Effects of Miosis on the Visual Acuity Space under Varying Conditions of Contrast and Ambient Luminance in Presbyopia
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. General Examinations
2.3. VA-CAL Procedure
2.4. Statistics
3. Results
3.1. Participants’ Demographics
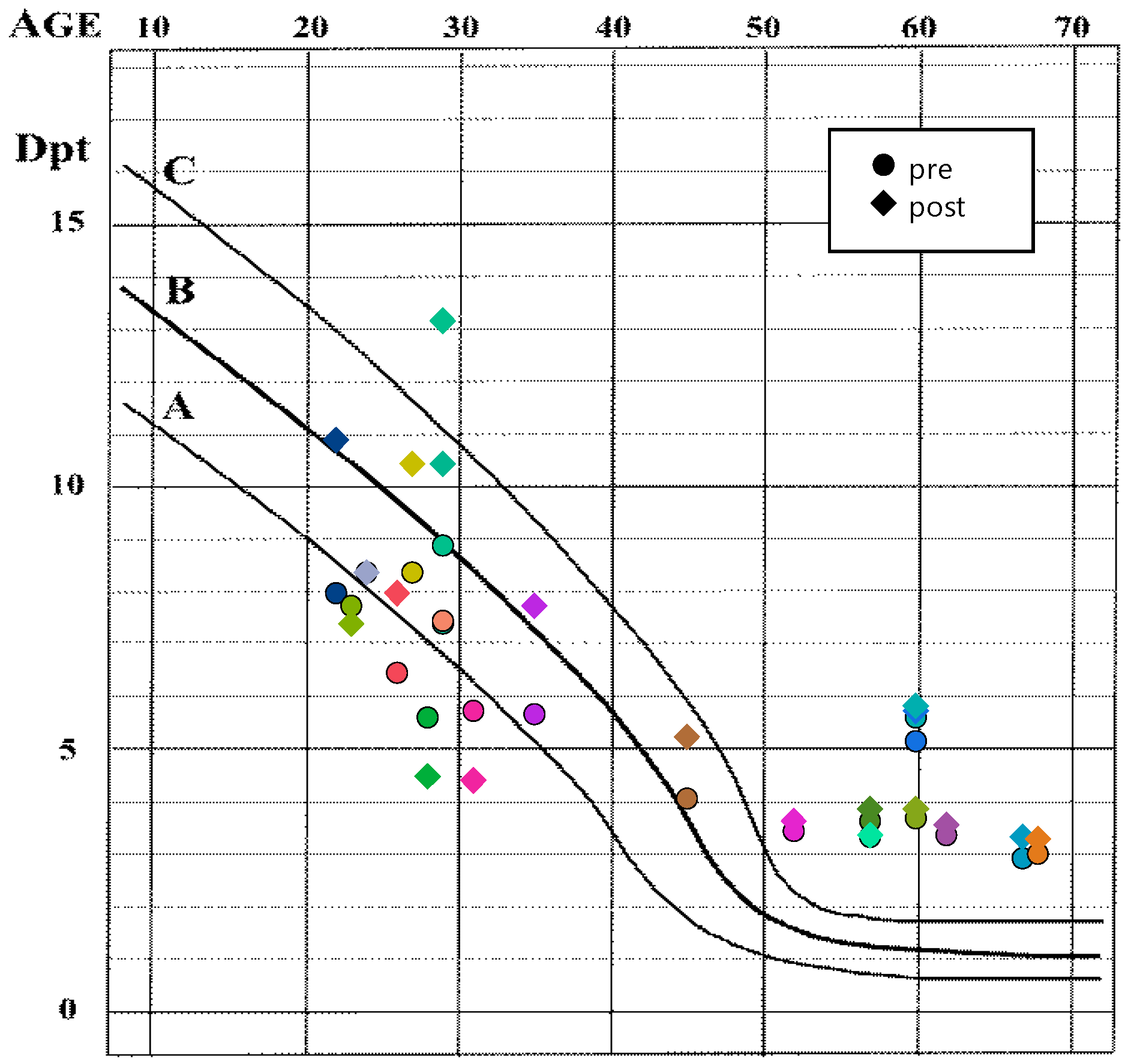
3.2. Effects of Refractive State and Time Point on Accommodation and Pupil Diameter
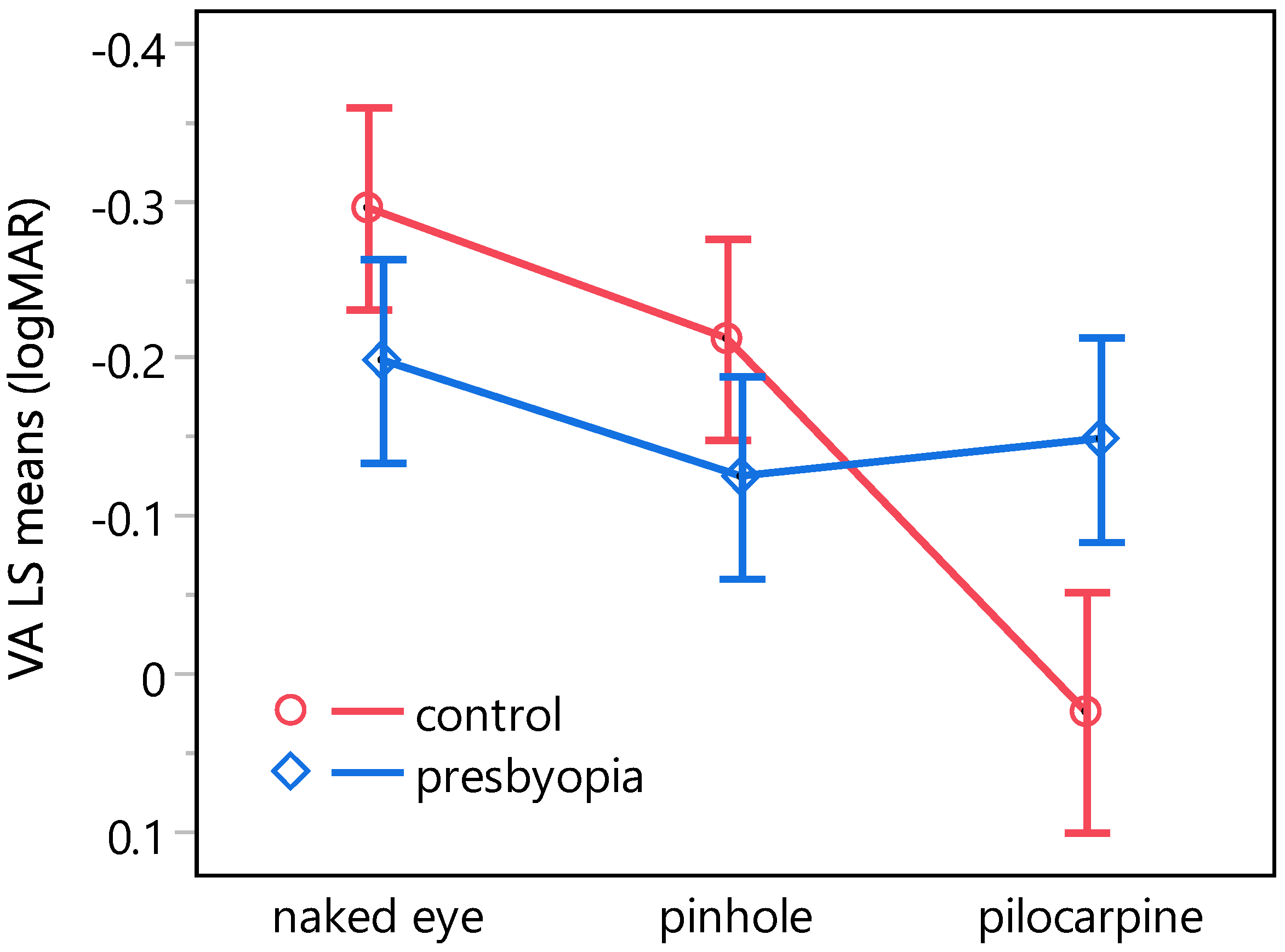
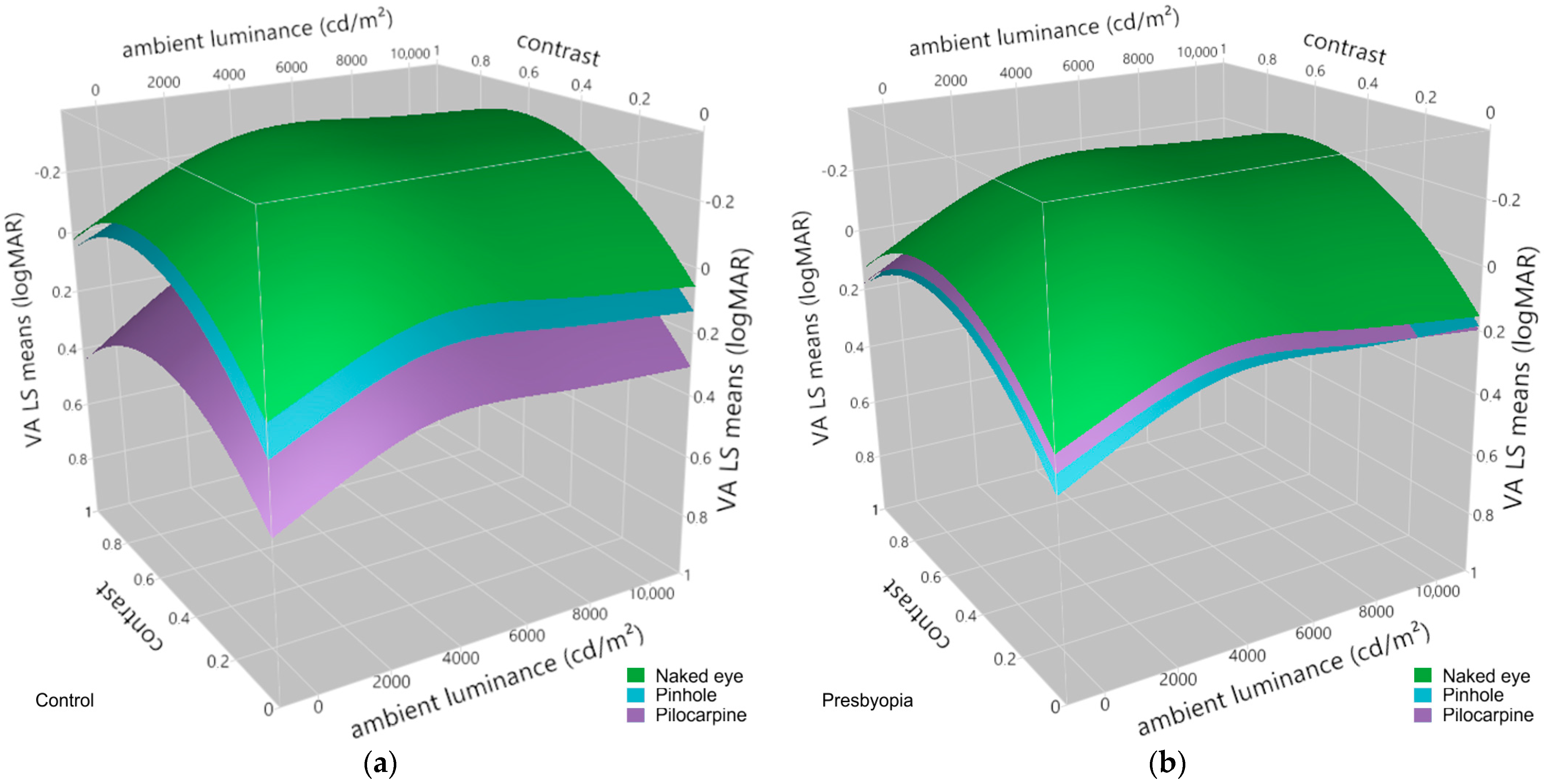
3.3. Effects of Pinhole Occluder and Pilocarpine on Visual Acuity at Varying Levels of Contrast and Ambient Luminance in Presbyopes and Emmetropic Controls
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duane, A. Studies in Monocular and Binocular Accommodation with their Clinical Applications. Am. J. Ophthalmol. 1922, 5, 865–877. [Google Scholar] [CrossRef]
- Glasser, A.; Campbell, M.C.W. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vis. Res. 1999, 39, 1991–2015. [Google Scholar] [CrossRef] [PubMed]
- Heys, K.R.; Cram, S.L.; Truscott, R.J.W. Massive increase in the stiffness of the human lens nucleus with age: The basis for presbyopia? Mol. Vis. 2004, 10, 956–963. [Google Scholar] [PubMed]
- Atchison, D.A. Accommodation and presbyopia. Ophthalmic Physiol. Opt. 1995, 15, 255–272. [Google Scholar] [CrossRef]
- Katz, J.A.; Karpecki, P.M.; Dorca, A.; Chiva-Razavi, S.; Floyd, H.; Barnes, E.; Wuttke, M.; Donnenfeld, E. Presbyopia—A Review of Current Treatment Options and Emerging Therapies. Clin. Ophthalmol. 2021, 15, 2167–2178. [Google Scholar] [CrossRef]
- Fricke, T.R.; Tahhan, N.; Resnikoff, S.; Papas, E.; Burnett, A.; Ho, S.M.; Naduvilath, T.; Naidoo, K.S. Global Prevalence of Presbyopia and Vision Impairment from Uncorrected Presbyopia. Ophthalmology 2018, 125, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Goertz, A.D.; Stewart, W.C.; Burns, W.R.; Stewart, J.A.; Nelson, L.A. Review of the impact of presbyopia on quality of life in the developing and developed world. Acta Ophthalmol. 2014, 92, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.F.; MacKenzie, G.E.; Kassalow, J.; Gudwin, E.; Congdon, N. Impact of Presbyopia and Its Correction in Low- and Middle-Income Countries. Asia-Pac. J. Ophthalmol. 2019, 7, 370–374. [Google Scholar] [CrossRef]
- Han, X.; Lee, P.Y.; Liu, C.; He, M. Distribution and progression of add power among people in need of near correction. Clin. Exp. Ophthalmol. 2018, 46, 882–887. [Google Scholar] [CrossRef]
- Holden, B.A.; Fricke, T.R.; Ho, S.M.; Wong, R.; Schlenther, G.; Cronjé, S.; Burnett, A.; Papas, E.; Naidoo, K.S.; Frick, K.D. Global Vision Impairment Due to Uncorrected Presbyopia. Arch. Ophthalmol. 2008, 126, 1731–1739. [Google Scholar] [CrossRef]
- Gajapati, C.V. Awareness of Presbyopia among Rural Female Population in North Karnataka. J. Clin. Diagn. Res. 2017, 11, NC01–NC05. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Davies, L.N. Presbyopia: Effectiveness of correction strategies. Prog. Retin. Eye Res. 2019, 68, 124–143. [Google Scholar] [CrossRef] [PubMed]
