Whole-Body MRI at Initial Presentation of Chronic Recurrent Multifocal Osteomyelitis, Juvenile Idiopathic Arthritis, Their Overlapping Syndrome, and Non-Specific Arthropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI Protocol and Interpretation of Imaging Features
2.3. Imaging Evaluation Criteria
2.4. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Imaging Findings in Patients with CRMO, JIA, OS, and NA
- In CRMO, a total of 263 lesions were recorded, ranging from 1 (in 3 patients) to 33 (in 1 patient);
- In JIA, a total of 296 lesions were recorded; the number of lesions ranged from 0 (in 14 patients) to 31 lesions (in 1 patient);
- In OS, a total of 82 lesions were recorded; the number of lesions ranged from 0 (in 1 patient) to 22 (in 1 patient);
- In NA, a total of 56 lesions were recorded; the number of lesions ranged from 0 (in 78 patients) to 21 (in 1 patient).
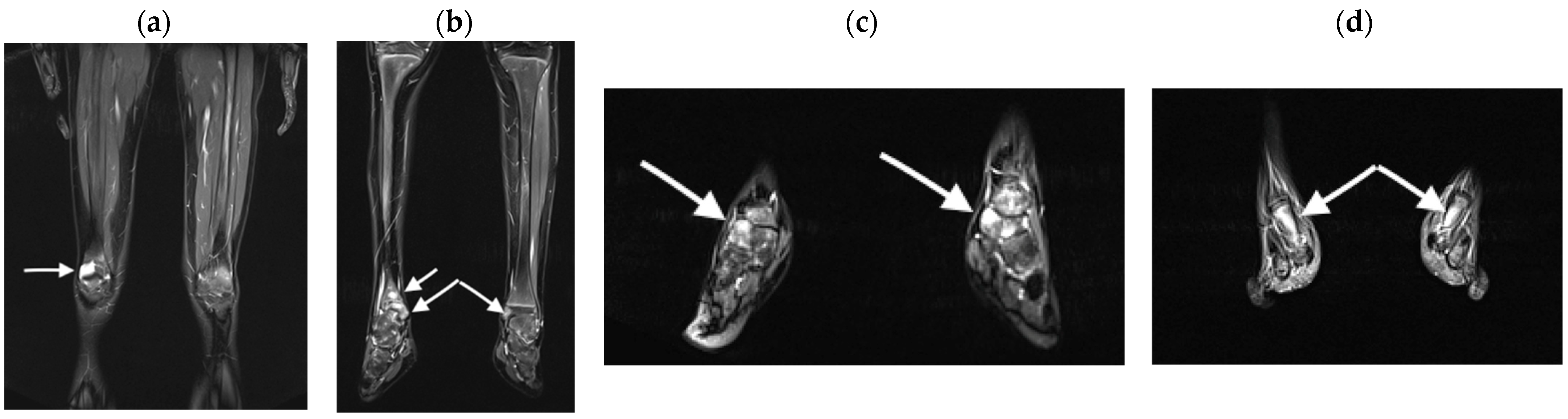
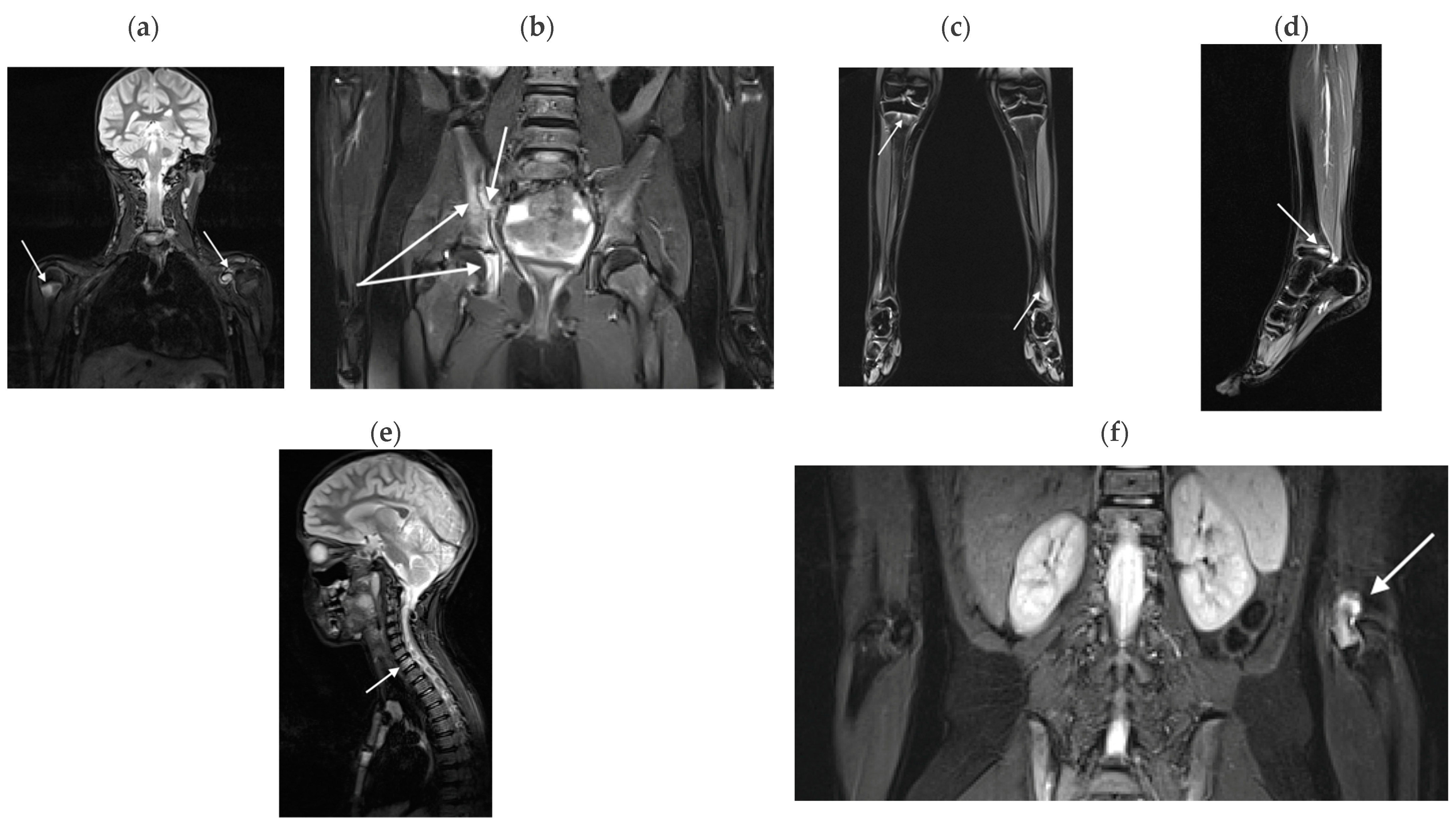
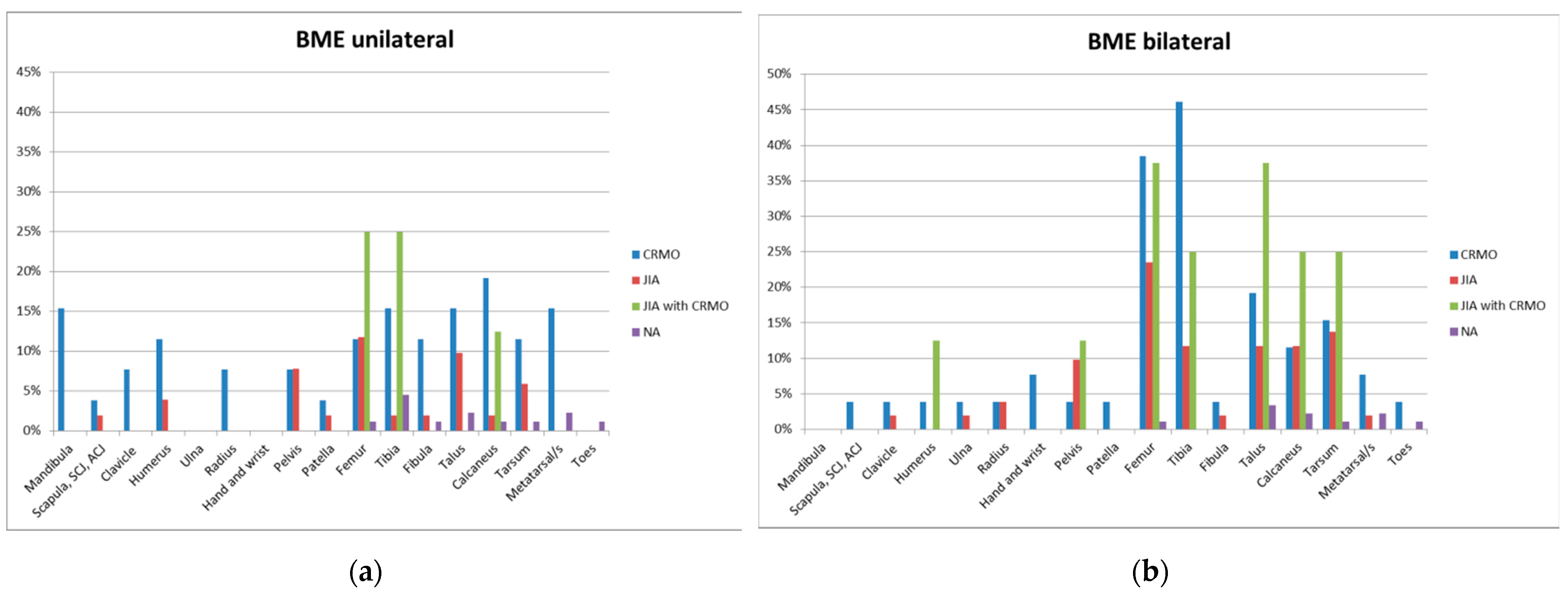
3.2.1. Bone Marrow Edema Lesions
3.2.2. Effusions
3.2.3. Myositis
3.2.4. Enthesitis
4. Discussion
4.1. WB-MRI in CRMO
4.2. WB-MRI in JIA
4.3. WB-MRI in OS
4.4. WB-MRI in NA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greer, M.L.C. Whole-body magnetic resonance imaging: Techniques and nononcologic indications. Pediatr. Radiol. 2018, 48, 1348–1363. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, J.F.; Berthold, L.D.; Hahn, G.; von Kalle, T.; Moritz, J.D.; Schröder, C.; Stegmann, J.; Steinborn, M.; Weidemann, J.; Wunsch, R.; et al. Whole-Body MRI in Children and Adolescents—S1 Guidelines. Fortschr. Röntgenstr. 2019, 191, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Giraudo, C.; Lecouvet, F.E.; Cotton, A.; Eshed, I.; Jans, L.; Jurik, A.G.; Maas, M.; Weber, M.; Sudoł-Szopińska, I. Whole-body magnetic resonance imaging in inflammatory diseases: Where are we now? Results of an International Survey by the European Society of Musculoskeletal Radiology. Eur. J. Radiol. 2021, 136, 109533. [Google Scholar] [CrossRef] [PubMed]
- Spalkit, S.; Sinha, A.; Prakash, M.; Sandhu, M.S. Dermatomyositis: Patterns of MRI findings in muscles, fascia and skin of pelvis and thigh. Eur. J. Radiol. 2021, 141, 109812. [Google Scholar] [CrossRef] [PubMed]
- Sreelal, T.V.; Bhatia, A.; Suri, D.; Singh, S.; Saxena, A.K.; Tao, T.Y.; Sodhi, K.S. Whole-body MR imaging in evaluation of children with juvenile dermatomyositis. Eur. J. Radiol. 2022, 155, 110475. [Google Scholar] [CrossRef] [PubMed]
