Subjective Complaints and Coping Strategies of Individuals with Reported Low-Frequency Noise Perceptions
Abstract
:1. Introduction
1.1. Low-Frequency Noise (LFN)
1.2. Cognitive Complaints Reported in Relation to LFN
1.3. Psychological Complaints Reported in Relation to LFN
1.3.1. Depression
1.3.2. Sleep
1.3.3. Fatigue
1.3.4. Stress
1.4. Coping
1.5. Research Aims and Questions
- What types and severity of complaints do individuals with LFN perceptions report?
- Do individuals with LFN perceptions report more complaints compared to individuals with no LFN perceptions?
- What proportion of individuals with and without LFN perceptions report complaints in a presumably clinically relevant range?
- What kind of coping mechanisms do individuals with LFN perceptions use compared to individuals with no LFN perceptions?
2. Methods
2.1. Procedure and Participants
2.2. Materials
2.3. Statistical Analysis
3. Results
3.1. LFN-Related Cognitive Complaints
3.1.1. Raw Scores
3.1.2. Proportion of Individuals with above Average Symptom Reporting
| LFN1 n = 181 | LFN2 LFN2-SA = 131 | CG CG-SA = 229 | LFN1–LFN2 | LFN1–CG | LFN2–CG | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | M ± SD | Range | M | N | M ± SD | Range | M | N | M ± SD | Range | M | H | p | η2 | r | r | r | |
| Cognition | ||||||||||||||||||
| FLei Sum | 169 | 43.2 ± 23.2 | 0–108 | 42 | 131 | 31.3 ± 19.1 | 0–81 | 30 | 229 | 18.9 ± 14.9 | 0–65 | 17 | 118.4 | <0.001 ** | 0.22 | 0.25 ** | 0.52 ** | 0.32 ** |
| FLei Attention | 170 | 15.6 ± 8.8 | 0–36 | 16 | 131 | 10.6± 6.9 | 0–32 | 10 | 229 | 6.0 ± 5.3 | 0–24 | 5 | 131.5 | <0.001 ** | 0.25 | 0.28 ** | 0.53 ** | 0.34 ** |
| FLei Memory | 177 | 15.5 ± 7.9 | 0–35 | 15 | 131 | 11.8 ± 6.5 | 0–28 | 11 | 229 | 7.7 ± 5.9 | 0–27 | 7 | 106.7 | <0.001 ** | 0.20 | 0.23 ** | 0.49 ** | 0.31 ** |
| Executive functions | ||||||||||||||||||
| FLei Executive functions | 180 | 11.7 ± 7.7 | 0–37 | 11 | 131 | 8.9 ± 6.5 | 0–25 | 8 | 229 | 5.2 ± 4.7 | 0–20 | 4 | 86.6 | <0.001 ** | 0.16 | 0.17 * | 0.45 ** | 0.28 ** |
| BRIEF-A Global | 167 | 109.9 ± 23.1 | 70–168 | 107 | 131 | 99.9 ± 22.7 | 70–162 | 98 | 229 | 89.7 ± 17.0 | 70–148 | 86 | 80.8 | <0.001 ** | 0.15 | 0.22 ** | 0.45 ** | 0.22 ** |
| BRIEF-A BR | 172 | 46.6 ± 10.3 | 30–77 | 46 | 131 | 42.8 ± 9.6 | 30–67 | 41 | 229 | 38.5 ± 7.7 | 30–67 | 37 | 70.0 | <0.001 ** | 0.13 | 0.19 ** | 0.41 ** | 0.23 ** |
| BRIEF-A MC | 172 | 63.0 ± 14.2 | 40–104 | 61 | 131 | 57.1 ± 14.1 | 40–95 | 55 | 229 | 51.2± 10.6 | 40–86 | 48 | 76.7 | <0.001 ** | 0.14 | 0.22 ** | 0.44 ** | 0.20 ** |
| Depressive symptoms | ||||||||||||||||||
| BDI-II Sum | 178 | 11.7 ± 8.3 | 0–42 | 10 | 131 | 7.2 ± 6.2 | 0–29 | 6 | 229 | 4.4 ± 4.3 | 0–27 | 3 | 104.7 | <0.001 ** | 0.19 | 0.29 ** | 0.50 ** | 0.24 ** |
| LFN2-SB = 108 | CG-SB = 239 | |||||||||||||||||
| Sleep (PSQI) | ||||||||||||||||||
| Global | 142 | 8.6 ± 4.7 | 1–19 | 8 | 102 | 7.7 ± 4.0 | 0–19 | 7 | 231 | 5.5 ± 3.3 | 0–19 | 5 | 49.0 | <0.001 ** | 0.10 | 0.08 | 0.33 ** | 0.27 ** |
| Sleep Quality | 178 | 1.6 ± 0.9 | 0–3 | 2 | 108 | 1.3 ± 0.8 | 0–3 | 1 | 239 | 0.9 ± 0.8 | 0–3 | 1 | 54.0 | <0.001 ** | 0.10 | 0.17 * | 0.35 ** | 0.19 ** |
| Sleep Latency | 164 | 1.5 ± 1.1 | 0–3 | 1 | 103 | 1.5 ± 1.1 | 0–3 | 1 | 232 | 1.0 ± 1.0 | 0–3 | 1 | 29.1 | <0.001 ** | 0.05 | 0.01 | 0.24 ** | 0.22 ** |
