Impact of Cardiac Magnetic Resonance on the Diagnosis of Left Ventricular Noncompaction—A 15-Year Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics Approval
2.3. CMR Protocol
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. CMR Diagnosis
3.3. Patients with Confirmed and Unconfirmed LVNC Diagnoses
3.4. Patients with Dilated Cardiomyopathy, Hypertrophic Cardiomyopathy, and Left Ventricular Noncompaction
4. Discussion
Study Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cuevas, J.; Ptaszynski, R.; Cigarrán, H.; Calvo, J.; Martín, M. Left ventricular noncompaction cardiomyopathy: Recent advances. Kardiol. Pol. 2022, 80, 529–539. [Google Scholar] [CrossRef]
- Stähli, B.E.; Gebhard, C.; Biaggi, P.; Klaassen, S.; Valsangiacomo Buechel, E.; Attenhofer Jost, C.H.; Jenni, R.; Tanner, F.C.; Greutmann, M. Left ventricular non-compaction: Prevalence in congenital heart disease. Int. J. Cardiol. 2013, 167, 2477–2481. [Google Scholar] [CrossRef]
- Ross, S.B.; Jones, K.; Blanch, B.; Puranik, R.; McGeechan, K.; Barratt, A.; Semsarian, C. A systematic review and meta-analysis of the prevalence of left ventricular non-compaction in adults. Eur. Heart J. 2020, 41, 1428–1436. [Google Scholar] [CrossRef]
- Bhatia, N.L.; Tajik, A.J.; Wilansky, S.; Steidley, D.E.; Mookadam, F. Isolated Noncompaction of the Left Ventricular Myocardium in Adults: A Systematic Overview. J. Card. Fail. 2011, 17, 771–778. [Google Scholar] [CrossRef]