- Schaal, M.C.; Gendler, L.; Ammann, B.; Eberhardt, N.; Janda, A.; Morbach, H.; Darge, K.; Girschick, H.; Beer, M. Imaging in non-bacterial osteomyelitis in children and adolescents: Diagnosis, differential diagnosis and follow-up—An educational review based on a literature survey and own clinical experiences. Insights Imaging 2021, 12, 113. [Google Scholar] [CrossRef]
- Malattia, C.; Tolend, M.; Mazzoni, M.; Panwar, J.; Zlotnik, M.; Otobo, T.; Vidarsson, L.; Doria, A.S. Current status of MR imaging of juvenile idiopathic arthritis. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101629. [Google Scholar] [CrossRef]
- Koneru, S.; Magid, M.S.; Fritz, J. Case of the Season: Asymmetric Chronic Recurrent Multifocal Osteomyelitis. Semin. Roentgenol. 2021, 57, 184–190. [Google Scholar] [CrossRef]
- Zhao, Y.; Sato, T.S.; Nielsen, S.M.; Beer, M.; Huang, M.; Iyer, R.S.; McGuire, M.; Ngo, A.; Otjen, J.P.; Panwar, J.; et al. Development of a Scoring Tool. for Chronic Nonbacterial Osteomyelitis Magnetic Resonance Imaging and Evaluation of its Interrater Reliability. J. Rheumatol. 2020, 47, 739–747. [Google Scholar] [CrossRef]
- Panwar, J.; Tolend, M.; Lim, L.; Shirley, M.; Tse, S.M.; Doria, A.S.; Laxer, R.M.; Stimec, J. Whole-body MRI Quantification for Assessment of Bone Lesions in Chronic Nonbacterial Osteomyelitis Patients Treated with Pamidronate: A Prevalence, Reproducibility, and Responsiveness Study. J. Rheumatol. 2021, 48, 751–759. [Google Scholar] [CrossRef]
- Aquino, M.R.; Tse, S.M.L.; Gupta, S.; Rachlis, A.C.; Stimec, J. Whole-body MRI of juvenile spondyloarthritis: Protocols and pictorial review of characteristic patterns. Pediatr. Radiol. 2015, 45, 754–762. [Google Scholar] [CrossRef]
- Sato, T.S.; Watal, P.; Ferguson, P.J. Imaging mimics of chronic recurrent multifocal osteomyelitis: Avoiding pitfalls in a diagnosis of exclusion. Pediatr. Radiol. 2020, 50, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Kieninger, A.; Schäfer, J.F.; Tsiflikas, I.; Moll, M.; Kümmerle-Deschner, J.; Kraus, M.S.; Esser, M. Early diagnosis and response assessment in chronic recurrent multifocal osteomyelitis: Changes in lesion volume and signal intensity assessed by whole-body MRI. Br. J. Radiol. 2021, 95, 20211091. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Rosenkranz, M.; Thapa, M. Review of spinal involvement in Chronic recurrent multifocal osteomyelitis (CRMO): What radiologists need to know about CRMO and its imitators. Clin. Imaging 2022, 81, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Damasio, M.B.; Magnaguagno, F.; Stagnaro, G. Whole-body MRI: Non-oncological applications in paediatrics. Radiol. Med. 2016, 121, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Koné-Paut, I.; Mannes, I.; Dusser, P. Chronic Recurrent Multifocal Osteomyelitis (CRMO) and Juvenile Spondyloarthritis (JSpA): To What Extent Are They Related? J. Clin. Med. 2023, 12, 453. [Google Scholar] [CrossRef] [PubMed]