- Pallikaris, I.K.; Plainis, S.; Charman, W.N. Presbyopia: Origins, Effects, and Treatment, 1st ed.; SLACK Incorporated: Thorofare, NJ, USA, 2012; ISBN 1617110264. [Google Scholar]
- Charman, W.N. Developments in the correction of presbyopia I: Spectacle and contact lenses. Ophthalmic Physiol. Opt. 2014, 34, 8–29. [Google Scholar] [CrossRef] [PubMed]
- Charman, W.N. Developments in the correction of presbyopia II: Surgical approaches. Ophthalmic Physiol. Opt. 2014, 34, 397–426. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cazorla, R.; Shah, S.; Naroo, S.A. A review of the surgical options for the correction of presbyopia. Br. J. Ophthalmol. 2016, 100, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, S.A.; Alió, J.L. Presbyopic correction on the cornea. Eye Vis. 2014, 1, 5. [Google Scholar] [CrossRef]
- Paley, G.L.; Chuck, R.S.; Tsai, L.M. Corneal-Based Surgical Presbyopic Therapies and Their Application in Pseudophakic Patients. J. Ophthalmol. 2016, 2016, 5263870. [Google Scholar] [CrossRef] [PubMed]
- Korenfeld, M.S.; Robertson, S.M.; Stein, J.M.; Evans, D.G.; Rauchman, S.H.; Sall, K.N.; Venkataraman, S.; Chen, B.-L.; Wuttke, M.; Burns, W. Topical lipoic acid choline ester eye drop for improvement of near visual acuity in subjects with presbyopia: A safety and preliminary efficacy trial. Eye 2021, 35, 3292–3301. [Google Scholar] [CrossRef]
- Hughes, P.C.; Neer, R.M. Lighting for the Elderly: A Psychobiological Approach to Lighting. Hum. Factors J. Hum. Factors Ergon. Soc. 1981, 23, 65–85. [Google Scholar] [CrossRef]
- Schwartz, J.T.; Ogle, K.N. The Depth of Focus of the Eye. Arch. Ophthalmol. 1959, 61, 578–588. [Google Scholar] [CrossRef]
- Wang, B.; Ciuffreda, K.J. Depth-of-Focus of the Human Eye: Theory and Clinical Implications. Surv. Ophthalmol. 2006, 51, 75–85. [Google Scholar] [CrossRef]
- Miller, D.; Johnson, R. Quantification of the pinhole effect. Surv. Ophthalmol. 1977, 21, 347–350. [Google Scholar] [CrossRef]
- Charman, W.N. Correcting presbyopia: The problem of pupil size. Ophthalmic Physiol. Opt. 2017, 37, 1–6. [Google Scholar] [CrossRef]
- Lebensohn, J.E. The Pinhole Test. Am. J. Ophthalmol. 1950, 33, 1612–1614. [Google Scholar] [CrossRef]
- Park, H.H.; Park, I.K.; Moon, N.J.; Chun, Y.S. Clinical feasibility of pinhole glasses in presbyopia. Eur. J. Ophthalmol. 2019, 29, 133–140. [Google Scholar] [CrossRef]
- Kim, W.S.; Park, I.K.; Park, Y.K.; Chun, Y.S. Comparison of Objective and Subjective Changes Induced by Multiple-Pinhole Glasses and Single-Pinhole Glasses. J. Korean Med. Sci. 2017, 32, 850–857. [Google Scholar] [CrossRef]
- Kim, W.S.; Park, I.K.; Chun, Y.S. Quantitative Analysis of Functional Changes Caused by Pinhole Glasses. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6679–6685. [Google Scholar] [CrossRef] [PubMed]
- Charman, W.N. Pinholes and presbyopia: Solution or sideshow? Ophthalmic Physiol. Opt. 2019, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E. Pinhole Contact Lenses. Optom. Vis. Sci. 1952, 29, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Abadi, R.V.; Papas, E. Visual performance with artificial iris contact lenses. J. Br. Contact Lens Assoc. 1987, 10, 10–15. [Google Scholar] [CrossRef]
