Cardioprotective Effects of PARP Inhibitors: A Re-Analysis of a Meta-Analysis and a Real-Word Data Analysis Using the FAERS Database
Abstract
1. Introduction
2. Materials and Methods
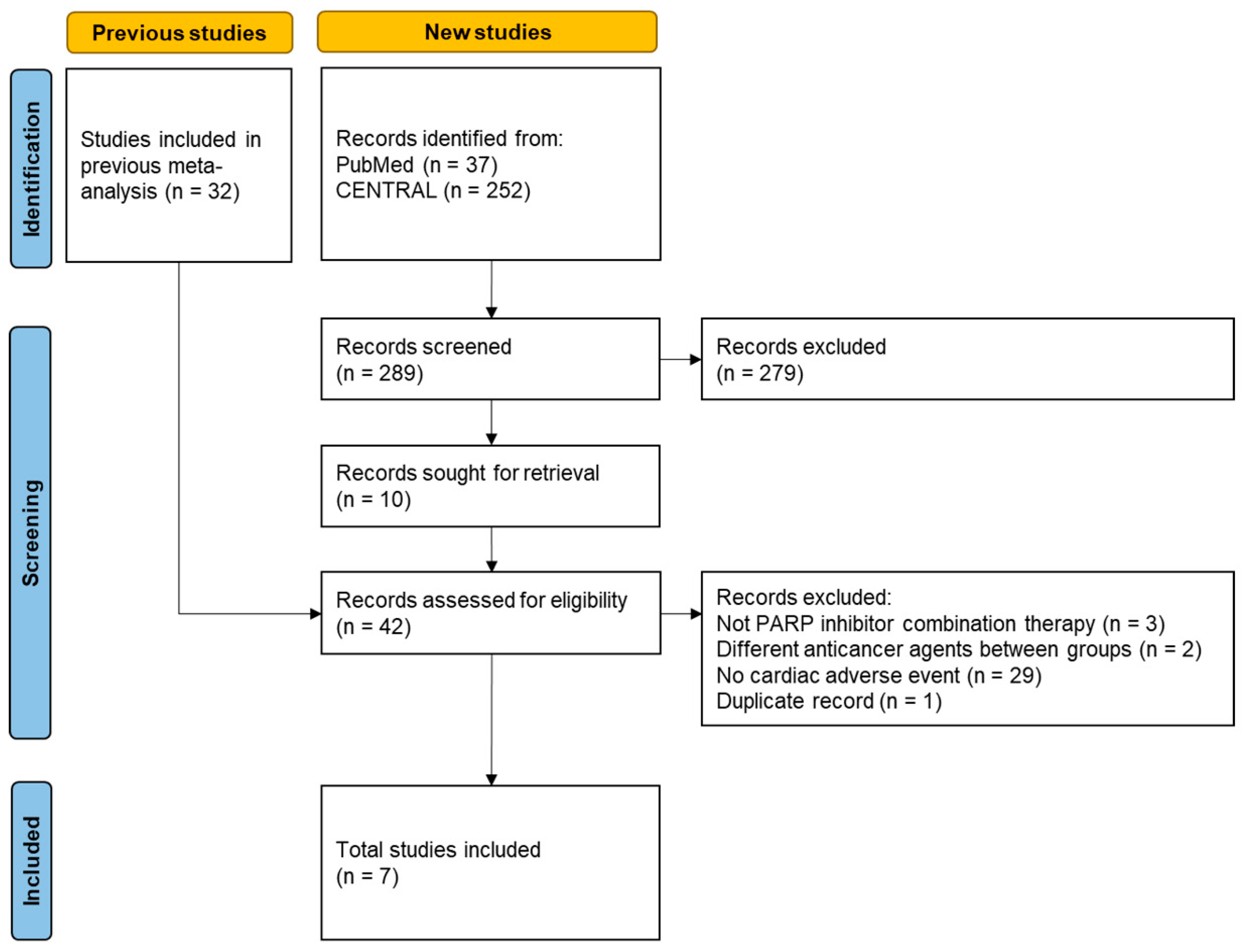
2.1. Re-Analysis of a Previous Meta-Analysis
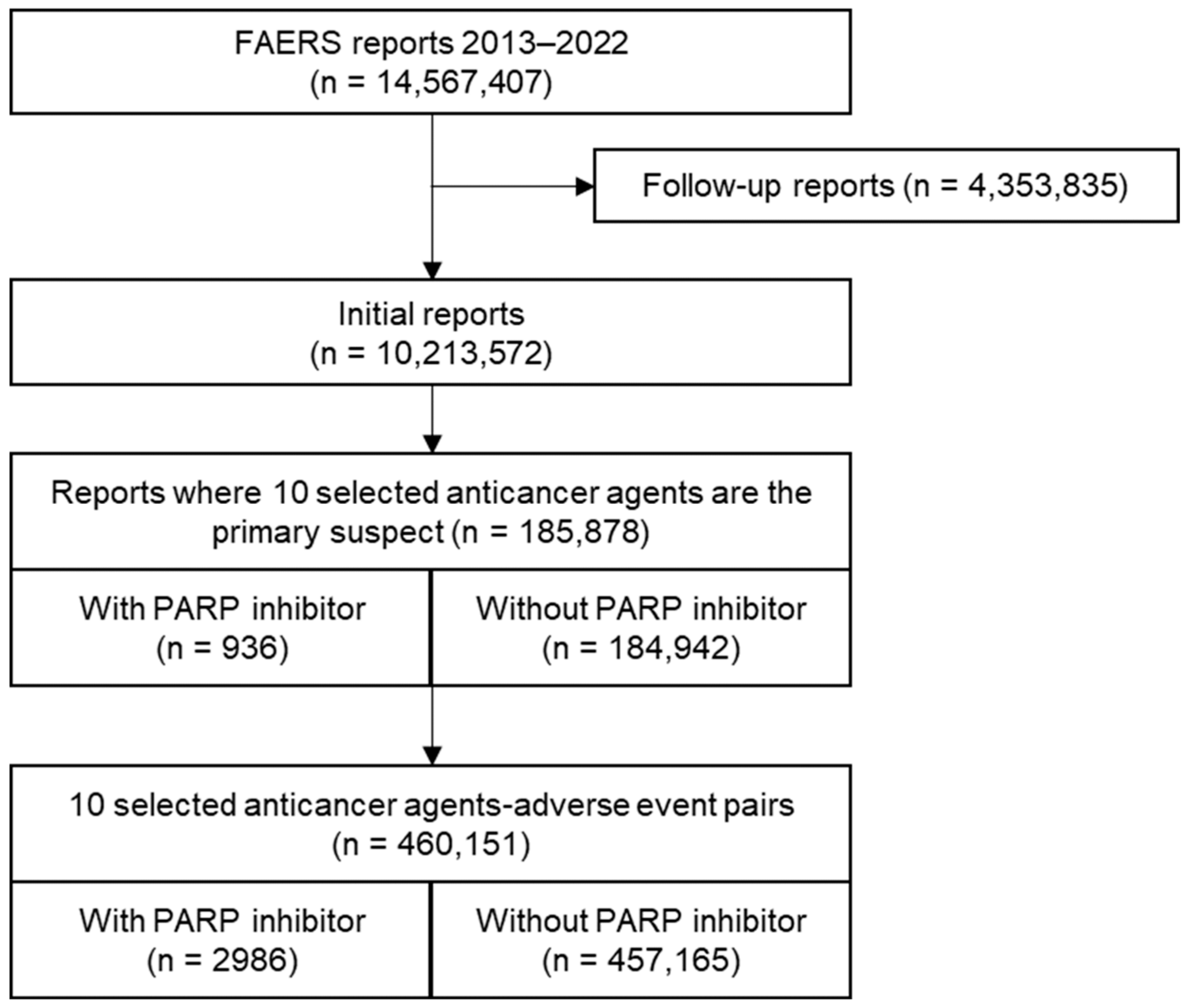
2.2. Analysis of the FAERS Database
3. Results
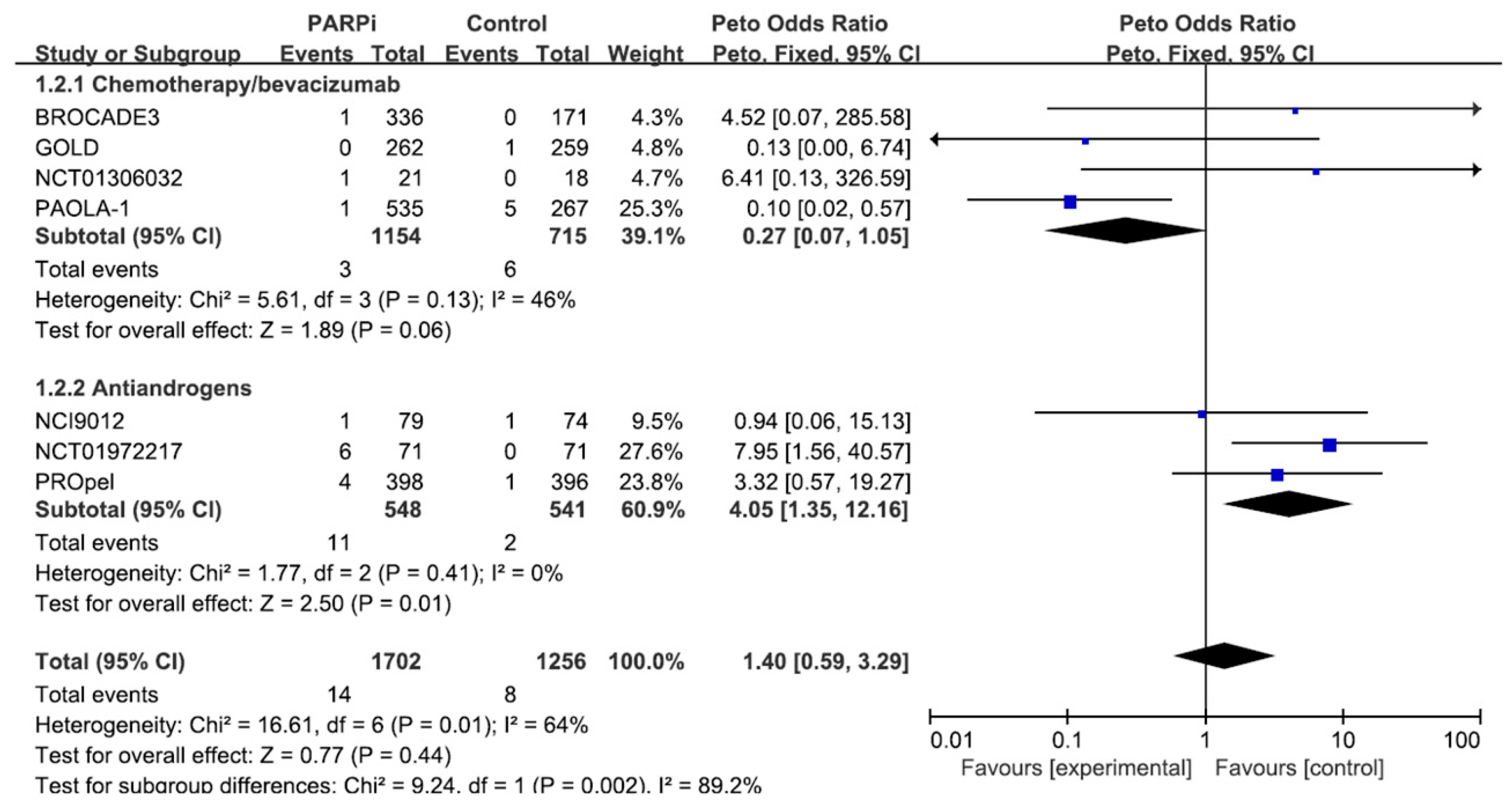
3.1. Re-Analysis of the Previous Meta-Analysis
3.2. Analysis of the FAERS Database
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hockings, H.; Miller, R.E. The role of PARP inhibitor combination therapy in ovarian cancer. Ther. Adv. Med. Oncol. 2023, 15, 17588359231173183. [Google Scholar] [CrossRef]
- Morelli, M.B.; Bongiovanni, C.; Da Pra, S.; Miano, C.; Sacchi, F.; Lauriola, M.; D’uva, G. Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms and Strategies for Cardioprotection. Front. Cardiovasc. Med. 2022, 9, 847012. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.J.; Bourgeois, M.; Harbison, R.D. Poly(ADP-ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders. Cardiovasc. Toxicol. 2018, 18, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.M.; Kong, X.; Moncada, E.; Chen, Y.; Imamura, H.; Wang, P.; Berns, M.W.; Yokomori, K.; Digman, M.A. NAD+ consumption by PARP1 in response to DNA damage triggers metabolic shift critical for damaged cell survival. Mol. Biol. Cell 2019, 30, 2584–2597. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Liaudet, L.; Bai, P.; Virag, L.; Mabley, J.G.; Hasko, G.; Szabo, C. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J. Pharmacol. Exp. Ther. 2002, 300, 862–867. [Google Scholar] [CrossRef]
- Pacher, P.; Liaudet, L.; Mabley, J.G.; Cziraki, A.; Hasko, G.; Szabo, C. Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure. Int. J. Mol. Med. 2006, 17, 369–375. [Google Scholar] [CrossRef][Green Version]