- Pracoń, G.; Simoni, O.P.; Gietka, P.; Aparisi, P.; Sudoł-Szopińska, I. Conventional Radiography and Ultrasound Imaging of Rheumatic Diseases Affecting the Pediatric Population. Semin. Musculoskelet. Radiol. 2021, 25, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Sudoł-Szopińska, I.; Gietka, P.; Znajdek, M.; Matuszewska, G.; Bogucevska, M.; Damjanovska-Krstikj, L.; Ivanoski, S. Imaging of Juvenile Spondyloarthritis. Part I: Classifications and Radiographs. J. Ultrason. 2017, 17, 167–175. [Google Scholar] [CrossRef]
- Sudoł-Szopińska, I.; Herregods, N.; Doria, A.S.; Taljanovic, M.S.; Gietka, P.; Tzaribachev, N.; Klauser, A.S. Advances in Musculoskeletal Imaging in Juvenile Idiopathic Arthritis. Biomedicines 2022, 10, 2417. [Google Scholar] [CrossRef]
- Sudoł-Szopińska, I.; Giiraudo, C.; Oei, E.H.G.; Jans, L. Imaging update in inflammatory arthritis. J. Clin. Orthop. Trauma 2021, 20, 101491. [Google Scholar] [CrossRef]
- Malievskiy, V. Arthralgia in children: The epidemiological study. Pediatr. Rheumatol. 2011, 9 (Suppl. S1), P144. [Google Scholar] [CrossRef]
- De Inocencio, J.; Carro, M.A.; Flores, M.; Carpio, C.; Mesa, S.; Marín, M. Epidemiology of musculoskeletal pain in a pediatric emergency department. Rheumatol. Int. 2016, 36, 83–89. [Google Scholar] [CrossRef]
- De Inocencio, J. Epidemiology of musculoskeletal pain in primary care. Arch. Dis. Child. 2004, 89, 431–434. [Google Scholar] [CrossRef]
- Remvig, L.; Jensen, D.V.; Ward, R.C. Epidemiology of General Joint Hypermobility and Basis for the Proposed Criteria for Benign Joint Hypermobility Syndrome: Review of the Literature. J. Rheumatol. 2007, 34, 804–809. [Google Scholar] [PubMed]
- Mohanta, M.P. Growing pains: Pratitioners’ dilemma. Indian Pediatr. 2014, 51, 379–383. [Google Scholar] [CrossRef]
- Weiss, J.; Stinson, J.N. Pediatric pain syndromes and noninflammatory musculoskeletal pain. Pediatr. Clin. N. Am. 2018, 65, 801–826. [Google Scholar] [CrossRef] [PubMed]
- Uziel, Y.; Hashkes, P.J. Growing pains in children. Pediatrics 2008, 147, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Scutter, S. Prevalence of “growing pains” in young children. J. Pediatr. 2004, 145, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Roderick, M.R.; Shah, R.; Rogers, V.; Finn, A.; Ramanan, A.V. Chronic recurrent multifocal osteomyelitis (CRMO)—Advancing the diagnosis. Pediatr. Rheumatol. Online J. 2016, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Petty, R.E.; TR Southwood, T.R.; Baum, J.; Betay, E.; Glass, D.N.; Manners, P.; Maldonado-Cocco, J.; Suarez-Almazor, M.; Orozco-Alcala, J.; Prieur, A.M.; et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J. Rheumatol. 1998, 25, 1991–1994. [Google Scholar] [PubMed]