- Kayvanpour, E.; Sedaghat-Hamedani, F.; Gi, W.T.; Tugrul, O.F.; Amr, A.; Haas, J.; Zhu, F.; Ehlermann, P.; Uhlmann, L.; Katus, H.A.; et al. Clinical and genetic insights into non-compaction: A meta-analysis and systematic review on 7598 individuals. Clin. Res. Cardiol. 2019, 108, 1297–1308. [Google Scholar] [CrossRef]
- Aung, N.; Doimo, S.; Ricci, F.; Sanghvi, M.M.; Pedrosa, C.; Woodbridge, S.P.; Al-Balah, A.; Zemrak, F.; Khanji, M.Y.; Munroe, P.B.; et al. Prognostic Significance of Left Ventricular Noncompaction. Circ. Cardiovasc. Imaging 2020, 13, e009712. [Google Scholar] [CrossRef] [PubMed]
- Femia, G.; Zhu, D.; Choudhary, P.; Ross, S.B.; Muthurangu, V.; Richmond, D.; Celermajer, D.S.; Semsarian, C.; Puranik, R. Long term clinical outcomes associated with CMR quantified isolated left ventricular non-compaction in adults. Int. J. Cardiol. 2021, 328, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Zemrak, F.; Raisi-Estabragh, Z.; Khanji, M.Y.; Mohiddin, S.A.; Bruder, O.; Wagner, A.; Lombardi, M.; Schwitter, J.; van Rossum, A.C.; Pilz, G.; et al. Left Ventricular Hypertrabeculation Is Not Associated with Cardiovascular Morbity or Mortality: Insights from the Eurocmr Registry. Front. Cardiovasc. Med. 