| Sleep Duration | 170 | 1.1 ± 1.1 | 0–3 | 2 | 106 | 0.9 ± 1.0 | 0–3 | 1 | 239 | 0.5 ± 0.8 | 0–3 | 0 | 32.2 | <0.001 ** | 0.06 | 0.08 | 0.27 ** | 0.19 ** |
| Habit Sleep Efficiency | 169 | 1.5 ± 1.2 | 0–3 | 1 | 105 | 1.5 ± 1.3 | 0–3 | 1 | 236 | 1.1 ± 1.2 | 0–3 | 1 | 10.2 | 0.006 * | 0.01 | 0.02 | 0.15 * | 0.11 |
| Sleep Disturbance | 161 | 1.4 ± 0.6 | 0–3 | 1 | 108 | 1.4 ± 0.5 | 0–3 | 1 | 239 | 1.2 ± 0.5 | 0–3 | 1 | 19.3 | <0.001 ** | 0.03 | 0.06 | 0.21 ** | 0.15 * |
| Sleep Medication | 181 | 0.8 ± 1.2 | 0–3 | 0 | 108 | 0.4 ± 0.9 | 0–3 | 0 | 239 | 0.3 ± 0.8 | 0–3 | 0 | 34.1 | <0.001 ** | 0.06 | 0.19 * | 0.28 ** | 0.08 |
| Daytime Dysfunction | 178 | 1.0 ± 0.8 | 0–3 | 1 | 108 | 0.8 ± 0.7 | 0–3 | 1 | 239 | 0.5 ± 0.6 | 0–3 | 0 | 39.1 | <0.001 ** | 0.07 | 0.06 | 0.28 ** | 0.23 ** |
| Fatigue | ||||||||||||||||||
| FSS Sum | 180 | 37.0 ± 13.9 | 9–63 | 38 | 108 | 36.0 ± 10.7 | 13–62 | 37 | 239 | 27.1 ± 11.6 | 9–61 | 25 | 68.4 | <0.001 ** | 0.12 | 0.05 | 0.35 ** | 0.35 ** |
| Daily stress (APLN) | ||||||||||||||||||
| All items | ||||||||||||||||||
| Total | 165 | 38.1 ± 32.2 | 0–172 | 27 | 108 | 41.7 ± 42.1 | 0–198 | 27 | 239 | 18.6 ± 35.7 | 0–248 | 8 | 110.0 | <0.001 ** | 0.21 | 0.01 | 0.48 ** | 0.40 ** |
| Frequency | 169 | 26.5 ± 17.9 | 0–114 | 22 | 108 | 45.4 ± 31.5 | 1–114 | 37 | 239 | 22.2 ± 22.4 | 0–114 | 16 | 71.8 | <0.001 ** | 0.14 | 0.32 ** | 0.20 ** | 0.43 ** |
| Intensity | 164 | 1.4 ± 0.6 | 0–3.0 | 1.3 | 108 | 0.9 ± 0.5 | 0.0–2.3 | 0.8 | 229 | 0.7 ± 0.5 | 0.0–3.0 | 0.6 | 141.6 | <0.001 ** | 0.28 | 0.44 ** | 0.58 ** | 0.21 ** |
| Dependent items | ||||||||||||||||||
| Total | 171 | 8.5 ± 8.6 | 0–37 | 6 | 108 | 9.4 ± 10.6 | 0–50 | 5 | 239 | 4.1 ± 8.6 | 0–63 | 2 | 77.7 | <0.001 ** | 0.15 | 0.01 | 0.38 ** | 0.37 ** |
| Frequency | 175 | 6.1 ± 5.1 | 0–28 | 5 | 108 | 10.9 ± 8.1 | 0–28 | 9 | 239 | 5.5 ± 5.7 | 0–28 | 4 | 53.2 | <0.001 ** | 0.10 | 0.32 ** | 0.10 | 0.39 ** |
| Intensity | 156 | 1.3 ± 0.7 | 0.0–3.0 | 1.3 | 106 | 0.8 ± 0.5 | 0.0–2.0 | 0.7 | 215 | 0.6 ± 0.6 | 0.0–3.0 | 0.4 | 118.6 | <0.001 ** | 0.25 | 0.42 ** | 0.54 ** | 0.21 ** |
| Independent items | ||||||||||||||||||
| Total | 172 | 8.4 ± 6.6 | 0–37 | 7 | 108 | 8.5 ± 8.4 | 0–40 | 6 | 239 | 4.0 ± 7.5 | 0–52 | 2 | 107.2 | <0.001 ** | 0.20 | 0.06 | 0.48 ** | 0.37 ** |
| Frequency | 174 | 5.3 ± 3.6 | 0–21 | 5 | 108 | 8.4 ± 5.9 | 0–21 | 6.5 | 239 | 4.3 ± 4.3 | 0–21 | 3 | 60.1 | <0.001 ** | 0.11 | 0.26 ** | 0.21 ** | 0.39 ** |
| Intensity | 167 | 1.6 ± 0.7 | 0–3.0 | 1.6 | 106 | 1.0 ± 0.6 | 0.0–2.7 | 1.0 | 211 | 0.7 ± 0.6 | 0.0–3.0 | 0.7 | 125.6 | <0.001 ** | 0.26 | 0.44 ** | 0.55 ** | 0.18 * |
| LFN1 n = 181 | LFN2 LFN2-SA = 131 | CG CG-SA = 229 | LFN1–LFN2 | LFN1–CG | LFN2–CG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | χ2 | df | p | V | V | V | V | |
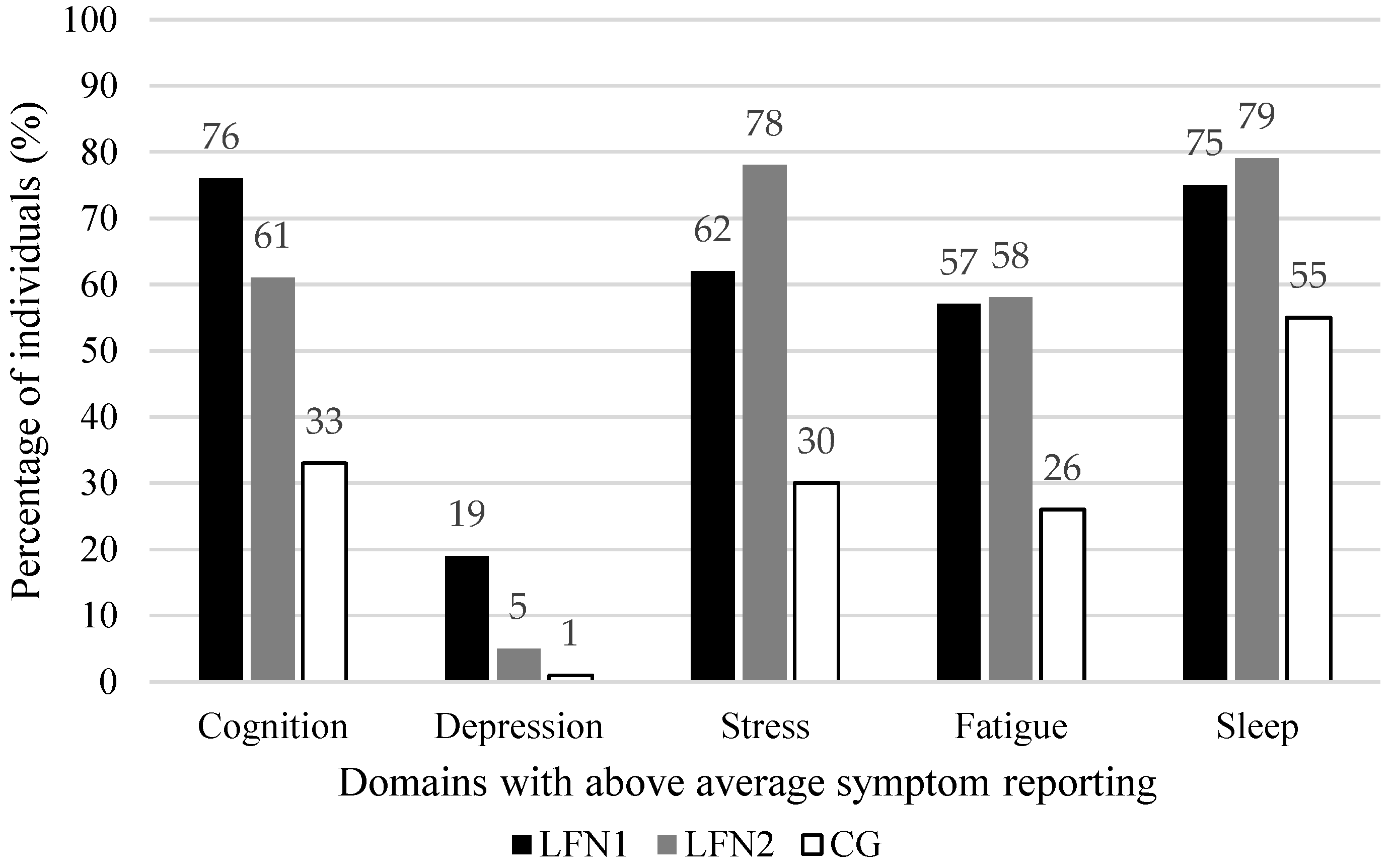
| Cognition a | 181 | 75.7 | 131 | 61.1 | 229 | 33.2 | 76.88 | 2 | <0.001 ** | 0.38 | 0.16 * | 0.42 ** | 0.27 ** |
| FLei Sum | 169 | 62.1 | 131 | 44.3 | 229 | 17.9 | 82.68 | 2 | <0.001 ** | 0.40 | 0.18 * | 0.45 ** | 0.28 ** |
| FLei Attention | 170 | 64.1 | 131 | 39.7 | 229 | 16.2 | 96.31 | 2 | <0.001 ** | 0.43 | 0.24 ** | 0.49 ** | 0.26 ** |
| FLei Memory | 177 | 58.8 | 131 | 41.2 | 229 | 14.8 | 86.05 | 2 | <0.001 ** | 0.40 | 0.17 * | 0.46 ** | 0.30 ** |
| Executive functions | |||||||||||||