- Bartha, E.; Solti, I.; Szabo, A.; Olah, G.; Magyar, K.; Szabados, E.; Kalai, T.; Hideg, K.; Toth, K.; Gero, D.; et al. Regulation of kinase cascade activation and heat shock protein expression by poly(ADP-ribose) polymerase inhibition in doxorubicin-induced heart failure. J. Cardiovasc. Pharmacol. 2011, 58, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Szenczi, O.; Kemecsei, P.; Holthuijsen, M.F.; van Riel, N.A.; van der Vusse, G.J.; Pacher, P.; Szabó, C.; Kollai, M.; Ligeti, L.; Ivanics, T. Poly(ADP-ribose) polymerase regulates myocardial calcium handling in doxorubicin-induced heart failure. Biochem. Pharmacol. 2005, 69, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Morrow, D.A.; Brickman, C.M.; Murphy, S.A.; Baran, K.; Krakover, R.; Dauerman, H.; Kumar, S.; Slomowitz, N.; Grip, L.; McCabe, C.H.; et al. A randomized, placebo-controlled trial to evaluate the tolerability, safety, pharmacokinetics, and pharmacodynamics of a potent inhibitor of poly(ADP-ribose) polymerase (INO-1001) in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: Results of the TIMI 37 trial. J. Thromb. Thrombolysis. 2009, 27, 359–364. [Google Scholar]
- Palazzo, A.; Ciccarese, C.; Iacovelli, R.; Cannizzaro, M.C.; Stefani, A.; Salvatore, L.; Bria, E.; Tortora, G. Major adverse cardiac events and cardiovascular toxicity with PARP inhibitors-based therapy for solid tumors: A systematic review and safety meta-analysis. ESMO Open 2023, 8, 101154. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing Data and Undertaking Meta-Analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023). Available online: https://training.cochrane.org/handbook/current/chapter-10#section-10-4-2 (accessed on 12 June 2023).
- Wittayanukorn, S.; Qian, J.; Johnson, B.S.; A Hansen, R. Cardiotoxicity in targeted therapy for breast cancer: A study of the FDA adverse event reporting system (FAERS). J. Oncol. Pharm. Pract. 2017, 23, 93–102. [Google Scholar] [CrossRef]
- Haldane, J. The mean and variance of the moments of chi-squared, when used as a test of homogeneity, when expectations are small. Biometrika 1940, 29, 133–134. [Google Scholar]