- Lindstrom, R.L.; MacRae, S.M.; Pepose, J.S.; Hoopes, P.C. Corneal inlays for presbyopia correction. Curr. Opin. Ophthalmol. 2013, 24, 281–287. [Google Scholar] [CrossRef]
- Arlt, E.; Krall, E.; Moussa, S.; Grabner, G.; Dexl, A. Implantable inlay devices for presbyopia: The evidence to date. Clin. Ophthalmol. 2015, 9, 129–137. [Google Scholar] [CrossRef]
- Srinivasan, S. Small aperture intraocular lenses: The new kids on the block. J. Cataract. Refract. Surg. 2018, 44, 927–928. [Google Scholar] [CrossRef]
- Vukich, J.A.; Durrie, D.S.; Pepose, J.S.; Thompson, V.; van de Pol, C.; Lin, L. Evaluation of the small-aperture intracorneal inlay: Three-year results from the cohort of the U.S. Food and Drug Administration clinical trial. J. Cataract. Refract. Surg. 2018, 44, 541–556. [Google Scholar] [CrossRef]
- Pluma-Jaramago, I.; Rocha-de-Lossada, C.; Rachwani-Anil, R.; Sánchez-González, J.-M. Small-aperture intracorneal inlay implantation in emmetropic presbyopic patients: A systematic review. Eye 2022, 36, 1747–1753. [Google Scholar] [CrossRef]
- Grabner, G.; Ang, R.E.; Vilupuru, S. The Small-Aperture IC-8 Intraocular Lens: A New Concept for Added Depth of Focus in Cataract Patients. Am. J. Ophthalmol. 2015, 160, 1176–1184.e1. [Google Scholar] [CrossRef]
- Dick, B.H.; Piovella, M.; Vukich, J.; Vilupuru, S.; Lin, L. Prospective multicenter trial of a small-aperture intraocular lens in cataract surgery. J. Cataract. Refract. Surg. 2017, 43, 956–968. [Google Scholar] [CrossRef]
- Trindade, C.L.C.; Trindade, B.L.C. Novel pinhole intraocular implant for the treatment of irregular corneal astigmatism and severe light sensitivity after penetrating keratoplasty. J. Cataract. Refract. Surg. Online Case Rep. 2015, 3, 4–7. [Google Scholar] [CrossRef]
- Trindade, C.C.; Trindade, B.C.; Trindade, F.C.; Werner, L.; Osher, R.; Santhiago, M.R. New pinhole sulcus implant for the correction of irregular corneal astigmatism. J. Cataract. Refract. Surg. 2017, 43, 1297–1306. [Google Scholar] [CrossRef]
- Benozzi, J.; Benozzi, G.; Orman, B. Presbyopia: A new potential pharmacological treatment. Med. Hypothesis Discov. Innov. Ophthalmol. 2012, 1, 3–5. [Google Scholar]
- Abdelkader, A. Improved Presbyopic Vision With Miotics. Eye Contact Lens Sci. Clin. Pract. 2015, 41, 323–327. [Google Scholar] [CrossRef]
- Renna, A.; Alió, J.L.; Vejarano, L.F. Pharmacological treatments of presbyopia: A review of modern perspectives. Eye Vis. 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Karanfil, F.Ç.; Turgut, B. Update on Presbyopia-correcting Drops. Eur. Ophthalmic Rev. 2017, 11, 99. [Google Scholar] [CrossRef]
- Mitchelson, F. Muscarinic Receptor Agonists and Antagonists: Effects on Ocular Function. In Handbook of Experimental Pharmacology; Kessinger Publishing: Whitefish, MT, USA, 2012; Volume 208, pp. 263–298. ISBN 9783642232732. [Google Scholar]
- Rosenfield, M. Pharmacological treatment of presbyopia. Ophthalmic Physiol. Opt. 2022, 42, 663–665. [Google Scholar] [CrossRef]
