Treatment and Rehabilitation of a Patient with Neuromyelitis Optica Spectrum Disorder-Induced Complete Spinal Cord Injury Following COVID-19 Vaccination: A Case Report
Abstract
:1. Introduction
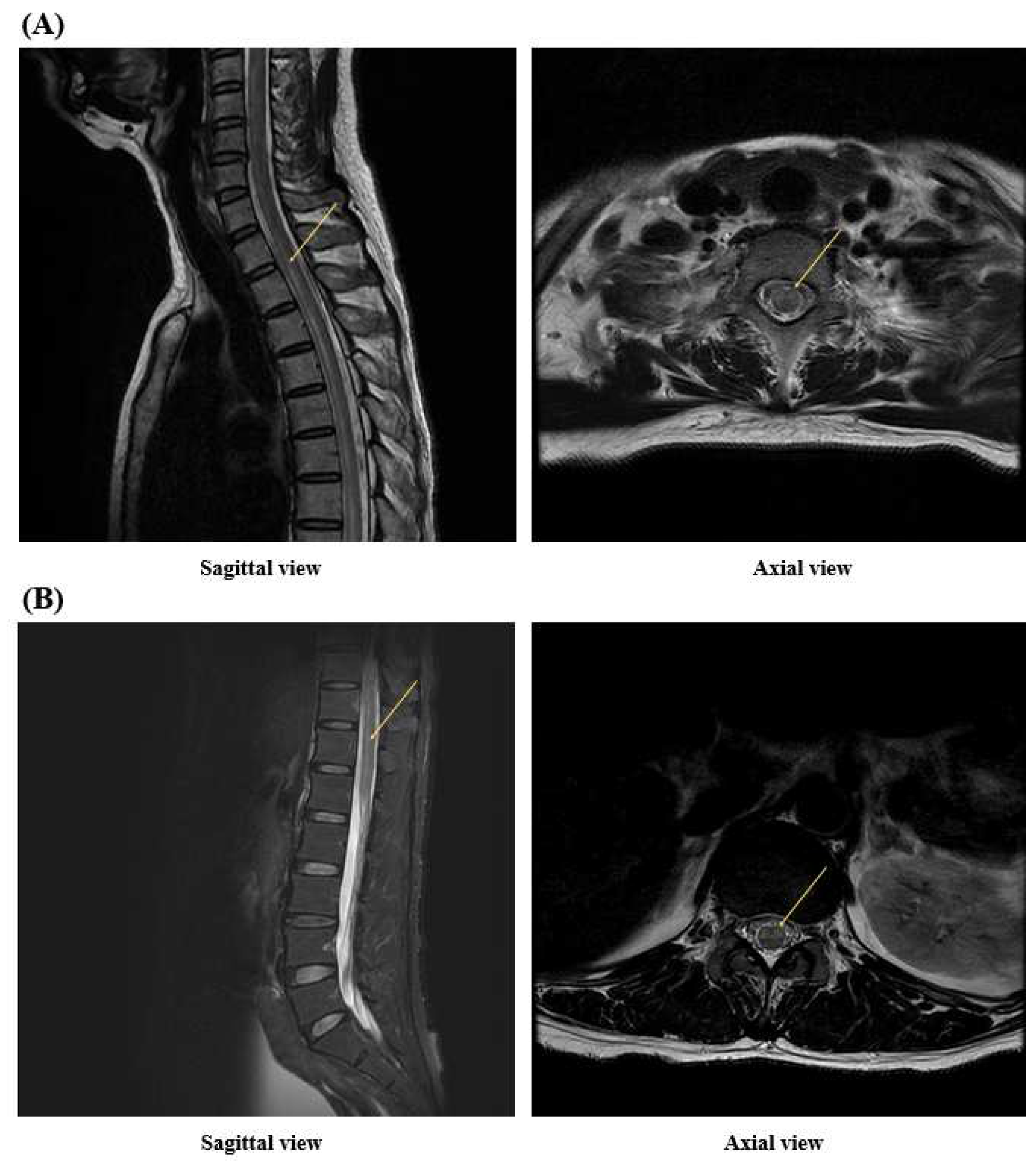
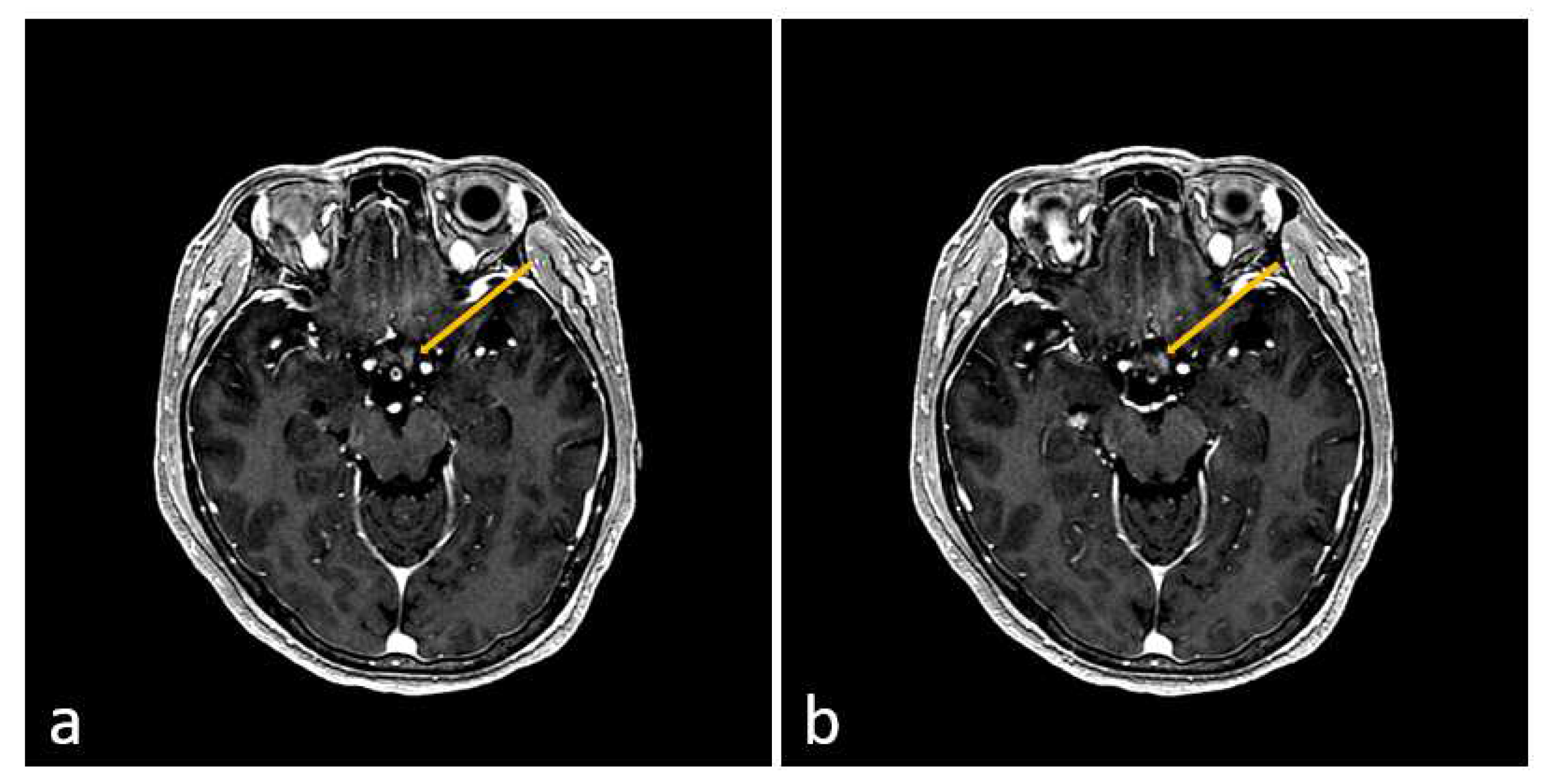
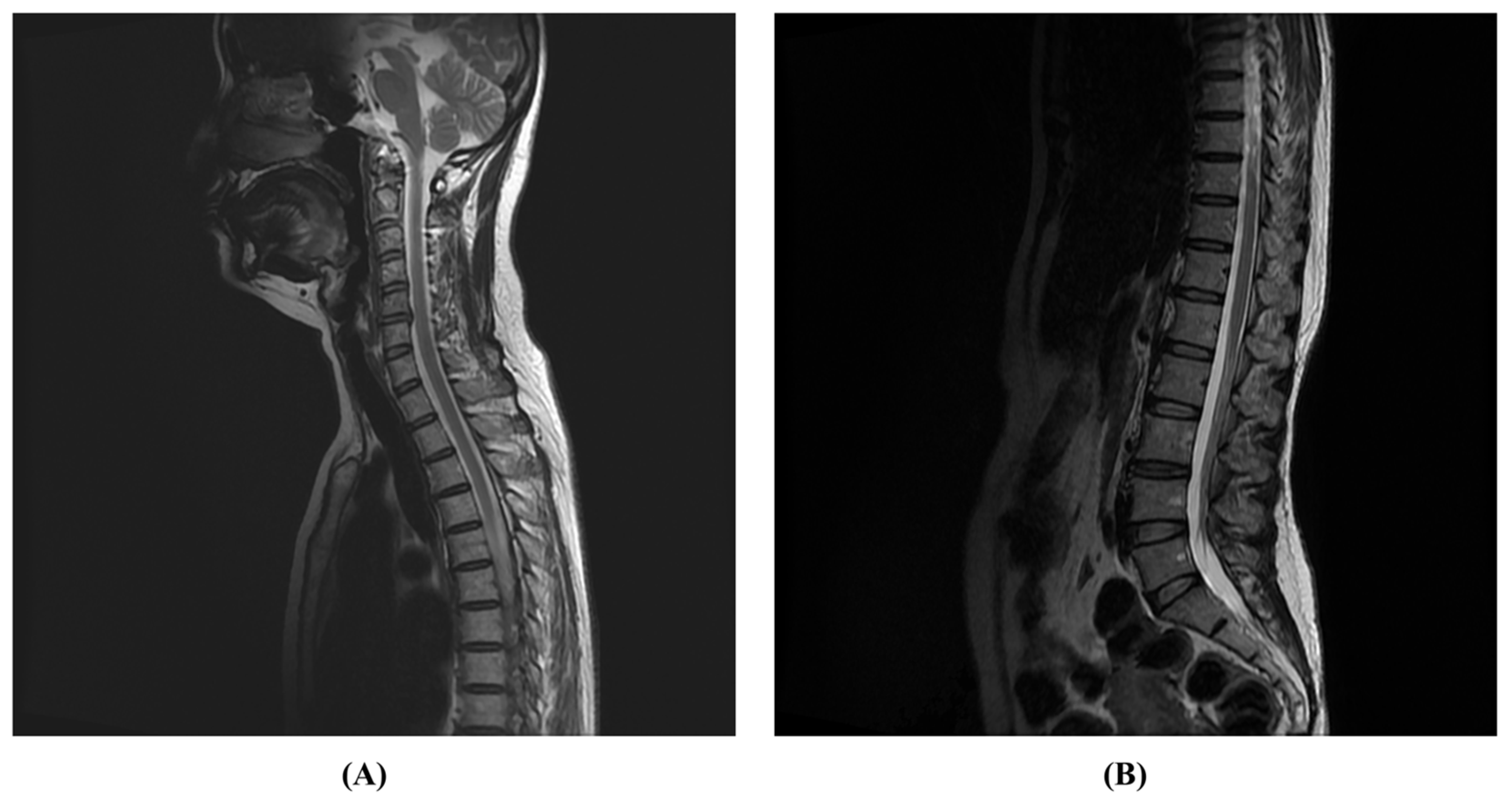
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paul, S.; Mondal, G.P.; Bhattacharyya, R.; Ghosh, K.C.; Bhat, I.A. Neuromyelitis optica spectrum disorders. J. Neurol. Sci. 2021, 420, 117225. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; De Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Traub, J.; Häusser-Kinzel, S.; Weber, M.S. Differential Effects of MS Therapeutics on B Cells—Implications for Their Use and Failure in AQP4-Positive NMOSD Patients. Int. J. Mol. Sci. 2020, 21, 5021. [Google Scholar] [CrossRef] [PubMed]
- Pittock, S.J.; Lucchinetti, C.F. Neuromyelitis optica and the evolving spectrum of autoimmune aquaporin-4 channelopathies: A decade later. Ann. N. Y. Acad. Sci. 2016, 1366, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Graf, J.; Mares, J.; Barnett, M.; Aktas, O.; Albrecht, P.; Zamvil, S.S.; Hartung, H.-P. Targeting B cells to modify MS, NMOSD, and MOGAD: Part 1. Neurol.-Neuroimmunol. Neuroinflamm. 2021, 8, e918. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.H.; Whittam, D.; Mutch, K.; Linaker, S.; Solomon, T.; Das, K.; Bhojak, M.; Jacob, A. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J. Neurol. 2017, 264, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Contentti, E.C.; Lopez, P.A.; Pettinicchi, J.P.; Pappolla, A.; Miguez, J.; Patrucco, L.; Cristiano, E.; Vrech, C.; Tkachuk, V.; Liwacki, S. What percentage of AQP4-ab-negative NMOSD patients are MOG-ab positive? A study from the Argentinean multiple sclerosis registry (RelevarEM). Mult. Scler. Relat. Disord. 2021, 49, 102742. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012, 11, 535–544. [Google Scholar] [CrossRef]
