.; Caronna, E.; Gine-Cipres, E.; Pozo-Rosich, P. The impact of anti-CGRP monoclonal antibodies in resistant migraine patients: A real-world evidence observational study. J. Neurol. 2021, 268, 3789–3798. [Google Scholar] [CrossRef]
- Sette, L.; Caponnetto, V.; Ornello, R.; Nežádal, T.; Čtrnáctá, D.; Šípková, J.; Matoušová, Z.; Sacco, S. Acute medication use in patients with migraine treated with monoclonal antibodies acting on the CGRP Pathway: Results from a multicenter study and proposal of a new index. Front. Neurol. 2022, 13, 846717. [Google Scholar] [CrossRef]
- Iannone, L.F.; De Cesaris, F.; Ferrari, A.; Benemei, S.; Fattori, D.; Chiarugi, A. Effectiveness of anti-CGRP monoclonal antibodies on central symptoms of migraine. Cephalalgia 2022, 42, 1323–1330. [Google Scholar] [CrossRef]
- di Cola, F.S.; Bolchini, M.; Ceccardi, G.; Caratozzolo, S.; Liberini, P.; Rao, R.; Padovani, A. An observational study on monoclonal antibodies against calcitonin-gene-related peptide and its receptor. Eur. J. Neurol. 2023, 30, 1764–1773. [Google Scholar] [CrossRef]
- Hepp, Z.; Bloudek, L.M.; Varon, S.F. Systematic review of migraine prophylaxis adherence and persistence. J. Manag. Care Pharm. 2014, 20, 22–33. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Reshef, S.; Cohen, J.M.; Gandhi, S.; Seminerio, M.; Campos, V.R.; Kessler, Y.; Thompson, S.F.; Blumenfeld, A. Adverse events reported with therapies targeting the CGRP pathway during the first 6 months post-launch: A retrospective analysis using the FDA Adverse Events Reporting System. Adv. Ther. 2022, 40, 445–459. [Google Scholar] [CrossRef]
- Kudrow, D.; Pascual, J.; Winner, P.K.; Dodick, D.W.; Tepper, S.J.; Reuter, U.; Hong, F.; Klatt, J.; Zhang, F.; Cheng, S.; et al. Vascular safety of erenumab for migraine prevention. Neurology 2020, 94, e497–e510. [Google Scholar] [CrossRef]