- Gil, D.W.; Krauss, H.A.; Bogardus, A.M.; WoldeMussie, E. Muscarinic receptor subtypes in human iris-ciliary body measured by immunoprecipitation. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1434–1442. [Google Scholar]
- Waring, G.O.; Price, F.W.; Wirta, D.; McCabe, C.; Moshirfar, M.; Guo, Q.; Gore, A.; Liu, H.; Safyan, E.; Robinson, M.R. Safety and Efficacy of AGN-190584 in Individuals With Presbyopia. JAMA Ophthalmol. 2022, 140, 363–371. [Google Scholar] [CrossRef]
- Price, F.W.; Hom, M.; Moshirfar, M.; Evans, D.; Liu, H.; Penzner, J.; Robinson, M.R.; Lee, S.; Wirta, D.L. Combinations of Pilocarpine and Oxymetazoline for the Pharmacological Treatment of Presbyopia: Two Randomized Phase 2 Studies. Ophthalmol. Sci. 2021, 1, 100065. [Google Scholar] [CrossRef]
- Woodhouse, J.M. The effect of pupil size on grating detection at various contrast levels. Vis. Res. 1975, 15, 645–648. [Google Scholar] [CrossRef]
- Campbell, F.W.; Gregory, A.H. Effect of Size of Pupil on Visual Acuity. Nature 1960, 187, 1121–1123. [Google Scholar] [CrossRef]
- Xu, R.; Wang, H.; Thibos, L.N.; Bradley, A. Interaction of aberrations, diffraction, and quantal fluctuations determine the impact of pupil size on visual quality. J. Opt. Soc. Am. A 2017, 34, 481–492. [Google Scholar] [CrossRef]
- Xu, R.; Thibos, L.; Bradley, A. Effect of Target Luminance on Optimum Pupil Diameter for Presbyopic Eyes. Optom. Vis. Sci. 2016, 93, 1409–1419. [Google Scholar] [CrossRef]
- Artigas, J.M.; Felipe, A.; Navea, A.; Fandiño, A.; Artigas, C. Spectral Transmission of the Human Crystalline Lens in Adult and Elderly Persons: Color and Total Transmission of Visible Light. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4076–4084. [Google Scholar] [CrossRef]
- Sample, P.A.; Esterson, F.D.; Weinreb, R.N.; Boynton, R.M. The aging lens: In vivo assessment of light absorption in 84 human eyes. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1306–1311. [Google Scholar]
- Hilmers, J.; Straßer, T.; Bach, M.; Stingl, K.; Zrenner, E. Quantification of the Dynamic Visual Acuity Space at Real-World Luminances and Contrasts: The VA-CAL Test. Transl. Vis. Sci. Technol. 2022, 11, 12. [Google Scholar] [CrossRef]
- Hilmers, J.; Bach, M.; Stingl, K.; Zrenner, E.; Straßer, T. The VA-CAL Test Quantifies Improvement of Visual Acuity in Achromatopsia by Means of Short-Wave Cutoff Filter Glasses in Daily Living Conditions. Transl. Vis. Sci. Technol. 2023, 12, 20. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Neely, J.C. The R.A.F. Near-point Rule. Br. J. Ophthalmol. 1956, 40, 636–637. [Google Scholar] [CrossRef]
- Watson, A.B.; Pelli, D.G. Quest: A Bayesian adaptive psychometric method. Percept. Psychophys. 1983, 33, 113–120. [Google Scholar] [CrossRef]
- Hair, J.F.J.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Multivariate Data Analysis, 3rd ed.; Macmillan: New York, NY, USA, 1995. [Google Scholar]
- Nobre, J.S.; da Motta Singer, J. Residual Analysis for Linear Mixed Models. Biom. J. 2007, 49, 863–875. [Google Scholar] [CrossRef]