- Gospe III, S.M.; Chen, J.J.; Bhatti, M.T. Neuromyelitis optica spectrum disorder and myelin oligodendrocyte glycoprotein associated disorder-optic neuritis: A comprehensive review of diagnosis and treatment. Eye 2021, 35, 753–768. [Google Scholar] [CrossRef]
- Fujihara, K.; Cook, L.J. Neuromyelitis optica spectrum disorders and myelin oligodendrocyte glycoprotein antibody-associated disease: Current topics. Curr. Opin. Neurol. 2020, 33, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Kitley, J.; Waters, P.; Woodhall, M.; Leite, M.I.; Murchison, A.; George, J.; Küker, W.; Chandratre, S.; Vincent, A.; Palace, J. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: A comparative study. JAMA Neurol. 2014, 71, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Sahraian, M.A.; Moghadasi, A.N.; Azimi, A.R.; Asgari, N.; Akhoundi, F.H.; Abolfazli, R.; Alaie, S.; Ashtari, F.; Ayromlou, H.; Baghbanian, S.M. Diagnosis and management of neuromyelitis optica spectrum disorder (NMOSD) in Iran: A consensus guideline and recommendations. Mult. Scler. Relat. Disord. 2017, 18, 144–151. [Google Scholar] [CrossRef]
- Abboud, H.; Petrak, A.; Mealy, M.; Sasidharan, S.; Siddique, L.; Levy, M. Treatment of acute relapses in neuromyelitis optica: Steroids alone versus steroids plus plasma exchange. Mult. Scler. J. 2016, 22, 185–192. [Google Scholar] [CrossRef]
- Akaishi, T.; Takeshita, T.; Himori, N.; Takahashi, T.; Misu, T.; Ogawa, R.; Kaneko, K.; Fujimori, J.; Abe, M.; Ishii, T. Rapid administration of high-dose intravenous methylprednisolone improves visual outcomes after optic neuritis in patients with AQP4-IgG-positive NMOSD. Front. Neurol. 2020, 11, 932. [Google Scholar] [CrossRef]