2020, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Grigoratos, C.; Barison, A.; Ivanov, A.; Andreini, D.; Amzulescu, M.S.; Mazurkiewicz, L.; De Luca, A.; Grzybowski, J.; Masci, P.G.; Marczak, M.; et al. Meta-Analysis of the Prognostic Role of Late Gadolinium Enhancement and Global Systolic Impairment in Left Ventricular Noncompaction. JACC Cardiovasc. Imaging 2019, 12 Pt 1, 2141–2151. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Jenni, R.; Oechslin, E.; Schneider, J.; Attenhofer Jost, C.; Kaufmann, P.A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart 2001, 86, 666–671. [Google Scholar] [CrossRef]
- Petersen, S.E.; Selvanayagam, J.B.; Wiesmann, F.; Robson, M.D.; Francis, J.M.; Anderson, R.H.; Watkins, H.; Neubauer, S. Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 2005, 46, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O’Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar] [CrossRef]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B.; American Heart Association; et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef]
- Petersen, S.E.; Jensen, B.; Aung, N.; Friedrich, M.G.; McMahon, C.J.; Mohiddin, S.A.; Pignatelli, R.H.; Ricci, F.; Anderson, R.H.; Bluemke, D.A. Excessive Trabeculation of the Left Ventricle: JACC: Cardiovascular Imaging Expert Panel Paper. JACC Cardiovasc. Imaging 2023, 16, 408–425. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Hou, Y. The prognostic value of late gadolinium enhancement in heart diseases: An umbrella review of meta-analyses of observational studies. Eur. Radiol. 2021, 31, 4528–4537. [Google Scholar] [CrossRef]
