Whole-Body MRI Screening for Carriers of Germline TP53 Mutations—A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Registration and Search Strategy
2.2. Inclusion/Exclusion Criteria
2.2.1. Inclusion Criteria
- (a)
- Report on patients with LFS who underwent WB-MRI radiological oncological screening.
- (b)
- Report on cancers picked up on WB-MRI.
- (c)
- Report with a well-defined research methodology.
2.2.2. Exclusion Criteria
- (a)
- Patients did not have a diagnosis of LFS.
- (b)
- Imaging was performed by another radiological modality, other than WB-MRI.
- (c)
- Outcomes of interest were not reported.
- (d)
- The methodology was not clearly reported.
2.3. Identification of Studies and Outcomes of Interest
- I.
- Population: LFS patients
- II.
- Intervention: LFS patient undergoing WB-MRI for cancer screening
- III.
- Comparison: asymptomatic non-LFS patients undergoing WB-MRI
- IV.
- Outcome:
2.4. Study Selection, Data Extraction and Critical Appraisal
2.5. Statistical Analysis
2.6. Risk of Bias
3. Results
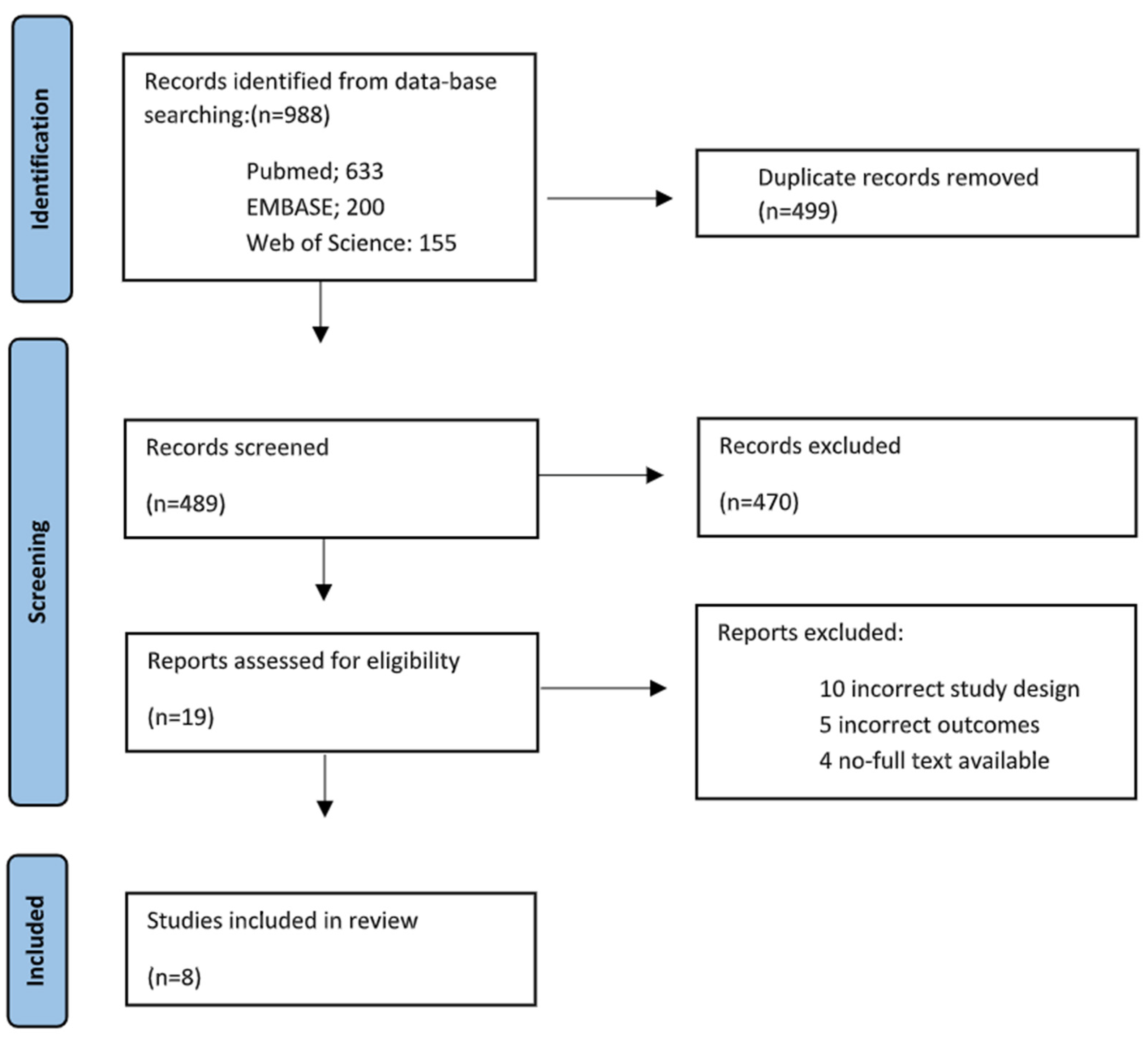
3.1. Search Results
3.2. Patient Characteristics
3.3. MRI Protocols/Acquisition Parameters
3.4. Incidence of New Cancer
3.5. Risk of Bias
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pasoglou, V.; Michoux, N.; Larbi, A.; Van Nieuwenhove, S.; Lecouvet, F. Whole Body MRI and oncology: Recent major advances. Br. J. Radiol. 2018, 91, 20170664. [Google Scholar] [CrossRef]
- Guha, T.; Malkin, D. Inherited TP53 Mutations and the Li-Fraumeni Syndrome. Cold Spring Harb. Perspect. Med. 2017, 7, a026187. [Google Scholar] [CrossRef]
- Varley, J.M. Germline TP53 mutations and Li-Fraumeni syndrome. Hum. Mutat. 2003, 21, 313–320, Erratum in Hum. Mutat. 2003, 21, 551. [Google Scholar] [CrossRef]
- Malkin, D.; Li, F.P.; Strong, L.C.; Fraumeni, J.F.; Nelson, C.E.; Kim, D.H.; Kassel, J.; Gryka, M.A.; Bischoff, F.Z.; Tainsky, M.A.; et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 1990, 250, 1233–1238. [Google Scholar] [CrossRef]
- Correa, H. Li-Fraumeni Syndrome. J. Pediatr. Genet. 2016, 5, 84–88. [Google Scholar] [CrossRef]
- Zhou, R.; Xu, A.; Gingold, J.; Strong, L.C.; Zhao, R.; Lee, D.F. Li-Fraumeni Syndrome Disease Model: A Platform to Develop Precision Cancer Therapy Targeting Oncogenic p53. Trends Pharmacol. Sci. 2017, 38, 908–927. [Google Scholar] [CrossRef]