| FLei Executive functions | 180 | 52.2 | 131 | 43.3 | 229 | 17.9 | 56.58 | 2 | <0.001 ** | 0.32 | 0.09 | 0.36 ** | 0.28 ** |
| BRIEF-A Global | 167 | 23.2 | 131 | 15.3 | 229 | 5.2 | 31.93 | 2 | <0.001 ** | 0.25 | 0.12 | 0.29 ** | 0.17 * |
| BRIEF-A BR | 172 | 23.8 | 131 | 14.5 | 229 | 6.1 | 25.82 | 2 | <0.001 ** | 0.22 | 0.12 | 0.26 ** | 0.14 * |
| BRIEF-A MC | 172 | 28.5 | 131 | 17.6 | 229 | 7.9 | 29.79 | 2 | <0.001 ** | 0.24 | 0.13 | 0.27 ** | 0.15 * |
| Depressive symptoms (BDI-II) | 178 | 18.5 | 131 | 5.3 | 229 | 0.9 | 44.89 | 2 | <0.001 ** | 0.29 | 0.19 ** | 0.31 ** | 0.14 * |
| Moderate symptoms | 178 | 14.6 | 131 | 4.6 | 229 | 0.9 | 32.79 | 2 | <0.001 ** | 0.25 | 0.16 * | 0.27 ** | 0.12 |
| Severe symptoms | 178 | 3.9 | 131 | 0.8 | 229 | 0 | 11.19 | 2 | 0.004 * | 0.14 | 0.10 | 0.15 * | 0.07 |
| LFN2-SB = 108 | CG-SB = 239 | ||||||||||||
| Sleep (PSQI) | 142 | 75.4 | 102 | 79.4 | 231 | 55.0 | 26.31 | 2 | <0.001 ** | 0.24 | 0.05 | 0.21 ** | 0.23 ** |
| Fatigue (FSS) | 180 | 56.7 | 108 | 58.3 | 239 | 26.4 | 50.99 | 2 | <0.001 ** | 0.31 | 0.02 | 0.31 ** | 0.31 ** |
| Daily stress (APLN) | 169 | 61.5 | 108 | 77.8 | 239 | 29.7 | 81.70 | 2 | <0.001 ** | 0.40 | 0.17 * | 0.32 ** | 0.45 ** |
| Total | 165 | 41.8 | 108 | 41.7 | 239 | 11.7 | 57.39 | 2 | <0.001 ** | 0.34 | 0.002 | 0.35 ** | 0.34 ** |
| Frequency | 169 | 46.2 | 108 | 75.0 | 239 | 28.9 | 64.58 | 2 | <0.001 ** | 0.35 | 0.29 ** | 0.18 ** | 0.43 ** |
| Intensity | 164 | 34.1 | 108 | 6.5 | 229 | 3.1 | 83.27 | 2 | <0.001 ** | 0.41 | 0.32 ** | 0.42 ** | 0.08 |
3.2. LFN-Related Psychological Complaints
3.2.1. Depressive Symptoms
Raw Scores
Proportion of Individuals with above Average Symptom Reporting
3.2.2. Sleep
Raw Scores
Proportion of Individuals with above Average Symptom Reporting
3.2.3. Fatigue
Raw Scores
Proportion of Individuals with above Average Symptom Reporting
3.2.4. Daily Stress
Raw Scores
Proportion of Individuals with above Average Symptom Reporting
3.3. Sum of Domains with above Average Symptom Reporting
3.4. Coping Strategies
| LFN1 n = 181 | LFN2 LFN2-SB = 108 | CG CG-SB = 239 | LFN1–LFN2 | LFN1–CG | LFN2–CG | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | M ± SD | Range | M | N | M ± SD | Range | M | N | M ± SD | Range | M | H | p | η2 | r | r | r | |
| Active problem-oriented coping a | 169 | 24.6 ± 6.5 | 10–39 | 24 | 108 | 21.8 ± 7.2 | 10–40 | 21 | 239 | 21.0 ± 7.3 | 10–40 | 21 | 24.7 | <0.001 ** | 0.04 | 0.21 ** | 0.24 ** | 0.04 |
| Active coping b | 179 | 6.0 ± 1.9 | 2–8 | 6 | 108 | 5.0 ± 1.9 | 2–8 | 5 | 239 | 4.8 ± 2.0 | 2–8 | 5 | 36.7 | <0.001 ** | 0.07 | 0.25 ** | 0.29 ** | 0.04 |
| Suppression of competing activities b | 179 | 4.2 ± 1.8 | 2–8 | 4 | 108 | 4.0 ± 1.8 | 2–8 | 4 | 239 | 3.9 ± 1.8 | 2–8 | 4 | 2.7 | 0.26 | <0.01 | 0.05 | 0.08 | 0.03 |
| Positive reframing c | 178 | 4.7 ± 2.0 | 2–8 | 4 | 108 | 4.3 ± 1.8 | 2–8 | 4 | 239 | 4.3 ± 1.9 | 2–8 | 4 | 4.5 | 0.10 | <0.01 | 0.09 | 0.10 | 0.02 |
| Planning b | 178 | 5.8 ± 1.9 | 2–8 | 6 | 108 | 4.5 ± 1.8 | 2–8 | 4 | 239 | 4.5 ± 1.9 | 2–8 | 4 | 53.8 | <0.001 ** | 0.10 | 0.33 ** | 0.33 ** | <0.01 |
| Restrain b | 169 | 4.0 ± 1.6 | 2–8 | 4 | 108 | 4.0 ± 1.6 | 2–8 | 4 | 239 | 3.6 ± 1.6 | 2–8 | 3 | 9.0 | 0.01 | 0.01 | <0.01 | 0.13 * | 0.12 |
| Support Seeking | 178 | 15.0 ± 4.4 | 6–24 | 15 | 108 | 11.0 ± 4.0 | 6–22 | 10 | 239 | 10.0 ± 4.0 | 6–24 | 9 | 122.6 | <0.001 ** | 0.23 | 0.43 ** | 0.52 ** | 0.14 * |