- Di Marco, A.; Anguera, I.; Schmitt, M.; Klem, I.; Neilan, T.G.; White, J.A.; Sramko, M.; Masci, P.G.; Barison, A.; Mckenna, P.; et al. Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy: Systematic Review and Meta-Analysis. JACC Heart Fail. 2017, 5, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Gati, S.; Chandra, N.; Bennett, R.L.; Reed, M.; Kervio, G.; Panoulas, V.F.; Ghani, S.; Sheikh, N.; Zaidi, A.; Wilson, M.; et al. Increased left ventricular trabeculation in highly trained athletes: Do we need more stringent criteria for the diagnosis of left ventricular non-compaction in athletes? Heart 2013, 99, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef] [PubMed]
- Bruder, O.; Wagner, A.; Lombardi, M.; Schwitter, J.; van Rossum, A.; Pilz, G.; Nothnagel, D.; Steen, H.; Petersen, S.; Nagel, E.; et al. European Cardiovascular Magnetic Resonance (EuroCMR) registry—Multi national results from 57 centers in 15 countries. J. Cardiovasc. Magn. Reson. 2013, 15, 9. [Google Scholar] [CrossRef]
- Ojrzyńska-Witek, N.; Marczak, M.; Mazurkiewicz, Ł.; Petryka-Mazurkiewicz, J.; Miłosz-Wieczorek, B.; Grzybowski, J.; Śpiewak, M. Role of cardiac magnetic resonance in heart failure of initially unknown etiology: A 10-year observational study. Kardiol. Pol. 2022, 80, 278–285. [Google Scholar] [CrossRef]