- Bougeard, G.; Renaux-Petel, M.; Flaman, J.; Charbonnier, C.; Fermey, P.; Belotti, M.; Gauthier-Villars, M.; Stoppa-Lyonnet, D.; Consolino, E.; Brugières, L.; et al. Revisiting Li-Fraumeni syndrome from TP53 Carriers. J. Clin. Oncol. 2015, 33, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Mai, P.L.; Best, A.F.; Peters, J.A.; DeCastro, R.M.; Khincha, P.P.; Loud, J.T.; Bremer, R.C.; Rosenberg, P.S.; Savage, S.A. Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer 2016, 122, 3673–3681. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Shore, A.; Wasserman, J.D.; Stephens, D.; Kim, R.H.; Druker, H.; Gallinger, B.; Naumer, A.; Kohlmann, W.; Novokmet, A.; et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncol. 2016, 17, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.E.; Magoon, S.; Forghani, I.; Alessandrino, F.; D’Amato, G.; Jonczak, E.; Subhawong, T.K. Radiologic screening and surveillance in hereditary cancers. Eur. J. Radiol. Open 2022, 9, 100422. [Google Scholar] [CrossRef] [PubMed]
- Consul, N.; Amini, B.; Ibarra-Rovira, J.J.; Blair, K.J.; Moseley, T.W.; Taher, A.; Shah, K.B.; Elsayes, K.M. Li-Fraumeni Syndrome and Whole-Body MRI Screening: Screening Guidelines, Imaging Features, and Impact on Patient Management. AJR Am. J. Roentgenol. 2021, 216, 252–263. [Google Scholar] [CrossRef]
- Kratz, C.P.; Achatz, M.I.; Brugières, L.; Frebourg, T.; Garber, J.E.; Greer, M.C.; Hansford, J.R.; Janeway, K.A.; Kohlmann, W.K.; McGee, R.; et al. Cancer screening recommendations for individuals with Li-Fraumeni syndrome. Clin. Cancer Res. 2018, 23, e38–e45. [Google Scholar] [CrossRef]
- Omran, M.; Tham, E.; Brandberg, Y.; Ahlström, H.; Lundgren, C.; Paulsson-Karlsson, Y.; Kuchinskaya, E.; Silander, G.; Rosén, A.; Persson, F.; et al. Whole-Body MRI Surveillance-Baseline Findings in the Swedish Multicentre Hereditary TP53-Related Cancer Syndrome Study (SWEP53). Cancers 2022, 14, 380. [Google Scholar] [CrossRef]
- Bojadzieva, J.; Amini, B.; Day, S.F.; Jackson, T.L.; Thomas, P.S.; Willis, B.J.; Throckmorton, W.R.; Daw, N.C.; Bevers, T.B.; Strong, L.C. Whole body magnetic resonance imaging (WB-MRI) and brain MRI baseline surveillance in TP53 germline mutation carriers: Experience from the Li-Fraumeni Syndrome Education and Early Detection (LEAD) clinic. Fam. Cancer 2018, 17, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, L.; Nordberg, G.F.; Nurchi, V.M.; Aaseth, J.O. Gadolinium in Medical Imaging-Usefulness, Toxic Reactions and Possible Countermeasures-A Review. Biomolecules. 2022, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, A.D.; Espenschied, C.R.; Culver, J.O.; Weitzel, J.N. Tumor protein p53 (TP53) testing and Li-Fraumeni syndrome: Current status of clinical applications and future directions. Mol. Diagn. Ther. 2013, 17, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Frankenthal, I.A.; Alves, M.C.; Tak, C.; Achatz, M.I. Cancer surveillance for patients with Li-Fraumeni Syndrome in Brazil: A cost-effectiveness analysis. Lancet Reg. Health Am. 2022, 12, 100265. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.T.; Vlatkovic, N. p53: A molecular marker for the detection of cancer. Expert Opin. Med. Diagn. 2008, 2, 1013–1024. [Google Scholar] [CrossRef]