- Cebecauerová, D.; Malcová, H.; Koukolská, V.; Kvíčalová, Z.; Souček, O.; Wagenknecht, L.; BronskÝ, J.; Šumník, Z.; Kynčl, M.; Cebecauer, M.; et al. Two phenotypes of chronic recurrent multifocal osteomyelitis with diferent patterns of bone involvement. J. Pediatr. Rheumatol. 2022, 20, 108. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.R.; Kapplush, F.; Girchick, H.J.; Morbach, H.; Pablik, J.; Ferguson, P.J.; Hedrich, C.H.M. Chronic Recurrent Multifocal Osteomyelitis (CRMO): Presentation, Pathogenesis and treatment. Curr. Osteoporos. Rep. 2017, 15, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Ravelli, A.; Avcin, T.; Beresford, M.W.; Burgos-Vargas, R.; Cuttica, R.; Ilowite, N.T.; Khubchandani, R.; Laxer, R.M.; Lovell, D.J.; et al. Toward New Classification Criteria for Juvenile Idiopathic Arthritis: First Steps, Pediatric Rheumatology International Trials Organization International Consensus. J. Rheumatol. 2019, 46, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.G.; Grom, A.A.; Thompson, S.D.; Griffin, T.A.; Luyrink, L.K.; Colbert, R.A.; Glass, D.N. Biologic similarities based on age at onset in oligoarticular and polyarticular subtypes of juvenile idiopathic arthritis. Arthritis Rheum. 2010, 62, 3249. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.F.; Colbert, R.A. Juvenile Spondyloarthritis: A Distinct Form of Juvenile Arthritis. Pediatr. Clin. N. Am. 2018, 65, 675–690. [Google Scholar] [CrossRef]
- Yıldız, M.; Haşlak, F.; Adroviç, A.; Şahin, S.; Barut, K.; Kasapçopur, Ö. Juvenile spondyloartropathies. Eur. J. Rheumatol. 2022, 9, 42–49. [Google Scholar] [CrossRef]
- Gunz, A.C.; Canizares, M.; MacKay, C.; Badley, A.M. Magnitude of impact and healthcare use for musculoskeletal disorders in the paediatric: A population-based study. BMC Musculoskelet. Disord. 2012, 13, 98. [Google Scholar] [CrossRef]
- King, S.; Chambers, C.T.; Huguet, A.; MacNevin, R.C.; McGrath, P.J.; Parker, L.; MacDonald, A.J. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain 2011, 152, 2729–2738. [Google Scholar] [CrossRef]
- Turecki, M.B.; Taljanovic, M.S.; Stubbs, A.Y.; Graham, A.R.; Holden, D.A.; Hunter, T.B.; Rogers, L.F. Imaging of musculoskeletal soft tissue infections. Skelet. Radiol. 2010, 39, 957–971. [Google Scholar] [CrossRef]