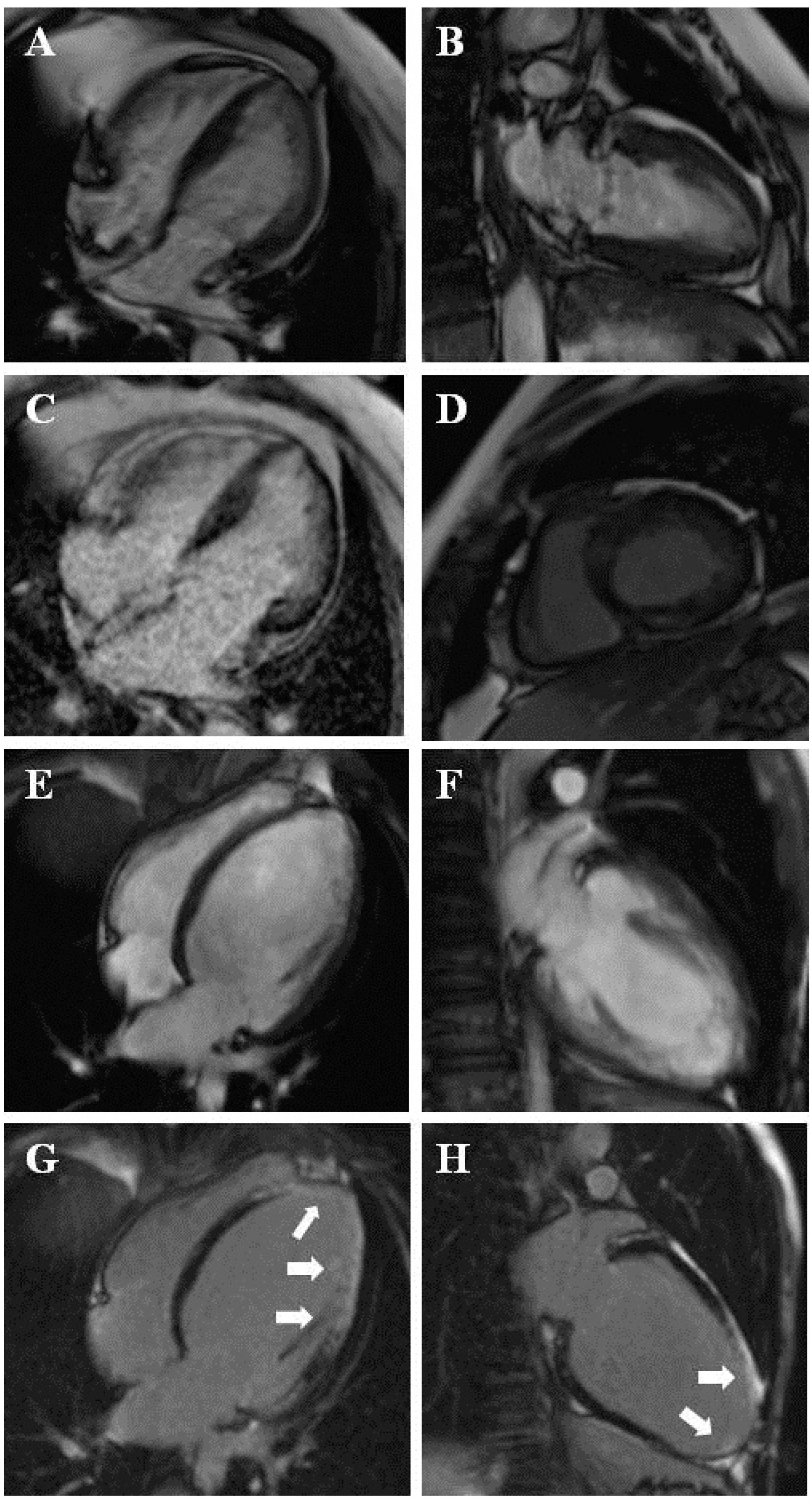
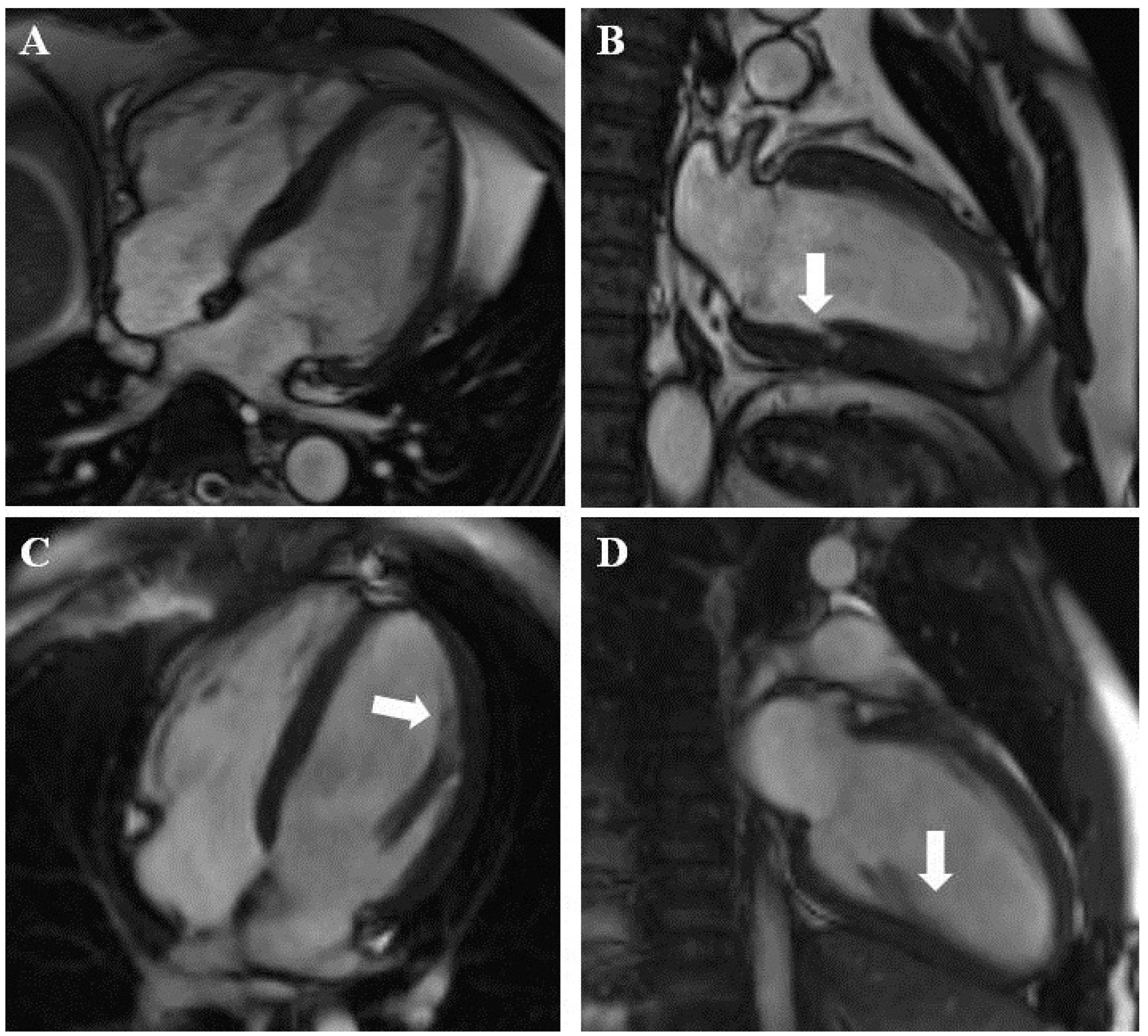
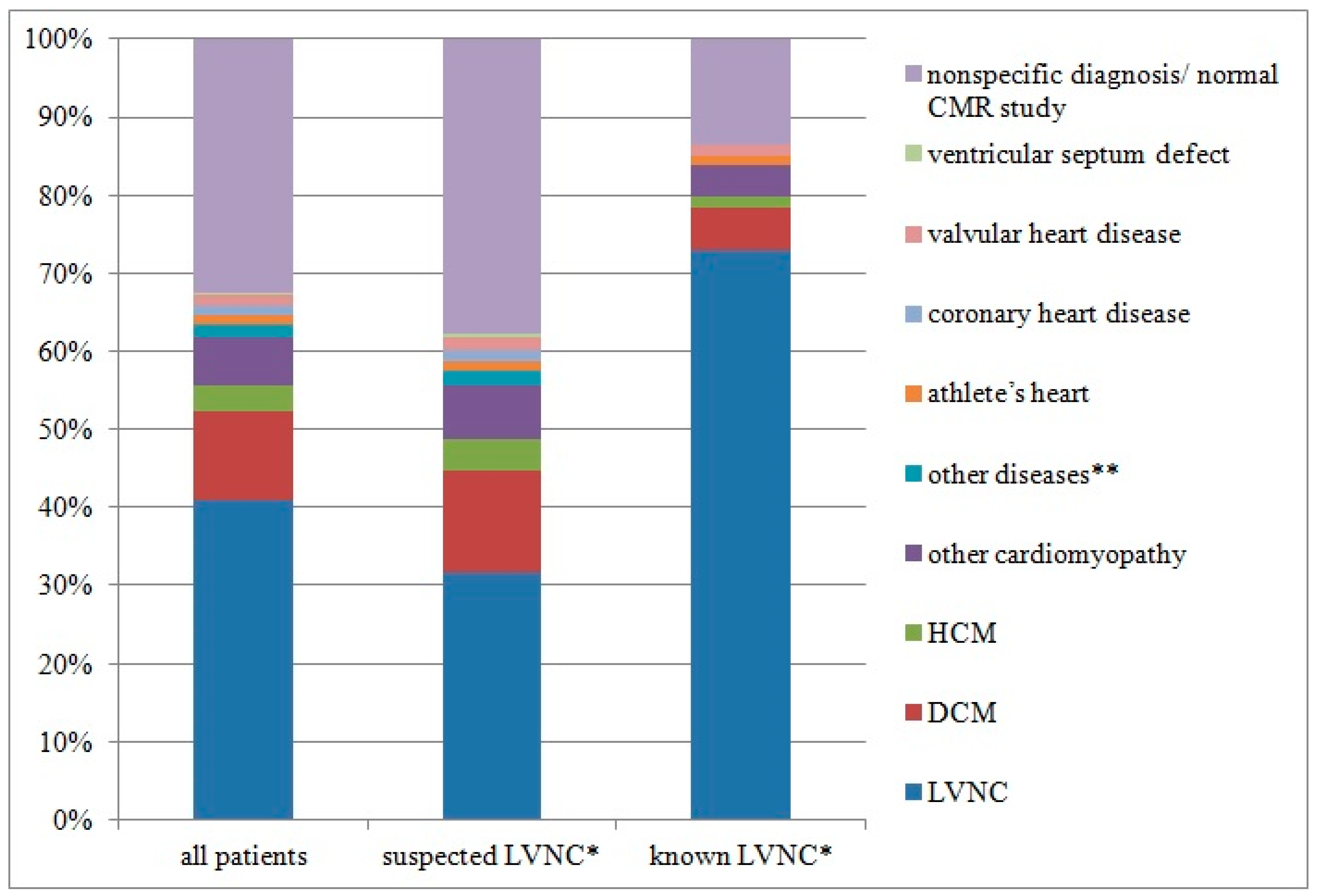
| Variable | All Patients (n = 333) | Suspected LVNC (n = 259) | Known LVNC (n = 74) | p Value (Suspected vs. Known LVNC) |
|---|---|---|---|---|
| Male sex, n (%) | 193 (58.0) | 149 (57.5) | 44 (59.5) | 0.88 |
| Age, years a | 39.0 (26.8–51.0) | 39.0 (27.0–51.8) | 34.5 (23.0–43.0) | 0.03 |
| LVEDV, mL/m2 a | 102.0 (85.0–127.3) | 99.0 (83.0–128.0) | 108.0 (93.0–126.0) | 0.05 |
| LVESV, mL/m2 a | 47.0 (35.0–68.5) | 44.5 (34.0–67.0) | 54.0 (41.0–73.0) | 0.008 |
| LVEF, % a | 54.1 (41.1–60.3) | 55.5 (42.4–61.0) | 49.8 (36.1–57.4) | 0.0049 |
| RVEDV, mL/m2 a | 92.0 (77.0–113.0) | 91.0 (77.0–113.0) | 95.0 (72.8–109.5) | 0.73 |
| RVESV, mL/m2 a | 41.0 (30.0–56.0) | 42.0 (30.0–56.0) | 40.0 (32.8–55.0) | 0.83 |
| RVEF, % a | 54.4 (48.6–60.3) | 54.3 (48.5–61.2) | 55.3 (48.9–59.4) | 0.92 |
| LGE, % (n) b | 44.8 (143/319) | 46.0 (115/250) | 40.6 (28/69) | 0.88 |
| LVM, g/ m2 a | 64.5 (54.0–82.0) | 66.0 (54.0–83.0) | 60.0 (53.3–79.3) | 0.19 |
| Variable | Suspected LVNC Based on Echocardiography (n = 259) | Known LVNC Based on Echocardiography (n = 74) | p Value | ||
|---|---|---|---|---|---|