- Kumamoto, T.; Yamazaki, F.; Nakano, Y.; Tamura, C.; Tashiro, S.; Hattori, H.; Nakagawara, A.; Tsunematsu, Y. Medical guidelines for Li-Fraumeni syndrome 2019, version 1.1. Int. J. Clin. Oncol. 2021, 26, 2161–2178, Erratum in Int. J. Clin. Oncol. 2022, 27, 262–263. [Google Scholar] [CrossRef]
- Bancroft, E.K.; Saya, S.; Brown, E.; Thomas, S.; Taylor, N.; Rothwell, J.; Pope, J.; Chamberlain, A.; Page, E.; Benafif, S.; et al. Psychosocial effects of whole-body MRI screening in adult high-risk pathogenic TP53 mutation carriers: A case-controlled study (SIGNIFY). J. Med. Genet. 2020, 57, 226–236. [Google Scholar] [CrossRef]
- Anupindi, S.A.; Bedoya, M.A.; Lindell, R.B.; Rambhatla, S.J.; Zelley, K.; Nichols, K.E.; Chauvin, N.A. Diagnostic Performance of Whole-Body MRI as a Tool for Cancer Screening in Children With Genetic Cancer-Predisposing Conditions. AJR Am. J. Roentgenol. 2015, 205, 400–408. [Google Scholar] [CrossRef]
- Ballinger, M.L.; Ferris, N.J.; Moodie, K.; Mitchell, G.; Shanley, S.; James, P.A.; Thomas, D.M. Surveillance in Germline TP53 Mutation Carriers Utilizing Whole-Body Magnetic Resonance Imaging. JAMA Oncol. 2017, 3, 1735–1736. [Google Scholar] [CrossRef]
- Schmidt, G.; Dinter, D.; Reiser, M.F.; Schoenberg, S.O. The uses and limitations of whole-body magnetic resonance imaging. Dtsch. Arztebl. Int. 2010, 107, 383–389. [Google Scholar]
- Goehde, S.C.; Hunold, P.; Vogt, F.M.; Ajaj, W.; Goyen, M.; Herborn, C.U.; Forsting, M.; Debatin, J.F.; Ruehm, S.G. Full-body cardiovascular and tumor MRI for early detection of disease: Feasibility and initial experience in 298 subjects. AJR 2005, 184, 598–611. [Google Scholar] [CrossRef]
- Kramer, H.; Michaely, K.; Nikolaou, K.; Reiser, M.F.; Schoenberg, S.O. State of the art cardiovascular imaging with parallel imaging techniques on a whole body MR scanner: Experience in more than 200 individuals. Eur. Radiol. 2006, 41, 141–147. [Google Scholar]
- Paixão, D.; Guimarães, M.D.; de Andrade, K.C.; Nóbrega, A.F.; Chojniak, R.; Achatz, M.I. Whole-body magnetic resonance imaging of Li-Fraumeni syndrome patients: Observations from a two rounds screening of Brazilian patients. Cancer Imaging 2018, 18, 27. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.C. The well-built clinical question: The key tofinding the best evidence efficiently. World Med. J. 1999, 98, 25–28. [Google Scholar]
- Covidence Systematic Review Software, version: 2.0; Veritas Health Innovation: Melbourne, Australia, 2023.