- Mahmoud, A.N.; Mentias, A.; Elgendy, A.Y.; Qazi, A.; Barakat, A.F.; Saad, M.; Mohsen, A.; Abuzaid, A.; Mansoor, H.; Mojadidi, M.K.; et al. Migraine and the risk of cardiovascular and cerebrovascular events: A meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open 2018, 8, e020498. [Google Scholar] [CrossRef] [PubMed]

| Assessed for Eligibility | 209 |
|---|---|
| Gender, n (%) | |
| Female | 179 (85.6%) |
| Male | 30 (14.3%) |
| F:M ratio | 6:1 |
| Age, yr. * | 51 (43–59) |
| ICHD diagnosis n (%) | |
| Chronic Migraine | 148 (70.8%) |
| Episodic Migraine | 61 (29.2%) |
| Migraine without aura | 196 (93.8%) |
| Migraine with aura | 13 (6.2%) |
| Actual migraine attack drugs, n (%) | |
| Triptans | 75 (35.9%) |
| NSAIDs | 44 (21.1%) |
| Assessed for Eligibility | 209 | ||
|---|---|---|---|
| Treatment, n (%) | Erenumab, 105 (50.2%) | Galcanezumab, 60 (28.7%) | Fremanezumab, 44 (21.05%) |
| Female, n (%) | 93 (88.6%) | 52 (86.7%) | 33 (76.7%) |
| Male, n (%) | 12 (11.4%) | 8 (13.3%) | 10 (23.3%) |
| F:M ratio | 8.7:1 | 6.5:1 | 3.3:1 |
| Age, yr. * | 50 (43–58) | 52 (40–64) | 53 (48–58) |
| ICHD diagnosis n (%) | |||
| Chronic Migraine (CM) | 78 (74.3%) | 49 (81.7%) | 21 (47.7%) |
| Episodic Migraine (EM) | 27 (25.7%) | 11 (18.3%) | 23 (52.3%) |
| Migraine without aura | 99 (94.3%) | 58 (96.7%) | 39 (88.6%) |
| Migraine with aura | 6 (5.7%) | 2 (3.3%) | 5 (11.4%) |
| Headache scores * | |||
| MIDAS (0–450) | 80 (50–135) | 107 (60–160) | 65 (39–104) |
| HIT-6 (36–78) | 68 (65–72) | 58 (48–63) | 66 (62–70) |
| MMDs (days) | 17 (12–28) | 16 (12–30) | 15 (10–22) |
| MAD (hours) | 24 (10–24) | 24 (10–48) | 10 (3–24) |
| TREATMENT | Time Course of Observation | Scores Reduction T3 vs. Baseline | ||||
|---|---|---|---|---|---|---|
| ERENUMAB | Baseline (n = 105) | T1 (n = 102) | T2 (n = 96) | T3 (n = 81) | ||
| ENDPOINTS | MIDAS (0–450) | 80 (50–135) | 25 (10–50) | 20 (8–36) | 25 (11–44) | 68.7% |
| HIT-6 (36–78) | 68 (65–72) | 62 (55–67) | 60 (56–65) | 61 (54–66) | 10.3% | |
| MMDs (days) | 17 (12–28) | 5.5 (4–10) | 5 (3–9) | 7 (3–10) | 58.8% | |