| Instrumental support b | 179 | 4.7 ± 1.8 | 2–8 | 5 | 108 | 3.4 ± 1.6 | 2–8 | 3 | 239 | 3.0 ± 1.5 | 2–8 | 2 | 95.1 | <0.001 ** | 0.18 | 0.34 ** | 0.47 ** | 0.14 |
| Focus on venting emotions d | 178 | 4.9 ± 1.8 | 2–8 | 5 | 108 | 3.7 ± 1.4 | 2–8 | 3 | 239 | 3.4 ± 1.5 | 2–8 | 3 | 84.1 | <0.001 ** | 0.16 | 0.35 ** | 0.43 ** | 0.11 |
| Use of emotional support c | 178 | 5.4 ± 1.9 | 2–8 | 6 | 108 | 3.8 ± 1.6 | 2–8 | 4 | 239 | 3.5 ± 1.7 | 2–8 | 3 | 99.4 | <0.001 ** | 0.19 | 0.39 ** | 0.47 ** | 0.11 |
| Avoidance Behavior | 176 | 10.7 ± 3.2 | 6–21 | 10 | 108 | 10.3 ± 3.3 | 6–23 | 10 | 239 | 9.1 ± 2.8 | 6–19 | 9 | 30.8 | <0.001 ** | 0.06 | 0.08 | 0.27 ** | 0.17 * |
| Self-distraction d | 178 | 4.7 ± 1.8 | 2–8 | 5 | 108 | 4.2 ± 1.8 | 2–8 | 4 | 239 | 3.6 ± 1.6 | 2–8 | 3 | 36.5 | <0.001 ** | 0.07 | 0.14 | 0.29 ** | 0.14 * |
| Behavioral disengagement d | 178 | 3.1 ± 1.5 | 2–8 | 2 | 108 | 3.0 ± 1.4 | 2–8 | 2 | 239 | 2.6 ± 1.0 | 2–8 | 2 | 9.5 | 0.009 * | 0.01 | 0.02 | 0.14 * | 0.11 |
| Denial c | 178 | 3.0 ± 1.5 | 2–8 | 2 | 108 | 3.1 ± 1.6 | 2–8 | 2 | 239 | 2.8 ± 1.5 | 2–8 | 2 | 7.0 | 0.03 | 0.01 | 0.06 | 0.09 | 0.14 |
| Non-dimension bound strategies | ||||||||||||||||||
| Religion c | 179 | 2.8 ± 1.7 | 2–8 | 2 | 108 | 3.0 ± 1.6 | 2–8 | 2 | 239 | 2.6 ± 1.5 | 2–8 | 2 | 7.7 | 0.02 | 0.01 | 0.10 | 0.05 | 0.15 * |
| Humor | 177 | 3.9 ± 1.7 | 2–8 | 4 | 108 | 4.0 ± 1.7 | 2–8 | 4 | 239 | 3.7 ± 1.7 | 2–8 | 4 | 1.8 | 0.40 | <0.01 | 0.03 | 0.04 | 0.07 |
| Acceptance c | 178 | 4.7 ± 1.8 | 2–8 | 4.5 | 108 | 4.9 ± 1.6 | 2–8 | 5 | 239 | 4.8 ± 2.0 | 2–8 | 5 | 1.2 | 0.54 | <0.01 | 0.07 | 0.01 | 0.05 |
| Substance use | 178 | 4.9 ± 2.1 | 4–16 | 4 | 108 | 4.9 ± 1.5 | 4–11 | 4 | 239 | 4.3 ± 0.7 | 4–9 | 4 | 24.2 | <0.001 ** | 0.04 | 0.05 | 0.19 ** | 0.24 ** |
3.4.1. Active Problem-Oriented Coping
3.4.2. Support Seeking
3.4.3. Avoidance Behavior
3.4.4. Coping Strategies Not Allocated to Any Dimension
4. Discussion
4.1. LFN-Related Cognitive Complaints
4.2. LFN-Related Psychological Complaints
4.2.1. Depressive Symptoms
4.2.2. Sleep
4.2.3. Fatigue
4.2.4. Stress
4.3. Summary LFN-Related Complaints
4.4. Coping
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hänninen, O.; Knol, A.B.; Jantunen, M.; Lim, T.A.; Conrad, A.; Rappolder, M.; Carrer, P.; Fanetti, A.C.; Kim, R.; Buekers, J.; et al. Environmental burden of disease in Europe: Assessing nine risk factors in six countries. Environ. Health Perspect. 2014, 122, 439–446. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Regional Office for Europe. Health and Environment in Europe: Progress Assessment. 2010. Available online: https://iris.who.int/bitstream/handle/10665/107983/9789289041980-eng.pdf?sequence=1&isAllowed=y (accessed on 11 December 2023).
- World Health Organization; Regional Office for Europe. Burden of Disease from Environmental Noise: Quantification of Healthy Life Years Lost in Europe. 2011. Available online: http://www.euro.who.int/__data/assets/pdf_file/0008/136466/e94888.pdf (accessed on 11 December 2023).
- Rijksinstituut voor Volksgezondheid en Milieu. Laagfrequentgeluid [Fact Sheet]. 2020. Available online: https://www.rivm.nl/sites/default/files/2020-09/Factsheet%20laagfrequent%20geluid.pdf (accessed on 11 December 2023).