- Barker, T.H.; Migliavaca, C.B.; Stein, C.; Colpani, V.; Falavigna, M.; Aromataris, E.; Munn, Z. Conducting proportional meta-analysis in different types of systematic reviews: A guide for synthesisers of evidence. BMC Med. Res. Methodol. 2021, 21, 189. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Stata Statistical Software; Release 17; StataCorp LLC: College Station, TX, USA, 2021. [Google Scholar]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, J.; Robertson, J.; Peterson, V.; Welch, V. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analysis. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 23 November 2023).
- Saya, S.; Killick, E.; Thomas, S.; Taylor, N.; Bancroft, E.K.; Rothwell, J.; Benafif, S.; Dias, A.; Mikropoulos, C.; Pope, J.; et al. Baseline results from the UK SIGNIFY study: A whole-body MRI screening study in TP53 mutation carriers and matched controls. Fam. Cancer 2017, 16, 433–440. [Google Scholar] [CrossRef]
- Kagami, L.A.T.; Du, Y.K.; Fernandes, C.J.; Le, A.N.; Good, M.; Duvall, M.M.; Baldino, S.E.; Powers, J.; Zelley, K.; States, L.J.; et al. Rates of Intervention and Cancer Detection on Initial versus Subsequent Whole-body MRI Screening in Li-Fraumeni Syndrome. Cancer Prev. Res. 2023, 16, 507–512. [Google Scholar] [CrossRef]
- Mai, P.L.; Khincha, P.P.; Loud, J.T.; DeCastro, R.M.; Bremer, R.C.; Peters, J.A.; Liu, C.Y.; Bluemke, D.A.; Malayeri, A.A.; Savage, S.A. Prevalence of Cancer at Baseline Screening in the National Cancer Institute Li-Fraumeni Syndrome Cohort. JAMA Oncol. 2017, 3, 1640–1645. [Google Scholar] [CrossRef]
- O’Neill, A.F.; Voss, S.D.; Jagannathan, J.P.; Kamihara, J.; Nibecker, C.; Itriago-Araujo, E.; Masciari, S.; Parker, E.; Barreto, M.; London, W.B.; et al. Screening with whole-body magnetic resonance imaging in pediatric subjects with Li-Fraumeni syndrome: A single institution pilot study. Pediatr. Blood Cancer 2018, 65, e26822. [Google Scholar] [CrossRef]
- Evans, D.G.; Woodward, E.R.; Bajalica-Lagercrantz, S.; Oliveira, C.; Frebourg, T. Germline TP53 Testing in Breast Cancers: Why, When and How? Cancers 2020, 12, 3762. [Google Scholar] [CrossRef]
- Weitzel, J.N.; Chao, E.C.; Nehoray, B.; Van Tongeren, L.R.; LaDuca, H.; Blazer, K.R.; Slavin, T.; Pesaran, T.; Rybak, C.; Solomon, I.; et al. Somatic TP53 variants frequently confound germ-line testing results. Genet. Med. 2018, 20, 809–816. [Google Scholar] [CrossRef]
- Frebourg, T.; Bajalica Lagercrantz, S.; Oliveira, C.; Magenheim, R.; Evans, D.G. Guidelines for the Li–Fraumeni and heritable TP53-related cancer syndromes. Eur. J. Hum. Genet. 2020, 28, 1379–1386. [Google Scholar] [CrossRef]
- Ballinger, M.L.; Best, A.; Mai, P.L.; Khincha, P.P.; Loud, J.T.; Peters, J.A.; Achatz, M.I.; Chojniak, R.; Balieiro da Costa, A.; Santiago, K.M.; et al. Baseline Surveillance in Li-Fraumeni Syndrome Using Whole-Body Magnetic Resonance Imaging: A Meta-analysis. JAMA Oncol. 2017, 3, 1634–1639, Erratum in JAMA Oncol. 2018, 4, 590. [Google Scholar] [CrossRef]
- Petralia, G.; Zugni, F.; Summers, P.E.; Colombo, A.; Pricolo, P.; Grazioli, L.; Colagrande, S.; Giovagnoni, A.; Padhani, A.R.; on behalf of the Italian Working Group on Magnetic Resonance. Whole-body magnetic resonance imaging (WB-MRI) for cancer screening: Recommendations for use. Radiol. Med. 2021, 126, 1434–1450. [Google Scholar] [CrossRef]