| MAD (hours) | 24 (10–24) | 10 (3–24) | 6 (2–24) | 4 (2–10) | 83.3% | |
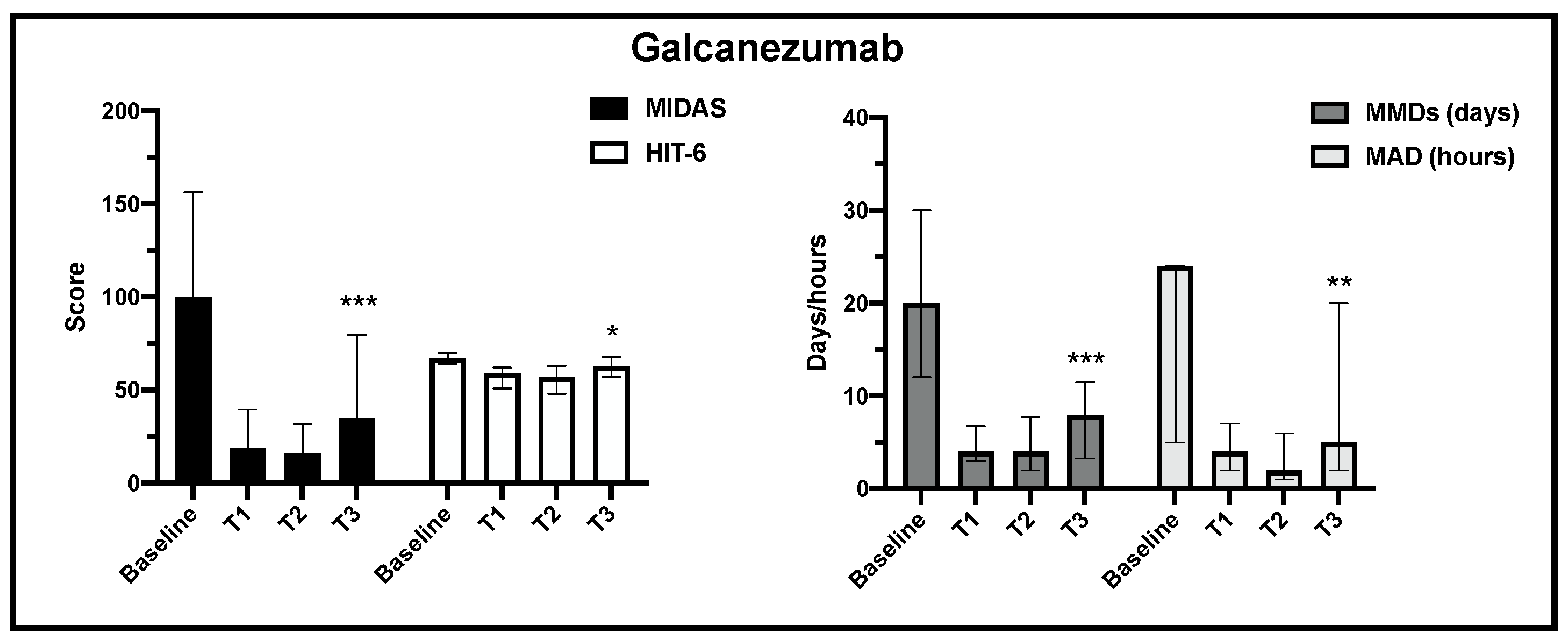
| GALCANEZUMAB | Baseline (n = 60) | T1 (n = 57) | T2 (n = 52) | T3 (n = 37) | ||
| ENDPOINTS | MIDAS (0–450) | 107 (60–160) | 18 (3–40) | 18.5 (2.5–31.5) | 35 (12–72) | 67.2% |
| HIT-6 (36–78) | 66 (64–70) | 58.5 (48–63) | 60 (49.5–65) | 63 (57–68) | 4.54% | |
| MMDs (days) | 16 (12–30) | 4.5 (2–8) | 4 (2–8) | 8 (3.5–11) | 50.0% | |
| MAD (hours) | 24 (10–48) | 5 (2–10) | 2 (2–6) | 5 (2–20) | 79.2% | |
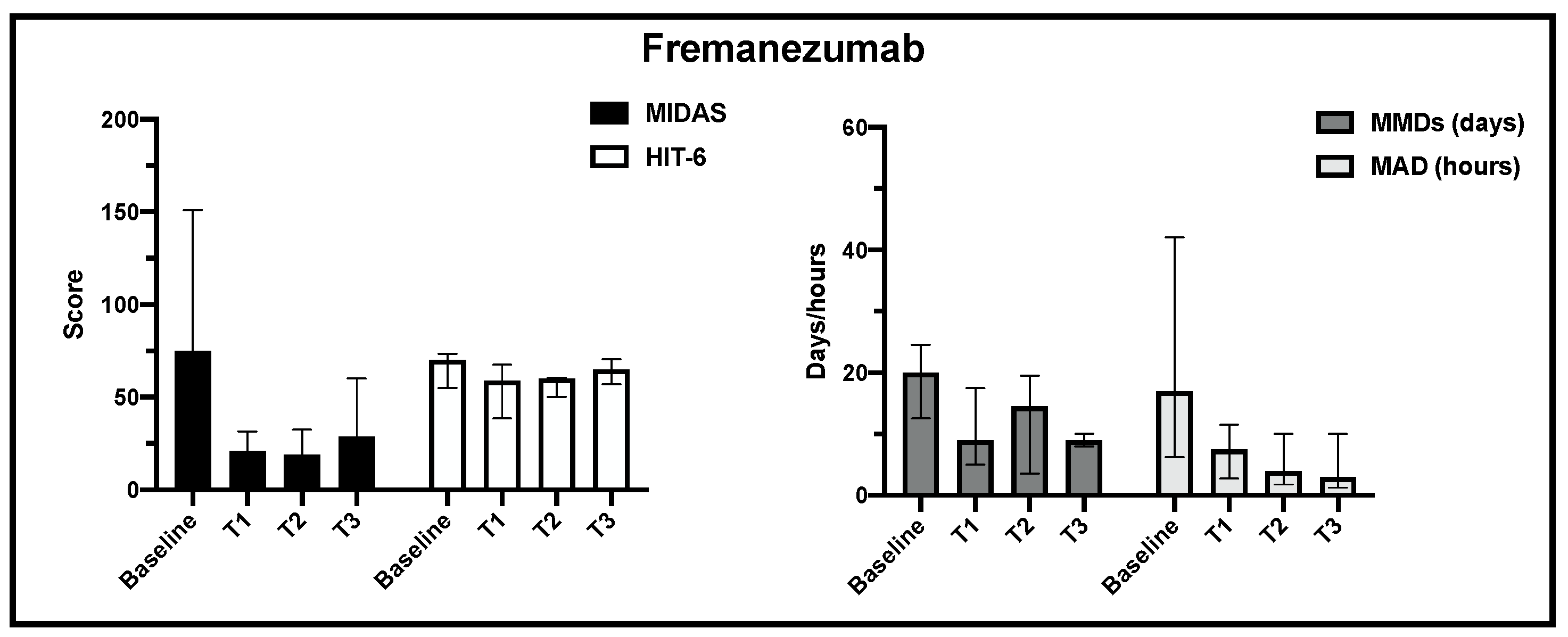
| FREMANEZUMAB | Baseline (n = 44) | T1 (n = 36) | T2 (n = 21) | T3 (n = 5) | ||
| ENDPOINTS | MIDAS (0–450) | 65 (39–104) | 10.5 (2.5–33.5) | 9 (3–17) | 29 (20–42) | 55.4% |
| HIT-6 (36–78) | 66 (62–70) | 55 (47–65) | 60 (50–62) | 65 (64–67) | 1.5% | |
| MMDs (days) | 15 (10–22) | 4.5 (3–8) | 4 (1–11) | 9 (8–10) | 40.0% | |
| MAD (hours) | 10 (3–24) | 3 (1–10) | 4 (2–9) | 3 (1.5–8) | 70.0% | |
| Treatment Discontinuation, n (%) | 24 | (11.7%) |
|---|---|---|
| Lack of efficacy | 15 | (7.3%) |
| Adverse Events (AEs) | 5 | (2.4%) |
| Personal choice | 2 | (0.9%) |
| Medical decision | 2 | (0.9%) |
| Adverse Events during Treatment, n (%) | 36 (7.5%) |
|---|---|
| Pain after injection | 21 (10.2%) |
| Injection site erythema | 18 (8.7%) |
| Nausea | 14 (6.8%) |
| Fatigue | 12 (5.8%) |
| Constipation | 12 (5.8%) |
| Paresthesia | 2 (0.9%) |
| Cerebrovascular events | 2 (0.9%) |
| Hair loss | 1 (0.4%) |
| Authors | Study Type | Patients (n) | mAbs Treatment | Follow-up (Months) | Dropout (n, %) | MIDAS Reduction (%) | HIT-6 Reduction (Points) | MMDs Reduction (%) |
|---|---|---|---|---|---|---|---|---|
| Goadsby P.J. et al, 2020 [24] | Prospective | 1890 | Fremanezumab | 12 | 396 (21%) | 73.7% | 8.4 | 62.5% (CM) 44.4% (EM) |
| Lambru G. et al, 2020 [25] | Prospective | 164 | Erenumab | 6 | 19 (12%) | n.a. | 4 | 35% |
| Torres-Ferrús M. et al. 2021 [26] | Prospective | 155 | Erenumab Galcanezumab | 3 | 45 (22.5%) | 65.2% | n.a. | 47% |