- Leventhall, G.; Pelmear, P.; Benton, S. A Review of Published Research on Low Frequency Noise and Its Effects; Department for Environment, Food and Rural Affairs: London, UK, 2003; 88p. [Google Scholar]
- Erasmus, M.; van der Plas, D. Jaarrapportage 2019 Laagfrequentgeluid. 2020. Available online: https://overijssel.notubiz.nl/document/8659360/1/Jaarrapportage+2019+Stichting+Laagfrequent+geluid%28gec%29 (accessed on 11 December 2023).
- Van Kamp, I.; Breugelmans, O.R.P.; van Poll, H.F.P.M.; Baliatsas, C.; van Kempen, E.E.M.M. Meldingen over en Hinder van Laagfrequent Geluid of Het Horen van een Bromtoon in Nederland: Inventarisatie. 2018. Available online: https://www.rivm.nl/bibliotheek/rapporten/2018-0119.pdf (accessed on 11 December 2023).
- Møller, H.; Lydolf, M. A questionnaire survey of complaints of infrasound and low-frequency noise. J. Low Freq. Noise Vib. Act. Control 2002, 21, 53–63. [Google Scholar] [CrossRef]
- Baliatsas, C.; van Kamp, I.; van Poll, R.; Yzermans, J. Health effects from low-frequency noise and infrasound in the general population: Is it time to listen? A systematic review of observational studies. Sci. Total Environ. 2016, 557–558, 163–169. [Google Scholar] [CrossRef]
- Persson Waye, K.; Rylander, R.; Benton, S.; Leventhall, H.G. Effects on performance and work quality due to low frequency ventilation noise. J. Sound Vib. 1997, 205, 467–474. [Google Scholar] [CrossRef]
- Alves, J.A.; Filipa, N.P.; Silva, L.T.; Paula, R. Low-frequency noise and its main effects on human health—A review of the literature between 2016 and 2019. J. Appl. Sci. 2020, 10, 5205. [Google Scholar] [CrossRef]
- Van Zomeren, A.; Brouwer, W. Clinical Neuropsychology of Attention; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Persson Waye, P.; Rylander, R. The prevalence of annoyance and effects after longterm exposure to low-frequency noise. J. Sound Vib. 2001, 240, 483–497. [Google Scholar] [CrossRef]
- Ising, H.; Ising, M. Chronic cortisol increases in the first half of the night caused by road traffic noise. Noise Health 2002, 4, 13–21. [Google Scholar] [PubMed]
- Pawlaczyk-Luszczyńiska, M.; Dudarewicz, A.; Waszkowska, M.; Szymczak, W.; Sliwińska-Kowalska, M. The Impact of Low-Frequency Noise on Human Mental Performance. Int. J. Occup. Med. Environ. Health 2005, 18, 185–198. [Google Scholar]
- Pawlaczyk-Luszczyńska, M.; Dudarewicz, A.; Waszkowska, M.; Szymczak, W.; Kamedula, M.; Sliwinska-Kowalska, M. Does low frequency noise at moderate levels influence human mental performance? J. Low Freq. Noise Vib. Act. Control 2005, 24, 25–42. [Google Scholar] [CrossRef]
- Alimohammadi, I.; Ebrahimi, H. Comparison between effects of low and high frequency noise on mental performance. Appl. Acoust. 2017, 126, 131–135. [Google Scholar] [CrossRef]
- Alimohammadi, I.; Sandrock, S.; Gohari, M.R. The effects of low frequency noise on mental performance and annoyance. Environ. Monit. Assess. 2013, 185, 7043–7051. [Google Scholar] [CrossRef]
- Gomes, L.M.; Martinho Pimenta, A.J.; Castelo Branco, N.A. Effects of occupational exposure to low frequency noise on cognition. Aviat. Space Environ. Med. 1999, 70 Pt 2, A115–A118. [Google Scholar]
- American Psychological Association. Memory. In APA Dictionary of Psychology; American Psychological Association: Washington, DC, USA; Available online: https://dictionary.apa.org/memory (accessed on 11 December 2023).