| LVNC Confirmed in CMR (n = 82) | LVNC Unconfirmed in CMR (n = 177) | LVNC Confirmed in CMR (n = 54) | LVNC Unconfirmed in CMR (n = 20) | ||
| Male sex, n (%) | 43 (52.4) | 106 (59.9) | 34 (63.0) | 10 (50.0) | 0.89 |
| Age, years a | 39.0 (32.0–51.0) | 39.0 (24.0–52.0) | 36.0 (27.0–43.0) | 28.5 (19.5–50.0) | 0.09 |
| LVEDV, mL/m2 a | 104.0 (87.0–130.0) | 97.0 (83.0– 127.3) | 108.0 (93.0–135.0) | 106.0 (94.0–123.5) | 0.12 |
| LVESV, mL/m2 a | 50.0 (38.0–67.3) | 40.0 (33.0–67.0) | 56.0 (42.0–81.0) | 49.0 (36.0–62.5) | 0.003 |
| LVEF, % a | 50.0 (41.7–59.4) | 56.7 (42.7–61.4) | 46.7 (35.1–55.7) | 54.0 (46.3–60.0) | 0.001 |
| RVEDV, mL/m2 a | 86.0 (70.8–114.3) | 92.0 (78.8–112.3) | 93.0 (72.5–107.0) | 104.0 (72.3–129.5) | 0.91 |
| RVESV, mL/m2 a | 40.0 (30.0–59.0) | 42.0 (29.0–56.0) | 38.5 (32.5–54.0) | 49.0 (33.5–58.5) | 0.98 |
| RVEF, % a | 53.8 (48.7–61.2) | 54.8 (48.3–61.2) | 55.7 (49.3–59.3) | 53.5 (48.2–60.9) | 0.98 |
| LGE, % (n) b | 48.1 (38/79) | 45.0 (77/171) | 43.1 (22/51) | 33.3 (6/18) | 0.50 |
| LVM, g/ m2 a | 64.0 (53.0–81.0) | 67.0 (55.0–83.0) | 61.5 (55.0–77.0) | 57.0 (47.5–82.3) | 0.35 |
| Variable | LVNC (n = 136) | Not LVNC (n = 197) | p Value |
|---|---|---|---|
| Male sex, n (%) | 77 (56.6) | 116 (58.9) | 0.83 |
| Age, years a | 38.5 (29.0–47.0) | 39.0 (24.0–52.0) | 0.63 |
| LVEDV, mL/m2 a | 105.5 (90.5–130.0) | 97.0 (83.0–126.0) | 0.047 |