- Zadig, P.; von Brandis, E.; Küfner, L.; Rosendahl, K.; Avenarius, D.; Ording Müller, L. Whole-body magnetic resonance imaging in children—How and why? A systematic review. Pediatr. Radiol. 2021, 51, 14–24. [Google Scholar] [CrossRef]
- Taşar, S.; Sözeri, B. Whole-body MRI in Pediatric Patients with Chronic Recurrent Multifocal Osteomyelitis. Med. J. Bakirkoy 2023, 19, 78–85. [Google Scholar] [CrossRef]
- Aden, S.; Won, S.; Yang, C.; Bui, T.; Higa, T.; Scheck, J.; Iyer, R.S.; Egbert, M.; Lindberg, A.; Zhao, Y. Increasing Cases of Chronic Nonbacterial Osteomyelitis in Children: A Series of 215 Cases from a Single Tertiary Referral Center. J. Rheumatol. 2022, 49, 929–934. [Google Scholar] [CrossRef]
- Menashe, S.J.; Iyer, R.S.; Ngo, A.; Rosenwasser, N.L.; Zhao, Y.; Maloney, E. Whole-body MRI at initial presentation of pediatric chronic recurrent multifocal osteomyelitis and correlation with clinical assessment. Pediatr. Radiol. 2022, 52, 2377–2387. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, P.; Prountzos, S.; Karavasilis, E.; Atsali, E.; Bizimi, V.; Alexopoulou, E.; Fotis, L. Whole-body magnetic resonance imaging findings and patterns of chronic nonbacterial osteomyelitis in a series of Greek pediatric Patients. Acta Radiol. Open 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Bhat, C.S.; Anderson, C.; Harbinson, A.; McCann, L.J.; Roderick, M.; Finn, A.; Davidson, J.E.; Ramanan, A.V. Chronic nonbacterial osteitis- a multicenter study. Pediatr. Rheumatol. 2018, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- D’ Angelo, P.; Tanturri de Horatio, L.; Toma, P.; Ording Miller, L.; Avenarius, D.; von Brandis, E.; Zadig, P.; Casazza, I.; Pardeo, M.; Pires-Marafon, D.; et al. Chronic nonbacterial osteomyelitis—Clinical and magnetic resonance imaging features. Pediatr. Radiol. 2021, 51, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Andronikou, S.; Kraft, J.K.; Offiah, A.C.; Jones, J.; Douis, H.; Thyagarajan, M.; Barrera, C.A.; Zouvani, A.; Ramanan, A.V. Whole-body MRI in the diagnosis of paediatric CNO/CRMO. Rheumtology 2020, 59, 2671–2680. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.Y.; Chen, K.C.; Huang, B.K.; Kavanaugh, A. Adult inflammatory arthritides: What the radiologist should know. Radiographics 2016, 36, 1849–1870. [Google Scholar] [CrossRef]
- Moradi, A.; Amin, R.M.; Thorne, J.E. The Role of Gender in Juvenile Idiopathic Arthritis-Associated Uveitis. J. Ophthalmol. 2014, 2014, 61078. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Guo, G. Enthesitis-related arthritis: The clinical characteristics and factors related to MRI remission of sacroiliitis. BMC Musculoskelet. Disord. 2022, 23, 1054. [Google Scholar] [CrossRef]