- Crosby, J.M.; Twohig, M.P.; Phelps, B.I.; Fornoff, A.; Boie, I.; Mazur-Mosiewicz, A.; Dean, R.S.; Mazur-Mosiewicz, A.; Dean, R.S.; Allen, R.L.; et al. Homoscedasticity. In Encyclopedia of Child Behavior and Development; Springer: Boston, MA, USA, 2011; p. 752. [Google Scholar]
- Gauthier, J.; Wu, Q.V.; Gooley, T.A. Cubic splines to model relationships between continuous variables and outcomes: A guide for clinicians. Bone Marrow Transplant. 2020, 55, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Schuster, N.A.; Rijnhart, J.J.M.; Twisk, J.W.R.; Heymans, M.W. Modeling non-linear relationships in epidemiological data: The application and interpretation of spline models. Front. Epidemiol. 2022, 2, 29. [Google Scholar] [CrossRef]
- Mandel, J. Estimation of Weighting Factors in Linear Regression and Analysis of Variance. Technometrics 1964, 6, 1–25. [Google Scholar] [CrossRef]
- Strutz, T. Data Fitting and Uncertainty; Vieweg+Teubner: Wiesbaden, Germany, 2011; ISBN 978-3-8348-1022-9. [Google Scholar]
- Aitkin, M. Modelling Variance Heterogeneity in Normal Regression Using GLIM. J. R. Stat. Soc. Ser. C Appl. Stat. 1987, 36, 332. [Google Scholar] [CrossRef]
- Dunnett, C.W. Pairwise Multiple Comparisons in the Homogeneous Variance, Unequal Sample Size Case. J. Am. Stat. Assoc. 1980, 75, 789–795. [Google Scholar] [CrossRef]
- Sauder, D.C.; DeMars, C.E. An Updated Recommendation for Multiple Comparisons. Adv. Methods Pract. Psychol. Sci. 2019, 2, 26–44. [Google Scholar] [CrossRef]
- Tahhan, N.; Papas, E.; Fricke, T.R.; Frick, K.D.; Holden, B.A. Utility and Uncorrected Refractive Error. Ophthalmology 2013, 120, 1736–1744. [Google Scholar] [CrossRef]
- Lu, Q.; Congdon, N.; He, X.; Murthy, G.V.S.; Yang, A.; He, W. Quality of Life and Near Vision Impairment Due to Functional Presbyopia among Rural Chinese Adults. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4118–4123. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, E.L.; Fenwick, E.; Moore, K.; Klaic, M.; Borschmann, K.; Hill, K. Impact of the Severity of Distance and Near-Vision Impairment on Depression and Vision-Specific Quality of Life in Older People Living in Residential Care. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4103–4109. [Google Scholar] [CrossRef]
- Patel, I.; Munoz, B.; Burke, A.G.; Kayongoya, A.; Mchiwa, W.; Schwarzwalder, A.W.; West, S.K. Impact of Presbyopia on Quality of Life in a Rural African Setting. Ophthalmology 2006, 113, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Luo, B. Associations of presbyopia with vision-targeted health-related quality of life. Evid.-Based Eye Care 2004, 5, 100–101. [Google Scholar] [CrossRef]
- Wade, N.J. A Natural History of Vision; The MIT Press: Cambridge, MA, USA, 2000; ISBN 9780262731294. [Google Scholar]
- Bennett, A.G. An historical review of optometric principles and techniques. Ophthalmic Physiol. Opt. 1986, 6, 3–21. [Google Scholar] [CrossRef]
- Marcos, S.; Moreno, E.; Navarro, R. The depth-of-field of the human eye from objective and subjective measurements. Vis. Res. 1999, 39, 2039–2049. [Google Scholar] [CrossRef]
- Guthrie, C.C. Physiologic Lensless Spectacles. Arch. Ophthalmol. 1934, 11, 254–261. [Google Scholar] [CrossRef]