| LVESV, mL/m2 a | 53.0 (40.0–70.0) | 41.0 (33.0–67.0) | 0.001 |
| LVEF, % a | 48.4 (37.1–57.1) | 56.5 (42.9–61.1) | 0.0002 |
| RVEDV, mL/m2 a | 91.0 (72.0–113.3) | 92.0 (78.0–113.0) | 0.65 |
| RVESV, mL/m2 a | 39.5 (31.0–57.0) | 42.0 (29.0–56.0) | 0.83 |
| RVEF, % a | 54.2 (48.7–59.3) | 54.7 (48.4–61.0) | 0.73 |
| LGE, % (n) b | 46.2 (60/130) | 43.9 (83/189) | 0.81 |
| LVM, g/ m2 a | 63.5 (53.5–79.0) | 66.0 (54.0–83.0) | 0.38 |
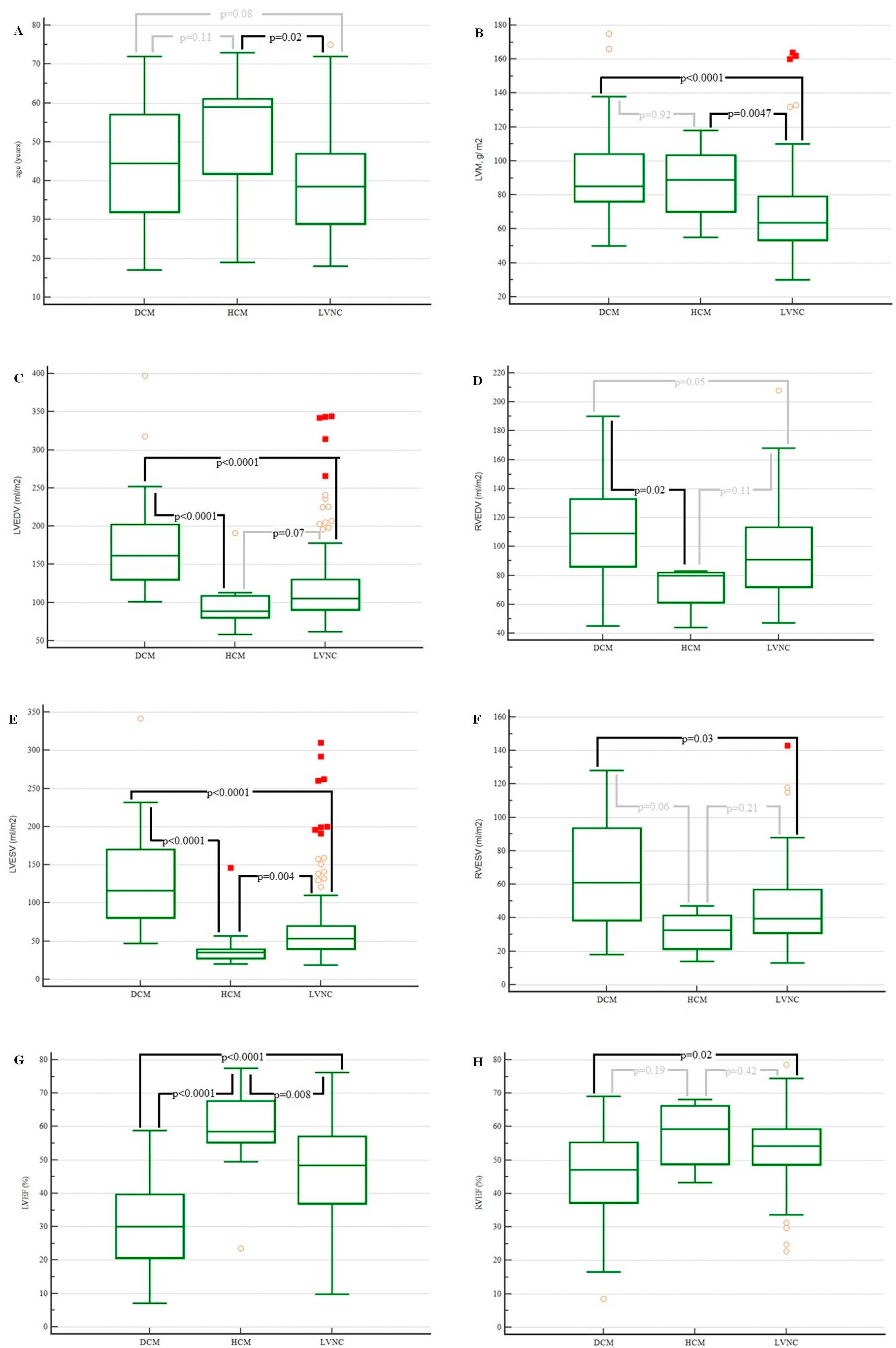
| Variable | DCM (n = 38) | HCM (n = 11) | LVNC (n = 136) | p Value |
|---|---|---|---|---|
| Male sex, n (%) | 24 (63.2) | 6 (54.5) | 77 (56.6) | 0.93 |
| Age, years a | 44.5 (32.0–57.0) | 59.0 (41.8–61.0) | 38.5 (29.0–47.0) | 0.02 |
| LVEDV, mL/m2 a | 161.5 (130.0–202.0) | 89.0 (80.5–109.0) | 105.5 (90.5–130.0) | <0.000001 |
| LVESV, mL/m2 a | 116.0 (81.0–170.5) | 35.0 (27.5–39.5) | 53.0 (40.0–70.0) | <0.000001 |
| LVEF, % a | 30.1 (20.7–39.7) | 58.4 (55.4–67.6) | 48.4 (37.0–57.1) | <0.000001 |
| RVEDV, mL/m2 a | 109.0 (86.3–132.8) | 80.0 (61.5–82.0) | 91.0 (72.0–113.3) | 0.03 |
| RVESV, mL/m2 a | 61.0 (38.5–93.8) | 32.5 (21.5–41.5) | 39.5 (31.0–57.0) | 0.04 |
| RVEF, % a | 47.1 (37.3–55.4) | 59.3 (48.9–66.2) | 54.2 (48.7–59.3) | 0.049 |
| LGE, % (n) b | 29/37 | 10/11 | 59/130 | 0.09 |