- Turco, E.C.; Curti, E.; Maffini, V.; Pisani, F.; Granella, F. Neuromyelitis optica spectrum disorder attack triggered by herpes zoster infection. Mult. Scler. Int. 2020, 2020, 6151258. [Google Scholar] [CrossRef] [PubMed]
- Vanood, A.; Wingerchuk, D. Systematic review investigating relationship between neuromyelitis optica spectrum disorder (NMOSD) and vaccination (P1. 2-003). Neurology 2019, 92. [Google Scholar] [CrossRef]
- Bchetnia, M.; Girard, C.; Duchaine, C.; Laprise, C. The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): A review of the current global status. J. Infect. Public Health 2020, 13, 1601–1610. [Google Scholar] [CrossRef]
- Rabail, R.; Ahmed, W.; Ilyas, M.; Rajoka, M.S.R.; Hassoun, A.; Khalid, A.R.; Khan, M.R.; Aadil, R.M. The side effects and adverse clinical cases reported after COVID-19 immunization. Vaccines 2022, 10, 488. [Google Scholar] [CrossRef]
- Müller-Jensen, L.; Ploner, C.J.; Kroneberg, D.; Schmidt, W.U. Clinical presentation and causes of non-traumatic spinal cord injury: An observational study in emergency patients. Front. Neurol. 2021, 12, 701927. [Google Scholar] [CrossRef]
- Burns, A.S.; Marino, R.J.; Flanders, A.E.; Flett, H. Clinical diagnosis and prognosis following spinal cord injury. Handb. Clin. Neurol. 2012, 109, 47–62. [Google Scholar]
- Halvorsen, A.; Pettersen, A.; Nilsen, S.; Halle, K.K.; Schaanning, E.E.; Rekand, T. Non-traumatic spinal cord injury in Norway 2012–2016: Analysis from a national registry and comparison with traumatic spinal cord injury. Spinal Cord 2019, 57, 324–330. [Google Scholar] [CrossRef]