- Seyeddain, O.; Bachernegg, A.; Riha, W.; Rückl, T.; Reitsamer, H.; Grabner, G.; Dexl, A.K. Femtosecond laser–assisted small-aperture corneal inlay implantation for corneal compensation of presbyopia: Two-year follow-up. J. Cataract. Refract. Surg. 2013, 39, 234–241. [Google Scholar] [CrossRef]
- Seyeddain, O.; Hohensinn, M.; Riha, W.; Nix, G.; Rückl, T.; Grabner, G.; Dexl, A.K. Small-aperture corneal inlay for the correction of presbyopia: 3-year follow-up. J. Cataract. Refract. Surg. 2012, 38, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.H.; Waring, G.O.; Hom, M.; Barnett, M. Presbyopia Treatments by Mechanism of Action: A New Classification System Based on a Review of the Literature. Clin. Ophthalmol. 2021, 15, 3733–3745. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, K.; Jin, Y.; Niu, Y.; Zuo, T. Changes of Higher Order Aberration With Various Pupil Sizes in the Myopic Eye. J. Refract. Surg. 2003, 19, S270–S274. [Google Scholar] [CrossRef] [PubMed]
- Kinney, M.; Johnson, A.D.; Reddix, M.; McCann, M.B. Temporal Effects of 2% Pilocarpine Ophthalmic Solution on Human Pupil Size and Accommodation. Mil. Med. 2020, 185, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Wesemann, W. Vuity Augentropfen—Eine Konkurrenz zur Lesebrille; DOZ: Heidelberg, Germany, 2022; pp. 72–77. [Google Scholar]
- Atchison, D.A.; Smith, G.; Efron, N. The Effect of Pupil Size on Visual Acuity in Uncorrected and Corrected Myopia. Optom. Vis. Sci. 1979, 56, 315–323. [Google Scholar] [CrossRef]
- Thibos, L.N.; Hong, X.; Bradley, A.; Cheng, X. Statistical variation of aberration structure and image quality in a normal population of healthy eyes. J. Opt. Soc. Am. A 2002, 19, 2329–2348. [Google Scholar] [CrossRef]
- Bradley, A.; Nam, J.; Xu, R.; Harman, L.; Thibos, L. Impact of contact lens zone geometry and ocular optics on bifocal retinal image quality. Ophthalmic Physiol. Opt. 2014, 34, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Guillon, M.; Dumbleton, K.; Theodoratos, P.; Gobbe, M.; Wooley, C.B.; Moody, K. The Effects of Age, Refractive Status, and Luminance on Pupil Size. Optom. Vis. Sci. 2016, 93, 1093–1100. [Google Scholar] [CrossRef]
- Winn, B.; Whitaker, D.; Elliott, D.B.; Phillips, N.J. Factors affecting light-adapted pupil size in normal human subjects. Investig. Ophthalmol. Vis. Sci. 1994, 35, 1132–1137. [Google Scholar]
- Telek, H.H. The Effects of Age Pupil Diameters at Different Light Amplitudes. Beyoglu Eye J. 2018, 3, 80–85. [Google Scholar] [CrossRef]
- Schielzeth, H.; Dingemanse, N.J.; Nakagawa, S.; Westneat, D.F.; Allegue, H.; Teplitsky, C.; Réale, D.; Dochtermann, N.A.; Garamszegi, L.Z.; Araya-Ajoy, Y.G. Robustness of linear mixed-effects models to violations of distributional assumptions. Methods Ecol. Evol. 2020, 11, 1141–1152. [Google Scholar] [CrossRef]
- Burns, D.H.; Allen, P.M.; Edgar, D.F.; Evans, B.J.W. A Review of Depth of Focus in Measurement of the Amplitude of Accommodation. Vision 2018, 2, 37. [Google Scholar] [CrossRef]
- Atchison, D.A.; Charman, W.N.; Woods, R.L. Subjective Depth-of-Focus of the Eye. Optom. Vis. Sci. 1997, 74, 511–520. [Google Scholar] [CrossRef]