- Leventhall, G.; Robertson, D.; Benton, S.; Leventhall, L. Helping Sufferers to Cope with Noise Using Distance Learning Cognitive Behaviour Therapy. J. Low Freq. Noise Vib. Act. Control 2012, 31, 193–203. [Google Scholar] [CrossRef]
- Goldberg, E. The Executive Brain: Frontal Lobes and the Civilized Mind; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Ward, J. The Student’s Guide to Cognitive Neuroscience, 4th ed.; Routledge: Oxford, UK, 2020. [Google Scholar]
- Abbasi, A.M.; Motamedzade, M.; Aliabadi, M.; Golmohammadi, R.; Tapak, L. Study of the physiological and mental health effects caused by exposure to low-frequency noise in a simulated control room. Build. Acoust. 2018, 25, 233–248. [Google Scholar] [CrossRef]
- Rossi, L.; Prato, A.; Lesina, L.; Schiavi, A. Effects of Low-Frequency Noise on Human Cognitive Performances in Laboratory. Build. Acoust. 2018, 25, 17–33. [Google Scholar] [CrossRef]
- Weichenberger, M.; Kühler, R.; Bauer, M.; Hensel, J.; Brühl, R.; Ihlenfeld, A.; Ittermann, B.; Gallinat, J.; Koch, C.; Sander, T.; et al. Brief Bursts of Infrasound May Improve Cognitive Function—An Fmri Study. Heart Res. 2015, 328, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Persson Waye, K.; Bengtsson, J.; Kjellberg, A.; Benton, S. Low frequency noise ‘pollution’ interferes with performance. Noise Health 2001, 4, 33–49. [Google Scholar] [PubMed]
- Liang, P.; Li, J.; Li, Z.; Wei, J.; Li, J.; Zhang, S.; Xu, S.; Liu, Z.; Wang, J. Effect of Low-Frequency Noise Exposure on Cognitive Function: A Systematic Review and Meta-Analysis. BMC Public Health 2024, 24, 125. [Google Scholar] [CrossRef] [PubMed]
- Erdélyi, K.; Fuermaier, A.B.M.; Koerts, J.; Tucha, L.; Tucha, O. Laagfrequent geluid als omgevingsstressor in relatie tot neuropsychologische beperkingen. Neuropraxis 2019, 23, 48–52. [Google Scholar] [CrossRef]
- Wittchen, H.; Jacobi, F.; Rehm, J.; Gustavsson, A.; Svensson, M.; Jönsson, B.; Olesen, J.; Allgulander, C.; Alonso, J.; Faravelli, C.; et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 655–679. [Google Scholar] [CrossRef] [PubMed]
- Mirowska, M.; Mroz, E. Effect of Low-frequency Noise at low levels on human health in light of questionnaire investigation. In Proceedings of the 29th International Congress and Exhibition on Noise Control Engineering, Nice, France, 27–30 August 2000. [Google Scholar]
- Leventhall, G.; Benton, S.; Robertson, D. Coping strategies for low frequency noise. J. Low Freq. Noise Vib. Act. Control 2008, 27, 35–52. [Google Scholar] [CrossRef]
- Lin, J.Y.; Cheng, W.J.; Wu, C.F.; Chang, T.Y. Associations of Road Traffic Noise and Its Frequency Spectrum with Prevalent Depression in Taichung, Taiwan. Front. Public Health 2023, 11, 1116345. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Monazzam, M.R.; Ebrahimi, M.H.; Zakerian, S.A.; Dehghan, S.F.; Akbarzadeh, A. Assessment of Noise Effects of Wind Turbine on the General Health of Staff at Wind Farm of Manjil, Iran. J. Low Freq. Noise Vib. Act. Cont. 2016, 35, 91–98. [Google Scholar] [CrossRef]
- Alves, J.A.; Torres Silva, L.; Remoaldo, P. How Can Low-Frequency Noise Exposure Interact with the Well-Being of a Population? Some Results from a Portuguese Municipality. Appl. Sci. 2019, 9, 5566. [Google Scholar] [CrossRef]
- Berglund, B.; Hassmén, P.; Job, R.F. Sources and effects of low-frequency noise. J. Acoust. Soc. Am. 1996, 99, 2985–3002. [Google Scholar] [CrossRef] [PubMed]
- Persson Waye, K. Effects of low frequency noise on sleep. Noise Health 2004, 6, 87–91. [Google Scholar] [PubMed]
- Persson Waye, K.; Clow, A.; Edwards, S.; Hucklebridge, F.; Rylander, R. Effects of nighttime low frequency noise on the cortisol response to awakening and subjective sleep quality. Life Sci. 2002, 72, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Persson Waye, K.; Agge, A.; Clow, A.; Hucklebridge, F. Cortisol response and subjective sleep disturbance after low-frequency noise exposure. J. Sound Vib. 2004, 277, 453–457. [Google Scholar] [CrossRef]
- Öhrström, E.; Skånberg, A. Sleep disturbances from road traffic and ventilation noise—Laboratory and field experiments. J. Sound Vib. 2004, 271, 279–296. [Google Scholar] [CrossRef]
- Inaba, R.; Okada, A. Study of the effect of infra- and low frequency sound on sleep by EEG recording. J. Low Freq. Noise Vib. Act. Control 1988, 7, 15–19. [Google Scholar] [CrossRef]
- Banks, S.; Dinges, D.F. Behavioral and physiological consequences of sleep restriction. J. Clin. Sleep Med. 2007, 3, 519–528. [Google Scholar] [CrossRef]
- Landström, U.; Byström, M.; Nordström, B. Changes in Wakefulness during Exposure to Noise at 42 Hz, 1000 Hz and Individual Eeg Frequencies. J. Low Freq. Noise Vib. Act. Control 1985, 4, 27–33. [Google Scholar] [CrossRef]
- Kaozmarska, A.; Luozak, A. A study of Annoyance caused by Low-Frequency Noise during mental work. Int. J. Occup. Saf. Ergon. 2007, 13, 117–125. [Google Scholar] [CrossRef]
- Schust, M. Effects of Low Frequency Noise Up to 100 Hz. Noise Health 2004, 6, 73–85. [Google Scholar]
- Bengtsson, J.; Persson Waye, K.; Kjellberg, A. Evaluations of effects due to low-frequency noise in a low demanding work situation. J. Sound Vib. 2004, 278, 83–99. [Google Scholar] [CrossRef]
- American Psychological Association. Fatigue. In APA Dictionary of Psychology; Available online: https://dictionary.apa.org/fatigue (accessed on 11 December 2023).