- Kummar, S.; Wade, J.L.; Oza, A.M.; Sullivan, D.; Chen, A.P.; Gandara, D.R.; Ji, J.; Kinders, R.J.; Wang, L.; Allen, D.; et al. Randomized phase II trial of cyclophosphamide and the oral poly (ADP-ribose) polymerase inhibitor veliparib in patients with recurrent, advanced triple-negative breast cancer. Investig. New Drugs 2016, 34, 355–363. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Xu, R.-H.; Chin, K.; Lee, K.-W.; Park, S.H.; Rha, S.Y.; Shen, L.; Qin, S.; Xu, N.; Im, S.-A.; et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1637–1651. [Google Scholar] [CrossRef]
- Hussain, M.; Daignault-Newton, S.; Twardowski, P.W.; Albany, C.; Stein, M.N.; Kunju, L.P.; Siddiqui, J.; Wu, Y.M.; Robinson, D.; Lonigro, R.J.; et al. Targeting Androgen Receptor and DNA Repair in Metastatic Castration-Resistant Prostate Cancer: Results From NCI 9012. J. Clin. Oncol. 2018, 36, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.; Wiechno, P.; Alekseev, B.; Sala, N.; Jones, R.; Kocak, I.; Chiuri, V.E.; Jassem, J.; Fléchon, A.; Redfern, C.; et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018, 19, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Diéras, V.; Han, H.S.; Kaufman, B.; Wildiers, H.; Friedlander, M.; Ayoub, J.-P.; Puhalla, S.L.; Bondarenko, I.; Campone, M.; Jakobsen, E.H.; et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Thiery-Vuillemin, A.; Saad, F.; Armstrong, A.J.; Oya, M.; Vianna, K.C.M.; Özgüroğlu, M.; Gedye, C.; Buchschacher, G.L.; Lee, J.Y.; Emmenegger, U.; et al. Tolerability of abiraterone (abi) combined with olaparib (ola) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): Further results from the phase III PROpel trial. J. Clin. Oncol. 2022, 40, 5019. [Google Scholar] [CrossRef]