| Sette L. et al. 2022 [27] | Retrospective | 90 | Erenumab Fremanezumab | 6 | 4 (4.5%) | n.a. | 13 | 72.7% |
| Iannone L.F. et al. 2022 [28] | Prospective | 203 | Erenumab Fremanezumab Galcanezumab | 12 | 35 (19%) | 74.2% | 15 | 41.6% |
| Schiano di Cola F. et al. 2023 [29] | Retrospective | 152 | Erenumab Galcanezumab Fremanezumab | 6 | n.a. | 63.2% | n.a. | 45.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweiger, V.; Bellamoli, P.; Taus, F.; Gottin, L.; Martini, A.; Nizzero, M.; Bonora, E.; Del Balzo, G.; Donadello, K.; Secchettin, E.; et al. Anti-CGRP and Anti-CGRP Receptor Monoclonal Antibodies for Migraine Prophylaxis: Retrospective Observational Study on 209 Patients. J. Clin. Med. 2024, 13, 1130. https://doi.org/10.3390/jcm13041130
Schweiger V, Bellamoli P, Taus F, Gottin L, Martini A, Nizzero M, Bonora E, Del Balzo G, Donadello K, Secchettin E, et al. Anti-CGRP and Anti-CGRP Receptor Monoclonal Antibodies for Migraine Prophylaxis: Retrospective Observational Study on 209 Patients. Journal of Clinical Medicine. 2024; 13(4):1130. https://doi.org/10.3390/jcm13041130
Chicago/Turabian StyleSchweiger, Vittorio, Paola Bellamoli, Francesco Taus, Leonardo Gottin, Alvise Martini, Marta Nizzero, Eleonora Bonora, Giovanna Del Balzo, Katia Donadello, Erica Secchettin, and et al. 2024. "Anti-CGRP and Anti-CGRP Receptor Monoclonal Antibodies for Migraine Prophylaxis: Retrospective Observational Study on 209 Patients" Journal of Clinical Medicine 13, no. 4: 1130. https://doi.org/10.3390/jcm13041130
APA StyleSchweiger, V., Bellamoli, P., Taus, F., Gottin, L., Martini, A., Nizzero, M., Bonora, E., Del Balzo, G., Donadello, K., Secchettin, E., Finco, G., Santis, D. D., & Polati, E. (2024). Anti-CGRP and Anti-CGRP Receptor Monoclonal Antibodies for Migraine Prophylaxis: Retrospective Observational Study on 209 Patients. Journal of Clinical Medicine, 13(4), 1130. https://doi.org/10.3390/jcm13041130