- Bruscolini, A.; Sacchetti, M.; La Cava, M.; Gharbiya, M.; Ralli, M.; Lambiase, A.; De Virgilio, A.; Greco, A. Diagnosis and management of neuromyelitis optica spectrum disorders-An update. Autoimmun. Rev. 2018, 17, 195–200. [Google Scholar] [CrossRef]
- Sepúlveda, M.; Armangué, T.; Sola-Valls, N.; Arrambide, G.; Meca-Lallana, J.E.; Oreja-Guevara, C.; Mendibe, M.; De Arcaya, A.A.; Aladro, Y.; Casanova, B. Neuromyelitis optica spectrum disorders: Comparison according to the phenotype and serostatus. Neurol.-Neuroimmunol. Neuroinflamm. 2016, 3, e225. [Google Scholar] [CrossRef]
- Kim, S.-M.; Kim, S.-J.; Lee, H.J.; Kuroda, H.; Palace, J.; Fujihara, K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther. Adv. Neurol. Disord. 2017, 10, 265–289. [Google Scholar] [CrossRef]
- Kleiter, I.; Gold, R. Present and future therapies in neuromyelitis optica spectrum disorders. Neurotherapeutics 2016, 13, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Carnero Contentti, E.; Correale, J. Neuromyelitis optica spectrum disorders: From pathophysiology to therapeutic strategies. J. Neuroinflamm. 2021, 18, 208. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Hyun, J.-W.; Kim, H.J. Individualized B cell-targeting therapy for neuromyelitis optica spectrum disorder. Neurochem. Int. 2019, 130, 104347. [Google Scholar] [CrossRef] [PubMed]
- Cacciaguerra, L.; Tortorella, P.; Rocca, M.A.; Filippi, M. Targeting neuromyelitis optica pathogenesis: Results from randomized controlled trials of biologics. Neurotherapeutics 2021, 18, 1623–1636. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.T.; Leonard, G.R.; Cepela, D.J. Classifications in brief: American spinal injury association (ASIA) impairment scale. Clin. Orthop. Relat. Res. 2017, 475, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.J.; Ditunno, J.F., Jr.; Donovan, W.H.; Maynard, F., Jr. Neurologic recovery after traumatic spinal cord injury: Data from the Model Spinal Cord Injury Systems. Arch. Phys. Med. Rehabil. 1999, 80, 1391–1396. [Google Scholar] [CrossRef]