- Benusiglio, P.R.; Brugières, L.; Caron, O. Whole-body MRI screening in children with Li-Fraumeni and other cancer predisposition syndromes. AJR 2016, 206, W52. [Google Scholar] [CrossRef]
- Tak, C.R.; Biltaji, E.; Kohlmann, W.; Maese, L.; Hainaut, P.; Villani, A.; Malkin, D.; Sherwin, C.M.T.; Brixner, D.I.; Schiffman, J.D. Cost-effectiveness of early cancer surveillance for patients with Li-Fraumeni syndrome. Pediatr. Blood Cancer 2019, 66, e27629. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Study Population | Country | Journal | Study Method | No. Patients | Age (Mean ± SD, Range) | Sex (M:F) | Previous Cancer | Primary Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Anupindi [20] | 2015 | Children diagnosed with either Li-Fraumeni syndrome (LFS), hereditary paraganglioma-pheochromocytoma syndrome, or rhabdoid tumour syndrome | USA | American Journal of Roentgenology | Retrospective cohort | 24 | 10.7 (± 4.6, 2.1–18.2) | 6:18 | 7/24 (29%) | Identification of lesions suspicious for malignancy in asymptomatic TP53 pv carriers |
| Saya [33] | 2017 | TP53 pathogenic variant carriers, and age- matched (±5 years) and sex-matched population controls. | UK | Familial Cancer | Prospective cohort | 88 (44 carrier and 44 control) | Carrier 38.1 (±11.3, 19–58) Control 39.4 (±10.7, 22–59) | Carrier 27:17, Control 27:17 | 18/44 (41%) | Identification of lesions suspicious for malignancy in asymptomatic TP53 pv carriers |
| Bojadzieva [13] | 2017 | Adults and children with Li-Fraumeni Syndrome | USA | Familial Cancer | Prospective cohort | 63 (49 adults, 14 children). 53 underwent baseline WB-MRI, 35 underwent brain MRI only. | N/a | 18:45 | 43 (68%) | Identification of lesions suspicious for malignancy in asymptomatic TP53 pv carriers |
| Mai [7] | 2017 | Patients with Li-Fraumeni syndrome aged 3 years or older at time of baseline screening and who had not received active cancer therapy at least 6 months prior to screening. | USA | JAMA Oncology | Prospective cohort | 116 | 38.5 (±17.9, 3–65) | 40:17 | 71/116 (61.2%) | Identification of lesions suspicious for malignancy in asymptomatic TP53 pv carriers |
| O’Neill [36] | 2017 | Paediatric patients with TP53 germline mutation, prior cancer patients in stable remission for at least 6 months post treatment | USA | Paediatric blood cancer | Prospective cohort | 22 | 9.5 (±4.0, 1–15) | 10:12 | 5/22 (33%) | Identification of lesions suspicious for malignancy in asymptomatic TP53 pv carriers |
| Paixiao [25] | 2018 | Patients with Li-Fraumeni syndrome (TP53 germline mutation) with no current disease | Brazil | Cancer Imaging | Prospective cohort | 59 | 38 ± 11.1 (2–71) | 24:35 | 27/59 (45%) | Identification of lesions suspicious for malignancy in asymptomatic TP53 pv carriers |
| Omran [12] | 2019 | Adult patients recruited by SWEP53 study, with verified likely pathogenic (class 4) or pathogenic (class 5) TP 53 variants | Sweden | Cancer | Prospective cohort | 61 | 40 (±7.9, 18–74) | 21:39 | 32/61 (52%) | Identification of lesions suspicious for malignancy in asymptomatic TP53 pv carriers |