- Burgos-Vargas, R.; Pacheco-Tena, C.; Vazquez-Mellado, J. A short-term follow-up of enthesitis and arthritis in the active phase of juvenile onset spondyloarthropathies. Clin. Exp. Rheumatol. 2002, 20, 727–731. [Google Scholar]
- Rachlis, A.C.; Babyn, P.S.; Lobo-Mueller, E.W.; Benseler, S.M.; Stimec, J. Whole body magnetic resonance imaging in juvenile spondyloarthritis: Will it provide vital information compared to clinical exam alone? Arthritis Rheum. 2011, 63, 29. [Google Scholar]
- Sudoł-Szopińska, I.; Matuszewska, G.; Pracoń, G. Juvenile spondyloarthritis. In Radiographic Atlas of Inflammatory Rheumatic Diseases, 1st ed.; Sudoł-Szopińska, I., Matuszewska, G., Pracoń, G., Eds.; Medisfera: Otwock, Poland, 2022; pp. 215–219. [Google Scholar]
- Coles, M.L.; Weissmann, R.; Uziel, Y. Juvenile primary fibromyalgia syndrome: Epidemiology, etiology, pathogenesis, clinical manifestations and diagnosis. Pediatr. Rheumatol. 2021, 19, 22. [Google Scholar] [CrossRef]
- Weissmann, R.; Uziel, Y. Pediatric complex regional pain syndrome: A review. Pediatr. Rheumatol. Online J. 2016, 14, 29. [Google Scholar] [CrossRef]
- Pavone, V.; Vescio, A.; Valenti, F.; Sapienza, M.; Sessa, G.; Testa, G. Growing pains: What do we know about etiology? A systematic review. World J. Orthop. 2019, 10, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, F.; Brachi, S.; Zulian, F.; Vittadello, F. Musculoskeletal pain in schoolchildren across puberty: A 3-year follow-up study. Pediatr. Rheumatol. 2015, 13, A723–A724. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, C.; Houghton, K. Noninflammatory musculosceletal pain conditions. In Textbook of Pediatric Rheumatology; Cassidy, J.T., Petty, R.E., Eds.; Elsevier Saunders: Amsterdam, The Netherlands, 2011; pp. 697–717. [Google Scholar]
- De Somer, L.; Wouters, C.; Pans, S. Total body MRI, a guide to diagnosis in patients with osteo-articular pain and inflammation. Pediatr. Rheumatol. Online J. 2014, 12 (Suppl. S1), P164. [Google Scholar] [CrossRef][Green Version]
- Pasoglou, V.; Van Nieuwenhove, S.; Peeters, F.; Duchêne, G.; Kirchgesner, T.; Lecouvet, F.E. 3D Whole-Body MRI of the Musculoskeletal System. Semin. Musculoskelet. Radiol. 2021, 25, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Zadig, P.K.; von Brandis, E.; Flatø, B.; Müller, L.S.O.; Nordal, E.B.; de Horatio, L.T.; Rosendahl, K.; Avenarius, D.F. Whole body magnetic resonance imaging in healthy children and adolescents Bone marrow appearances of the appendicular skeleton. Eur. J. Radiol. 2022, 153, 110365. [Google Scholar] [CrossRef] [PubMed]