| LVM, g/ m2 a | 85.0 (76.3–104.0) | 89.0 (70.3–103.5) | 63.5 (53.5–79.0) | 0.000002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojrzyńska-Witek, N.; Marczak, M.; Mazurkiewicz, Ł.; Petryka-Mazurkiewicz, J.; Miłosz, B.; Grzybowski, J.; Śpiewak, M. Impact of Cardiac Magnetic Resonance on the Diagnosis of Left Ventricular Noncompaction—A 15-Year Experience. J. Clin. Med. 2024, 13, 949. https://doi.org/10.3390/jcm13040949
Ojrzyńska-Witek N, Marczak M, Mazurkiewicz Ł, Petryka-Mazurkiewicz J, Miłosz B, Grzybowski J, Śpiewak M. Impact of Cardiac Magnetic Resonance on the Diagnosis of Left Ventricular Noncompaction—A 15-Year Experience. Journal of Clinical Medicine. 2024; 13(4):949. https://doi.org/10.3390/jcm13040949
Chicago/Turabian StyleOjrzyńska-Witek, Natalia, Magdalena Marczak, Łukasz Mazurkiewicz, Joanna Petryka-Mazurkiewicz, Barbara Miłosz, Jacek Grzybowski, and Mateusz Śpiewak. 2024. "Impact of Cardiac Magnetic Resonance on the Diagnosis of Left Ventricular Noncompaction—A 15-Year Experience" Journal of Clinical Medicine 13, no. 4: 949. https://doi.org/10.3390/jcm13040949
APA StyleOjrzyńska-Witek, N., Marczak, M., Mazurkiewicz, Ł., Petryka-Mazurkiewicz, J., Miłosz, B., Grzybowski, J., & Śpiewak, M. (2024). Impact of Cardiac Magnetic Resonance on the Diagnosis of Left Ventricular Noncompaction—A 15-Year Experience. Journal of Clinical Medicine, 13(4), 949. https://doi.org/10.3390/jcm13040949