- Campbell, F.W. The depth of focus of the human eye. J. Physiol. 1954, 125, 29–30P. [Google Scholar]
- Cufflin, M.P.; Mankowska, A.; Mallen, E.A.H. Effect of Blur Adaptation on Blur Sensitivity and Discrimination in Emmetropes and Myopes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2932–2939. [Google Scholar] [CrossRef]
- Burns, D.H.; Allen, P.M.; Edgar, D.F.; Evans, B.J.W. Sources of error in clinical measurement of the amplitude of accommodation. J. Optom. 2020, 13, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Hauser, B.; Ochsner, H.; Zrenner, E. Der “Blendvisus”—Teil 1: Physiologische Grundlagen der Visusänderung bei steigender Testfeldleuchtdichte. Klin. Monbl. Augenheilkd. 1992, 200, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, C.; Cui, R.; He, X.; Shen, M.; Lesmes, L.A.; Lu, Z.-L.; Qu, J.; Hou, F. Measuring the Contrast Sensitivity Function Using the qCSF Method With 10 Digits. Transl. Vis. Sci. Technol. 2018, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, T.J.; Wheeler, T.M. Side Effects and Ways to Avoid Them. Ophthalmology 1982, 89, 76–80. [Google Scholar] [CrossRef] [PubMed]
- François, J.; Goes, F.; Zagorski, Z. Comparative Ultrasonographic Study of the Effect of Pilocarpine 2% and Ocusert P 20 on the Eye Components. Am. J. Ophthalmol. 1978, 86, 233–238. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, M.; Cong, Y.; Cheng, M.; Wang, S.; Wang, G. The pilocarpine-induced ciliary body contraction affects the elastic modulus and collagen of cornea and sclera in early development. Biomed. Pharmacother. 2018, 108, 1816–1824. [Google Scholar] [CrossRef]
- Mansoori, T. Pilocarpine 1.25% for the treatment of presbyopia. Indian J. Ophthalmol. 2023, 71, 308–309. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Emmetropes |
|
|
| Presbyopes |
|
|
| Fixed Effects and Interaction | Near Point (n = 41, R2adj. = 0.97) | Pupil Diameter (n = 43, R2adj. = 0.89) | ||
|---|---|---|---|---|
| F-Statistic | p-Value 1 | F-Statistic | p-Value 1 | |
| group | F(1, 19.13) = 41.32 | <0.0001 *** | F(1, 20.28) = 0.39 | 0.5399 |
| time point | F(1, 18.29) = 6.86 | 0.0172 * | F(1, 19.86) = 189.51 | <0.0001 *** |
| group × time point | F(1, 18.29) = 0.88 | 0.3593 | F(1, 19.86) = 0.54 | 0.4692 |
| Comparison (LS Means ± SE) | Diff. | 95% CI | t-Value | p-Value 1 | |
|---|---|---|---|---|---|
| Near point (cm) | |||||
| control (13.7 ± 1.4) | presbyope (26.7 ± 1.5) | 12.9 | [8.7, 17.1] | 6.53 | <0.0001 *** |
| pre (21.0 ± 1.0) | post (19.4 ± 1.1) | −1.6 | [−3.0, −0.3] | −2.62 | 0.0172 * |
| Pupil diameter (mm) | |||||
| pre (3.3 ± 0.1) | post (2.3 ± 0.1) | −1.0 | [−1.1, −0.8] | −13.77 | <0.0001 *** |
| Fixed Effects and Interactions 1 | F-Statistic | p-Value 2 |
|---|---|---|
| group | F(1, 21.06) = 0.01 | 0.9304 |
| condition | F(2, 1141.10) = 143.40 | <0.0001 *** |
| ambient luminance | F(3, 1140.92) = 142.88 | <0.0001 *** |
| contrast | F(1, 1140.92) = 472.61 | <0.0001 *** |
| contrast × contrast | F(1, 1140.92) = 158.77 | <0.0001 *** |
| group × condition | F(2, 1141.10) = 56.19 | <0.0001 *** |