- von Brandis, E.; Zadig, P.K.; Avenarius, D.F.M.; Flatø, B.; Knudsen, P.K.; Lilleby, V.; Nguyen, B.; Rosendahl, K.; Müller, L.S.O. Whole body magnetic resonance imaging in healthy children and adolescents. Bone marrow appearances of the axial skeleton. Eur. J. Radiol. 2022, 154, 110425. [Google Scholar] [CrossRef]

| TIRM Sequence | TR (ms) | TE (ms) | Stacks | FOV | Phase Oversampling | Phase Encode Direction | Slice Thickness | Matrix | Time of Acquisition (min:s) |
|---|---|---|---|---|---|---|---|---|---|
| coronal whole body | 5500 | 42 | 5 | 500 mm per stack | 60% | right to left |
5 mm, 1.5 mm gap | 384 × 384 | 3:30 per stack |
| sagittal whole body | 4590 | 41 | 5 | 500 mm per stack | 20% | head to foot |
5 mm, 1.5 mm gap | 226 × 320 | 3:00 per stack |
| CRMO n = 26 | JIA n = 51 | OS n = 8 | NA n = 88 | |
|---|---|---|---|---|
| age | 13.5 (10–15) | 13 (11–15) | 12.5 (10.5–15) | 14 (11–16) |
| gender (boys) | 14 (54%) | 18 (35%) | 4 (50%) | 21 (24%) |
| Lesion | CRMO 1 n = 26 | JIA n = 51 | OS n = 8 | NA n = 88 | ||||
|---|---|---|---|---|---|---|---|---|
| Unilateral | Bilateral | Unilateral | Bilateral | Unilateral | Bilateral | Unilateral | Bilateral | |
| Mandibula | 4 (15.4%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Scapula, SCJ, ACJ | 1 (3.8%) | 1 (3.8%) | 1 (2.0%) | 0 | 0 | 0 | 0 | 0 |
| Clavicle | 2 (7.7%) | 1 (3.8%) | 0 | 1 (2.0%) | 0 | 0 | 0 | 0 |
| Humerus | 3 (11.5%) | 1 (3.8%) | 2 (3.9%) | 0 | 0 | 1 (12.5%) | 0 | 0 |
| Ulna | 0 | 1 (3.8%) | 0 | 1 (2.0%) | 0 | 0 | 0 | 0 |
| Radius | 2 (7.7%) | 1 (3.8%) | 0 | 2 (3.9%) | 0 | 0 | 0 | 0 |
| Hand and wrist | 0 | 2 (7.7%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Pelvis | 2 (7.7%) | 1 (3.8%) | 4 (7.8%) | 5 (9.8%) | 1 (12.5%) | |||
| Patella | 1 (3.8%) | 1 (3.8%) | 1 (2.0%) | 0 | 0 | 0 | 0 | 0 |
| Femur | 3 (11.5%) | 10 (38.5%) | 6 (11.8%) | 12(23.5%) | 2 (25.0%) | 3 (37.5%) | 1 (1.1%) | 1 (1.1%) |
| Tibia | 4 (15.4%) | 12 (46.2%) | 1 (2.0%) | 6 (11.8%) | 2 (25.0%) | 2 (25.0%) | 4 (4.4%) | 0 |
| Fibula | 3 (11.5%) | 1 (3.8%) | 1 (2.0%) | 1 (2.0%) | 0 | 0 | 1 (1.1%) | 0 |
| Talus | 4 (15.4%) | 5 (19.2%) | 5 (9.8%) | 6 (11.8%) | 0 | 3 (37.5%) | 2 (2.2%) | 3 (3.3%) |
| Calcaneus | 5 (19.2%) | 3 (11.5%) | 1 (2.0%) | 6 (11.8%) | 1 (12.5%) | 2 (25.0%) | 1 (1.1%) | 2 (2.2%) |
| Midfoot | 3 (11.5%) | 4 (15.4%) | 3 (5.9%) | 7 (13.7%) | 0 | 2 (25.0%) | 1 (1.1%) | 1 (1.1%) |
| Metatarsal/s | 4 (15.4%) | 2 (7.7%) | 0 | 1 (2.0%) | 0 | 0 | 2 (2.2%) | 2 (2.2%) |
| Toes | 0 | 1 (3.8%) | 0 | 0 | 0 | 0 | 1 (1.1%) | 1 (1.1%) |
| An Affected Bone | Proximal Metaphysis | Proximal Epiphysis | Distal Metaphysis | Distal Epiphysis | ||||