| Kagami [34] | 2023 | Adults and children with Li-Fraumeni Syndrome | USA | American Association for Cancer Research | Retrospective cohort | 118 (68 adult, 50 children) | N/a | 41:18 | 64/118 (54%)—total 50/68 (74%)—adult 14/50 (28%)—paediatric | Radiological findings leading to follow-up imaging or intervention in asymptomatic TP53 pv carriers |
| Author | Scanning Time (minutes) | Identical WB-MRI Protocol in All Subjects (Y/N) | MRI Interpreter (Number of Rads/Level/Experience) | Phase | Model | Field Strength | FOV | TR/TE (ms) | ST (mm) | Matrix | Contrast |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anupindi [20] | 39–150 min (range) | No | Two board certified radiologists with at least 5 year’s paediatric full body MRI experience | coronal STIR, coronal T1-weighted, axial STIR, and axial T2-weighted fat-suppressed sequences (protocol changed during study) | Avanto or Symphony, Siemens Healthcare (n = 30/50 scans) Skyra, Verio, or Trio, Siemens Health-care) (20/50 scans) | 1.5 T (30/50 scans), 3T (20/50 Scans) | Variable based on patient size | 1.5 T Coronal STIR 2800–6371/33–70, Coronal T1-weighted 100–1610/10–12, Axial STIR 4000–9830/32–65, Axial T2-weighted fast suppressed 2500–13177/71–105, Coronal HASTE 900/82–86, Axial HASTE 900/79–85, Sagittal HASTE 900/83–87 3 T Coronal STIR 2900–5050/30–41, Coronal T1-weighted 480–820/9–10, Axial STIR 2500–9860/27–38, Axial T2 weighted fast suppressed 4240–17264/77–113, Coronal HASTE 1000/83–87, Axial HASTE 1230/87, Sagittal HASTE 1000/87 | 1.5 T Coronal STIR 4–6.6, Coronal T1-weighted 4–6.6, Axial STIR 4–8, Axial T2-weighted fast suppressed 4–8, Coronal HASTE 6–7, Axial HASTE 8–10, Sagittal HASTE 7–10 3 T Coronal STIR 4–5.5, Coronal T1-weighted 4–5.5, Axial STIR 4–7, Axial T2-weighted fast suppressed 4–7, Coronal HASTE 8–20, Axial HASTE 8, Sagittal HASTE 8.8 | 1.5 T Coronal STIR 256 × 256, Coronal T1-weighted 320 × 256, Axial STIR 256 × 256, Axial T2-weighted fast suppressed 320 × 320, Coronal HASTE 256 × 243, Axial HASTE 256 × 243, Sagittal HASTE 256 × 243 3 T Coronal STIR 256 × 256, Coronal T1-weighted 384 × 288, Axial STIR 256 × 256, Axial T2-weighted fast suppressed 320 × 320, Coronal HASTE 256 × 256, Axial HASTE 256 × 256, Sagittal HASTE 256 × 192 | No |
| Saya [33] | / | Yes | Two independent radiologist with at least 5 years’ experience | Axial T1-weighted gradient echo, axial fat-suppressed T2-weighted HASTE, axial DWIBS whole body, coronal T1-weighted VIBE DIXON | 1.5 T MRI machine (Siemens, Erlangen, Germany) | 1.5 T | 38–40 cm | Axial T1-weighted gradient echo 247/4.36, axial fat-suppressed T2-weighted HASTE 1000/84, axial DWIBS whole body 8600/72, coronal T1-weighted VIBE DIXON 6.97/2.39 | Axial T1-weighted gradient echo 8, axial fat-suppressed T2-weighted HASTE 8, axial DWIBS whole-body 8, coronal T1-weighted VIBE DIXON 5 | Axial T1 weighted gradient echo 182 × 320, axial fat-suppressed T2-weighted HASTE 208 × 256, axial DWIBS whole-body 128 × 128, coronal T1-weighted VIBE DIXON 192 × 192 | No |