- Korkmaz-Icöz, S.; Szczesny, B.; Marcatti, M.; Li, S.; Ruppert, M.; Lasitschka, F.; Loganathan, S.; Szabó, C.; Szabó, G. Olaparib protects cardiomyocytes against oxidative stress and improves graft contractility during the early phase after heart transplantation in rats. Br. J. Pharmacol. 2018, 175, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Y.; Rosenberg, J.E. Abiraterone acetate: Oral androgen biosynthesis inhibitor for treatment of castration-resistant prostate cancer. Drug Des. Devel Ther. 2012, 6, 13–18. [Google Scholar] [CrossRef]
- Tsao, P.A.; Estes, J.P.; Griggs, J.J.; Smith, D.C.; Caram, M.E.V. Cardiovascular and Metabolic Toxicity of Abiraterone in Castration-resistant Prostate Cancer: Post-marketing Experience. Clin. Genitourin. Cancer 2019, 17, e592–e601. [Google Scholar] [CrossRef]
- Bretagne, M.; Lebrun-Vignes, B.; Pariente, A.; Shaffer, C.M.; Malouf, G.G.; Dureau, P.; Potey, C.; Funck-Brentano, C.; Roden, D.M.; Moslehi, J.J.; et al. Heart failure and atrial tachyarrhythmia on abiraterone: A pharmacovigilance study. Arch. Cardiovasc. Dis. 2020, 113, 9–21. [Google Scholar] [CrossRef]
- Shalata, W.; Abu-Salman, A.; Steckbeck, R.; Jacob, B.M.; Massalha, I.; Yakobson, A. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review. Cancers 2021, 13, 5218. [Google Scholar] [CrossRef]
- Quagliariello, V.; Passariello, M.; Di Mauro, A.; Cipullo, C.; Paccone, A.; Barbieri, A.; Palma, G.; Luciano, A.; Buccolo, S.; Bisceglia, I.; et al. Immune checkpoint inhibitor therapy increases systemic SDF-1, cardiac DAMPs Fibronectin-EDA, S100/Calgranulin, galectine-3, and NLRP3-MyD88-chemokine pathways. Front. Cardiovasc. Med. 2022, 9, 930797. [Google Scholar] [CrossRef] [PubMed]
- Nso, N.; Antwi-Amoabeng, D.; Beutler, B.D.; Ulanja, M.B.; Ghuman, J.; Hanfy, A.; Nimo-Boampong, J.; Atanga, S.; Doshi, R.; Enoru, S.; et al. Cardiac adverse events of immune checkpoint inhibitors in oncology patients: A systematic review and meta-analysis. World J. Cardiol. 2020, 12, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Pai Bellare, G.; Sankar Patro, B. Resveratrol sensitizes breast cancer to PARP inhibitor, talazoparib through dual inhibition of AKT and autophagy flux. Biochem. Pharmacol. 