- Khorasanizadeh, M.; Yousefifard, M.; Eskian, M.; Lu, Y.; Chalangari, M.; Harrop, J.S.; Jazayeri, S.B.; Seyedpour, S.; Khodaei, B.; Hosseini, M. Neurological recovery following traumatic spinal cord injury: A systematic review and meta-analysis. J. Neurosurg. Spine 2019, 30, 683–699. [Google Scholar] [CrossRef]
- Carnero Contentti, E.; Daccach Marques, V.; Soto de Castillo, I.; Tkachuk, V.; Ariel, B.; Castillo, M.C.; Cristiano, E.; Diégues Serva, G.B.; Dos Santos, A.C.; Finkelsteyn, A.M. Clinical features and prognosis of late-onset neuromyelitis optica spectrum disorders in a Latin American cohort. J. Neurol. 2020, 267, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Collongues, N.; Marignier, R.; Jacob, A.; Leite, M.; Siva, A.; Paul, F.; Zephir, H.; Akman-Demir, G.; Elsone, L.; Jarius, S. Characterization of neuromyelitis optica and neuromyelitis optica spectrum disorder patients with a late onset. Mult. Scler. J. 2014, 20, 1086–1094. [Google Scholar] [CrossRef]
- Badrawi, N.; Kumar, N.; Albastaki, U. Post COVID-19 vaccination neuromyelitis optica spectrum disorder: Case report & MRI findings. Radiol. Case Rep. 2021, 16, 3864–3867. [Google Scholar] [PubMed]
- Fujimori, J.; Miyazawa, K.; Nakashima, I. Initial clinical manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J. Neuroimmunol. 2021, 361, 577755. [Google Scholar] [CrossRef] [PubMed]