- Benton, S.; Leventhall, H.G. The Role of ‘background Stressors’ in the Formation of Annoyance and Stress Responses. J. Low Freq. Noise Vib. Act. Cont. 1994, 13, 95–101. [Google Scholar] [CrossRef]
- Persson Waye, K.; Bengtsson, J.; Rylander, R.; Hucklebridge, F.; Evans, P.; Clow, A. Low Frequency Noise Enhances Cortisol among Noise Sensitive Subjects during Work Performance. Life Sci. 2002, 70, 745–758. [Google Scholar] [CrossRef]
- Lo Martire, V.; Caruso, D.; Palagini, L.; Zoccoli, G.; Bastianini, S. Stress & Sleep: A Relationship Lasting a Lifetime. Neurosci. Biobehav. Rev. 2020, 117, 65–77. [Google Scholar] [CrossRef]
- American Psychological Association. Coping. In APA Dictionary of Psychology; Available online: https://dictionary.apa.org/coping (accessed on 11 December 2023).
- Carver, C.S.; Scheier, M.F.; Weintraub, J.K. Assessing Coping Strategies: A Theoretically Based Approach. J. Pers. Soc. Psychol. 1989, 56, 267–283. [Google Scholar] [CrossRef]
- Leventhall, G. Low Frequency Noise. What We Know, What We Do Not Know, and What We Would Like to Know. J. Low Freq. Noise Vibr. Act. Cont. 2009, 28, 79–104. [Google Scholar] [CrossRef]
- Guski, R.; Schreckenberg, D.; Schuemer, R. Who Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Annoyance. Int. J. Environ. Res. Public Health 2017, 14, 1539. [Google Scholar] [CrossRef]
- Guski, R. Personal and social variables as co-determinants of noise annoyance. Noise Health 1999, 1, 45–56. [Google Scholar]
- Job, R.F.S. Community response to noise: A review of factors influencing the relationship between noise exposure and reaction. J. Acoust. Soc. Am. 1988, 83, 991–1001. [Google Scholar] [CrossRef]
- Stansfeld, S.A.; Shipley, M. Noise sensitivity and future risk of illness and mortality. Sci. Total Environ. 2015, 520, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Flindell, I.H.; Stallen, P.J.M. Non-acoustical factors in environmental noise. Noise Health 1999, 1, 11–16. [Google Scholar] [PubMed]
- Bartels, S.; Richard, I.; Ohlenforst, B.; Jeram, S.; Kuhlmann, J.; Benz, S.; Hauptvogel, D.; Schreckenberg, D. Coping with Aviation Noise: Non-Acoustic Factors Influencing Annoyance and Sleep Disturbance from Noise. In Aviation Noise Impact Management: Technologies, Regulations, and Societal Well-Being in Europe; Leylekian, L., Covrig, A., Maximova, A., Eds.; Springer Open Access: Cham, Switzerland, 2022; pp. 197–218. [Google Scholar]
- Veldboom, E.; van der Werf, C.H.; Incedalci, Z.; van den Berg, F. The effect of masking noise on persons suffering from a low frequency sound. Appl. Acoust. 2022, 191, 108681. [Google Scholar] [CrossRef]
- Moorhouse, A.; Baguley, D.; Banks, C.; Comiskey, P.; Kay, T.; Kenyon, A.; McFerran, D.; Penney, F.; Whalley, C.; Smith, K. Trials of a Protocol to Support LFN Sufferers in the UK. In Proceedings of the Euro Noise, Maastricht, The Netherlands, 31 May–3 June 2015. [Google Scholar]
- Erdélyi, K.H.; Fuermaier, A.B.M.; Tucha, L.; Tucha, O.; Koerts, J. Low-Frequency Noise: Experiences from a Low-Frequency Noise Perceiving Population. Int. J. Environ. Res. Public Health 2023, 20, 3916. [Google Scholar] [CrossRef]
- Beblo, T.; Kunz, M.; Brokate, B.; Scheurich, A.; Weber, B.; Albert, A.; Richter, P.; Lautenbacher, S. Construction of a questionnaire for complaints of cognitive disturbances in patients with mental disorders. Z. Neuropsychol. 2010, 21, 143–151. [Google Scholar] [CrossRef]
- Roth, R.M.; Isquith, P.K.; Gioia, G.A. BRIEF-A: Behavior Rating Inventory of Executive Function-Adult Version. Nederlandse Bewerking; Scholte, E.; Noens, I., Translators; Hogrefe Uitgevers B.V.: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Roth, R.M.; Isquith, P.K.; Gioia, G.A. BRIEF-A: Behavior Rating Inventory of Executive Function-Adult Version; Psychological Assessment Resources: Lutz, FL, USA, 2005. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II (BDI-II); [Database Record]; APA PsycTests; American Psychological Association: Washington, DC, USA, 1996. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory Second Edition (BDI-2-NL) Nederlandse Versie (Manual); van der Does, W., Translator; Ipskamp: Enschede, The Netherlands, 2002. [Google Scholar]
- Buysse, D.; Reynolds, C.; Monk, T.; Berman, S.; Kupfer, D. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The Fatigue Severity Scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef]
- Rietberg, M.B.; van Wegen, E.E.H.; Kwakkel, G. Measuring fatigue in patients with multiple sclerosis: Reproducibility, responsiveness and concurrent validity of three Dutch self-report questionnaires. Disabil. Rehabil. 2010, 32, 1870–1876. [Google Scholar] [CrossRef]
- Vingerhoets, A.J.J.M.; van Tilburg, M.A.L. Alledaagse Problemen Lijst; Swets en Zeitlinger b.v.: Lisse, The Netherlands, 1994. [Google Scholar]
- Kleijn, W.C.; van Heck, G.L.; van Waning, A. Ervaringen met een Nederlandse bewerking van de COPE copingvragenlijst: De COPE-Easy. Gedrag Gezondh. 2000, 28, 213–226. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect Size Estimates: Current Use, Calculations, and Interpretation. J. Exp. Psychol. 2012, 141, 2–18. [Google Scholar] [CrossRef]
- Trimbos-Institut. Netherlands Mental Health Survey and Incidence Study-3 (NEMESIS-3). [Data Set] Ministry of Health, Welfare and Sport. 2023. Available online: https://cijfers.trimbos.nl/nemesis/verdiepende-informatie-psychische-aandoeningen/depressieve-stoornis/ (accessed on 11 December 2023).