2022, 199, 115024. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Alam, A.S.; Takahara, S.; Soni, S.; Ferdaoussi, M.; Matsumura, N.; Zordoky, B.N.; Eisenstat, D.D.; Dyck, J.R. Resveratrol reduces cardiac NLRP3-inflammasome activation and systemic inflammation to lessen doxorubicin-induced cardiotoxicity in juvenile mice. FEBS Lett. 2021, 595, 1681–1695. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, H.; Wilson, M.A.; Matsushita, H.; Zhu, C.; Lange, M.; Gustavsson, M.; Poitras, M.F.; Dawson, T.M.; Dawson, V.L.; Northington, F.; et al. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J. Neurochem. 2004, 90, 1068–1075. [Google Scholar] [CrossRef]
- Mabley, J.G.; Horváth, E.M.; Murthy, K.G.K.; Zsengellér, Z.; Vaslin, A.; Benko, R.; Kollai, M.; Szabó, C. Gender differences in the endotoxin-induced inflammatory and vascular responses: Potential role of poly(ADP-ribose) polymerase activation. J. Pharmacol. Exp. Ther. 2005, 315, 812–820. [Google Scholar] [CrossRef]
- Booz, G.W. PARP inhibitors and heart failure? Translational medicine caught in the act. Congest. Heart Fail. 2007, 13, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A. The flaws and human harms of animal experimentation. Camb. Q. Healthc. Ethics 2015, 24, 407–419. [Google Scholar] [CrossRef] [PubMed]

| Study Name (First Author, Year) | Cancer Type | With a PARP Inhibitor | Without a PARP Inhibitor | Number of Patients |
|---|---|---|---|---|
| NCT01306032 (Kummar et al., 2016) [14] | Breast | Cyclophosphamide + Veliparib (60 mg once daily) | Cyclophosphamide | 39 |
| GOLD (Bang et al., 2017) [15] | Gastric | Paclitaxel + Olaparib (100 mg twice daily) | Paclitaxel + Placebo | 521 |
| NCI9012 (Hussain et al., 2018) [16] | Prostate | Abiraterone + Veliparib (300 mg twice daily) | Abiraterone | 153 |