|---|---|---|---|---|---|---|---|---|
| CRMO | JIA | CRMO | JIA | CRMO | JIA | CRMO | JIA | |
| Humerus | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 |
| Ulna | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| Radius | 2 | 0 | 2 | 0 | 1 | 2 | 2 | 2 |
| Femur | 5 | 6 | 3 | 2 | 10 | 8 | 9 | 12 |
| Tibia | 6 | 5 | 4 | 3 | 10 | 5 | 10 | 1 |
| Fibula | 0 | 1 | 0 | 1 | 2 | 1 | 2 | 0 |
| Lesion | CRMO n =26 | JIA n = 51 | OS n = 8 | NA n = 88 | ||||
|---|---|---|---|---|---|---|---|---|
| Unilateral | Bilateral | Unilateral | Bilateral | Unilateral | Bilateral | Unilateral | Bilateral | |
| Shoulder | 0 | 0 | 5 (9.8%) | 0 | 1 (12.5%) | 0 | 0 | 0 |
| Elbow | 0 | 0 | 1 (2.0%) | 1 (2.0%) | 1 (12.5%) | 1 (12.5%) | 1 (1.1%) | 0 |
| Wrist | 0 | 0 | 1 (2.0%) | 0 | 0 | 0 | 1 (1.1%) | 0 |
| SIJ | 0 | 0 | 1 (2.0%) | 0 | 0 | 0 | 0 | 0 |
| Hip | 0 | 0 | 4 (7.8%) | 8 (15.7%) | 1 (12.5%) | 3 (37.5%) | 0 | 0 |
| Knee | 0 | 2 (7.7%) | 9 (17.6%) | 8 (15.7%) | 0 | 1 (12.5%) | 1 (1.1%) | 0 |
| Ankle | 0 | 1 (3.8%) | 3 (5.9%) | 3 (5.9%) | 1 (12.5%) | 1 (12.5%) | 1 (1.1%) | 0 |
| Foot | 0 | 0 | 1 (2.0%) | 0 | 1 (12.5%) | 1 (12.5%) | 0 | 0 |
| Symphysis pubis | 0 | 0 | 1 (2.0%) | 0 | 1 (12.5%) | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanckoroński, M.; Gietka, P.; Mańczak, M.; Sudoł-Szopińska, I. Whole-Body MRI at Initial Presentation of Chronic Recurrent Multifocal Osteomyelitis, Juvenile Idiopathic Arthritis, Their Overlapping Syndrome, and Non-Specific Arthropathy. J. Clin. Med. 2024, 13, 998. https://doi.org/10.3390/jcm13040998
Lanckoroński M, Gietka P, Mańczak M, Sudoł-Szopińska I. Whole-Body MRI at Initial Presentation of Chronic Recurrent Multifocal Osteomyelitis, Juvenile Idiopathic Arthritis, Their Overlapping Syndrome, and Non-Specific Arthropathy. Journal of Clinical Medicine. 2024; 13(4):998. https://doi.org/10.3390/jcm13040998
Chicago/Turabian StyleLanckoroński, Michał, Piotr Gietka, Małgorzata Mańczak, and Iwona Sudoł-Szopińska. 2024. "Whole-Body MRI at Initial Presentation of Chronic Recurrent Multifocal Osteomyelitis, Juvenile Idiopathic Arthritis, Their Overlapping Syndrome, and Non-Specific Arthropathy" Journal of Clinical Medicine 13, no. 4: 998. https://doi.org/10.3390/jcm13040998
APA StyleLanckoroński, M., Gietka, P., Mańczak, M., & Sudoł-Szopińska, I. (2024). Whole-Body MRI at Initial Presentation of Chronic Recurrent Multifocal Osteomyelitis, Juvenile Idiopathic Arthritis, Their Overlapping Syndrome, and Non-Specific Arthropathy. Journal of Clinical Medicine, 13(4), 998. https://doi.org/10.3390/jcm13040998