- Statistics Netherlands (CBS). Gezondheidsenquête [Data Set]. 2023. Available online: https://www.vzinfo.nl/slapen/totale-bevolking#:~:text=Ruim%20%C3%A9%C3%A9n%20op%20de%20vijf,van%20de%20kwaliteit%20van%20slaap (accessed on 11 December 2023).
- Statistics Netherlands (CBS). Gezondheidsenquête [Data Set]. 2023. Available online: https://www.rivm.nl/nieuws/slechte-slaap-en-gezondheid-meer-aandacht-voor-preventie-van-belang (accessed on 11 December 2023).
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef]
| LFN1 (n = 181) | LFN2-SA (n = 131) | LFN2-SB (n = 108) | CG-SA (n = 229) | CG-SB (n = 239) | |
|---|---|---|---|---|---|
| Sex | |||||
| Females (%) | 124 (68.5) | 93 (71.0) | 71 (65.7) | 144 (62.9) | 158 (66.1) |
| Education (%) | |||||
| Low a | 14 (7.7) | 1 (0.8) | 0 (0) | 3 (1.3) | 2 (0.8) |
| Middle | 61 (33.7) | 56 (42.7) | 47 (43.5) | 88 (38.4) | 80 (33.5) |
| High | 106 (58.6) | 74 (56.5) | 61 (56.5) | 138 (60.3) | 157 (65.7) |
| Marital status (%) | |||||
| Married b | 87 (48.1) | 80 (61.1) | 48 (44.4) | 124 (54.1) | 142 (59.4) |
| Unmarried c No partner Partner, living together Partner, not living together | 64 (35.4) 34 (18.8) 26 (14.4) 4 (2.2) | 31 (23.7) 13 (9.9) 15 (11.5) 3 (2.3) | 35 (32.4) 15 (13.9) 16 (14.8) 4 (3.7) | 54 (23.6) 24 (10.5) 22 (9.6) 8 (3.5) | 55 (23.0) 32 (13.4) 20 (8.4) 3 (1.3) |
| Divorced | 22 (12.2) | 16 (12.2) | 13 (12.0) | 28 (12.2) | 24 (10.0) |
| Widowed d | 6 (3.3) | 4 (3.1) | 12 (11.1) | 23 (10.0) | 17 (7.1) |
| Mean ± SD (Range) | |||||
| Age in years e | 57.4 ± 11.3 (25–87) | 54.5 ± 13.5 (18–86) | 56.6 ± 13.0 (24–90) | 62.7 ± 11.8 (26–89) | 62.0 ± 11.5 (25–85) |
| Frequency of LFN complaints | 3.2 ± 0.8 (1–4) | 1.6 ± 0.9 (0–4) | 1.7 ± 0.9 (0–4) | 0.5 ± 0.5 (0–1) | 0.4 ± 0.5 (0–1) |
| Extent of LFN hindrance | 7.3 ± 2.3 (1–10) | 4.8 ± 1.8 (1–10) | 4.8 ± 1.8 (1–10) | 1.3 ± 0.5 (1–2) | 1.3 ± 0.5 (1–2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdelyi, K.H.; Fuermaier, A.B.M.; Tucha, L.; Tucha, O.; Koerts, J. Subjective Complaints and Coping Strategies of Individuals with Reported Low-Frequency Noise Perceptions. J. Clin. Med. 2024, 13, 935. https://doi.org/10.3390/jcm13040935
Erdelyi KH, Fuermaier ABM, Tucha L, Tucha O, Koerts J. Subjective Complaints and Coping Strategies of Individuals with Reported Low-Frequency Noise Perceptions. Journal of Clinical Medicine. 2024; 13(4):935. https://doi.org/10.3390/jcm13040935
Chicago/Turabian StyleErdelyi, Kristina H., Anselm B. M. Fuermaier, Lara Tucha, Oliver Tucha, and Janneke Koerts. 2024. "Subjective Complaints and Coping Strategies of Individuals with Reported Low-Frequency Noise Perceptions" Journal of Clinical Medicine 13, no. 4: 935. https://doi.org/10.3390/jcm13040935
APA StyleErdelyi, K. H., Fuermaier, A. B. M., Tucha, L., Tucha, O., & Koerts, J. (2024). Subjective Complaints and Coping Strategies of Individuals with Reported Low-Frequency Noise Perceptions. Journal of Clinical Medicine, 13(4), 935. https://doi.org/10.3390/jcm13040935