| NCT01972217 (Clarke et al., 2018) [17] | Prostate | Abiraterone + Olaparib (300 mg twice daily) | Abiraterone + Placebo | 142 |
| PAOLA-1 (Ray-Coquard et al., 2019) [18] | Ovarian | Bevacizumab + Olaparib (300 mg twice daily) | Bevacizumab + Placebo | 802 |
| BROCADE3 (Diéras et al., 2020) [19] | Breast | Carboplatin + Paclitaxel + Veliparib (120 mg twice daily) | Carboplatin + Paclitaxel + Placebo | 507 |
| PROpel (Thiery-Vuillemin et al., 2022) [20] | Prostate | Abiraterone + Olaparib (300 mg twice daily) | Abiraterone + Placebo | 794 |
| Number of Cardiac Adverse Events with PARP Inhibitors | ROR (95% CI) | |
|---|---|---|
| Total | 19 | 0.585 (0.372–0.919) |
| Chemotherapy/bevacizumab | 11 | 0.352 (0.194–0.637) |
| Immune checkpoint inhibitors | 2 | 0.606 (0.151–2.432) |
| Antiandrogens | 6 | 3.496 (1.539–7.942) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.-Y.; Seo, Y.-E.; Kwon, J.-H.; Kim, J.H.; Kim, M.G. Cardioprotective Effects of PARP Inhibitors: A Re-Analysis of a Meta-Analysis and a Real-Word Data Analysis Using the FAERS Database. J. Clin. Med. 2024, 13, 1218. https://doi.org/10.3390/jcm13051218
Han J-Y, Seo Y-E, Kwon J-H, Kim JH, Kim MG. Cardioprotective Effects of PARP Inhibitors: A Re-Analysis of a Meta-Analysis and a Real-Word Data Analysis Using the FAERS Database. Journal of Clinical Medicine. 2024; 13(5):1218. https://doi.org/10.3390/jcm13051218
Chicago/Turabian StyleHan, Ja-Young, Young-Eun Seo, Jae-Hee Kwon, Jae Hyun Kim, and Myeong Gyu Kim. 2024. "Cardioprotective Effects of PARP Inhibitors: A Re-Analysis of a Meta-Analysis and a Real-Word Data Analysis Using the FAERS Database" Journal of Clinical Medicine 13, no. 5: 1218. https://doi.org/10.3390/jcm13051218
APA StyleHan, J.-Y., Seo, Y.-E., Kwon, J.-H., Kim, J. H., & Kim, M. G. (2024). Cardioprotective Effects of PARP Inhibitors: A Re-Analysis of a Meta-Analysis and a Real-Word Data Analysis Using the FAERS Database. Journal of Clinical Medicine, 13(5), 1218. https://doi.org/10.3390/jcm13051218