| group × contrast | F(1, 1140.92) = 0.21 | 0.6456 |
| group × ambient luminance | F(1, 1140.92) = 0.05 | 0.8193 |
| condition × contrast | F(2, 1140.92) = 1.50 | 0.2251 |
| condition × ambient luminance | F(2, 1140.92) = 1.27 | 0.2801 |
| contrast × ambient luminance | F(1, 1140.92) = 2.66 | 0.1034 |
| group × condition × contrast | F(2, 1140.92) = 0.14 | 0.8662 |
| group × condition × ambient luminance | F(2, 1140.92) = 3.85 | 0.0215 * |
| group × contrast × ambient luminance | F(1, 1140.92) = 0.00 | 0.9770 |
| condition × contrast × ambient luminance | F(2, 1140.92) = 0.94 | 0.3917 |
| group × condition × contrast × ambient luminance | F(2, 1140.92) = 0.19 | 0.8252 |
| Comparison | VA LS Means ± SE (logMAR) | Diff. [95% CI] 1 (logMAR) | ||
|---|---|---|---|---|
| control, naked eye | control, pinhole | −0.30 ± 0.03 | −0.21 ± 0.03 | −0.08 [−0.21, 0.04] |
| control, pilocarpine | 0.02 ± 0.04 | −0.32 [−0.46, −0.18] * | ||
| presbyopia, naked eye | −0.20 ± 0.03 | −0.10 [−0.22, 0.03] | ||
| presbyopia, pinhole | −0.12 ± 0.03 | −0.17 [−0.30, −0.04] * | ||
| presbyopia, pilocarpine | −0.15 ± 0.03 | −0.15 [−0.27, −0.02] * | ||
| control, pinhole | control, pilocarpine | −0.21 ± 0.03 | 0.02 ± 0.04 | −0.24 [−0.38, −0.10] * |
| presbyopia, naked eye | −0.20 ± 0.03 | −0.01 [−0.14, 0.11] | ||
| presbyopia, pinhole | −0.12 ± 0.03 | −0.09 [−0.21, 0.04] | ||
| presbyopia, pilocarpine | −0.15 ± 0.03 | −0.06 [−0.19, 0.06] | ||
| control, pilocarpine | presbyopia, naked eye | 0.02 ± 0.04 | −0.20 ± 0.03 | 0.22 [0.08, 0.36] * |
| presbyopia, pinhole | −0.12 ± 0.03 | 0.15 [0.01, 0.29] * | ||
| presbyopia, pilocarpine | −0.15 ± 0.03 | 0.17 [0.03, 0.32] * | ||
| presbyopia, naked eye | presbyopia, pinhole | −0.20 ± 0.03 | −0.12 ± 0.03 | −0.07 [−0.20, 0.05] |
| presbyopia, pilocarpine | −0.15 ± 0.03 | −0.05 [−0.18, 0.08] | ||
| presbyopia, pinhole | presbyopia, pilocarpine | −0.12 ± 0.03 | −0.15 ± 0.03 | 0.02 [−0.10, 0.15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onyszkiewicz, M.; Hilmers, J.; Rejdak, R.; Zrenner, E.; Straßer, T. Effects of Miosis on the Visual Acuity Space under Varying Conditions of Contrast and Ambient Luminance in Presbyopia. J. Clin. Med. 2024, 13, 1209. https://doi.org/10.3390/jcm13051209
Onyszkiewicz M, Hilmers J, Rejdak R, Zrenner E, Straßer T. Effects of Miosis on the Visual Acuity Space under Varying Conditions of Contrast and Ambient Luminance in Presbyopia. Journal of Clinical Medicine. 2024; 13(5):1209. https://doi.org/10.3390/jcm13051209
Chicago/Turabian StyleOnyszkiewicz, Maksymilian, Julian Hilmers, Robert Rejdak, Eberhart Zrenner, and Torsten Straßer. 2024. "Effects of Miosis on the Visual Acuity Space under Varying Conditions of Contrast and Ambient Luminance in Presbyopia" Journal of Clinical Medicine 13, no. 5: 1209. https://doi.org/10.3390/jcm13051209
APA StyleOnyszkiewicz, M., Hilmers, J., Rejdak, R., Zrenner, E., & Straßer, T. (2024). Effects of Miosis on the Visual Acuity Space under Varying Conditions of Contrast and Ambient Luminance in Presbyopia. Journal of Clinical Medicine, 13(5), 1209. https://doi.org/10.3390/jcm13051209