- Mirmosayyeb, O.; Ghaffary, E.; Vaheb, S.; Pourkazemi, R.; Shaygannejad, V. Multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) following COVID-19 vaccines: A systematic review. Rev. Neurol. 2023, 179, 265–281. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Kharrazian, D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: Implications for autoimmune diseases. Front. Immunol. 2021, 11, 3679. [Google Scholar] [CrossRef]
| Admission | HD at 20 Days (Transfer to RM) | HD at 2 Months | HD at 4 Months (Discharge) | 3 Months after Discharge | ||
|---|---|---|---|---|---|---|
| BBS | NC | 3 | 4 | 54 | 56 | |
| K-MBI | NC | 23 | 30 | 49 | 98 | |
| AIS | NC | A | B | D | E | |
| MRS | 5 | 5 | 5 | 2 | 0 | |
| EDSS | 8.5 | NC | 8 | NC | 1 | |
| Dynamic test | TUG | NC | NT | NT | 28.17 s | 8.30 s |
| 10 m walk test | NC | NT | NT | 28.95 s | 7.99 s | |
| 6 min walk test | NC | NT | NT | 90 m | 415 m | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.-S.; Ryu, S.-M.; Lim, Y.-H.; Kim, A.-R.; Jung, T.-D. Treatment and Rehabilitation of a Patient with Neuromyelitis Optica Spectrum Disorder-Induced Complete Spinal Cord Injury Following COVID-19 Vaccination: A Case Report. J. Clin. Med. 2024, 13, 1175. https://doi.org/10.3390/jcm13041175
Han J-S, Ryu S-M, Lim Y-H, Kim A-R, Jung T-D. Treatment and Rehabilitation of a Patient with Neuromyelitis Optica Spectrum Disorder-Induced Complete Spinal Cord Injury Following COVID-19 Vaccination: A Case Report. Journal of Clinical Medicine. 2024; 13(4):1175. https://doi.org/10.3390/jcm13041175
Chicago/Turabian StyleHan, Jun-Sang, Seong-Mun Ryu, Young-Hwan Lim, Ae-Ryoung Kim, and Tae-Du Jung. 2024. "Treatment and Rehabilitation of a Patient with Neuromyelitis Optica Spectrum Disorder-Induced Complete Spinal Cord Injury Following COVID-19 Vaccination: A Case Report" Journal of Clinical Medicine 13, no. 4: 1175. https://doi.org/10.3390/jcm13041175