| Bojadzieva [13] | / | Yes | All scans reviewed by primary author, lesions reviewed by diagnostic radiologist | WB-MRI—Scout, DWI, T2 TIRM, T1FS Post VIBE, T1FS Post. Brain MRI—DWI, T2, FLAIR, T2, T1, T1 Post | WB-MRI—Siemens Aera 1.5T, brain MRI—GE SIgna HD 1.5T, Siemens Aera 1.5T | 1.5T | WB-MRI—([45 cm × 45 cm for Scout, DWI, T2 TIRM], [24 cm × 24 cm for T1FS post VIBE head, 44cm × 44 cm for T1FS post VIBE chest to pelvis, 38cm × 38 cm for T1FS post VIBE thighs to toes], [34cm × 34 cm for T1FS post]). Brain MRI—22 cm for all sequence except for 24 cm for T1 post sagittal view. | WB-MRI TR—1500, 8300, 5130 (H&N) 3000 (ch/ab) 5500 (pel-toes), 12, 4.09 (ch) 4.38 (ab) 4.51 (pel), 4.51, <500 for Scout, DWI, T2 TIRM, T1FS Post VIBE, T1FS Post, respectively. Brain MRI TR- 8000, 3600–4400, 10000, 675–825, 500–600, 500–600 for DWI, T2, FLAIR, T2, T1, T1 Post, respectively | WBMRI—6, 5, 6 (chest/ab) 5 (others), 5, 5, 5, 4 for Scout, DWI, T2 TIRM, T1FS Post VIBE, T1FS Post, respectively. Brain MRI—5 for all phases. | WBMRI—320 × 256 160 × 108 384 × 207 256 × 192 384 × 171 288 × 132 320 × 256 for Scout DWI T2 TIRM T1FS Post VIBE (ch, abd, pelv) T1FS Post, respectively. Brain MRI—128 × 128 320 × 224 256 × 192 256 × 192 256 × 192 256 × 192 256 × 192 256 × 192 for DWI, T2, FLAIR T2, T T1 Post, respectively. | Yes |
| Mai [35] | 45 min (mean) | No | / | Cor STIR, Cor 3D T1, Ax STIR | - | - | N/a | TR—3750, 6.36, 4990 for Cor STIR, Cor 3D T1, Ax STIR, respectively. TE—60, 4.77, 61 for Cor STIR, Cor 3D T1, Ax STIR, respectively | N/a | N/a | Yes |
| O’Neill [36] | 60–90 min (range) | Yes | Neuroradiology for brain, body radiologist for body, study radiologist independently reviewed all scans for accurate representation | N/a | - | 3T | N/a | N/a | N/a | N/a | No |
| Paixiao [25] | 25–35 min (range) | Yes | Experienced radiologists | Coronal T1 weighted, short TI inversion recovery, axial diffusion weighted | Signa Excite HT | 1.5T | N/a | N/a | N/a | N/a | No |
| Omran [12] | / | Yes | Two consultant radiologist | SSFSE/Haste Fat ST T2, DIXON T1, T2/EPI b50_400_800 | 1.5T Siemens | 1.5T | 400 × 275, 416/315, 420/338 | 1000/82, 165/60, 5600/60 | Five for all three phases | 256/123, 256/134, 192/156 | No |
| Kagami [34] | / | No | / | Adult—T2-weighted imaging with and without fat suppression using HASTE technique, whole-body diffusion-weighted imaging (DWI), and pre and post-T1 weighted imaging using DIXON technique. Children—short tau inversion recovery (STIR) or T2-weighted fat sup-pressed images and T1-weighted images. | 1.5T scanner (Aera, Sola, and Espree, Siemens Healthcare) for adults, WB-MRI examinations were per-formed on a 1.5-Tesla or a 3.0-Tesla scanner (Siemens Health) for children | 1.5T (adult), 1.5T or 3T (children) | N/a | N/a | N/a | N/a | No |
| Author | Patients with Subsequent Cancer on MRI | Location of Lesion (Multiple Lesions Found in Some Patients) | Critical Findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdomen | Pelvis | Chest | Head | Neck | LL | UL | Spine | Breast | Other | ||||
| No. | % | ||||||||||||
| Anupindi [20] | 1 | 4 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Sensitivity of WB-MRI—100% Specificity of WB-MRI—94% PPV of WB-MRI—25% NPV of WB-MRI—100% |
| Saya [33] | 6 | 13.6 | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 6/44 (13.6, 95% CI 5.2–27.4%) of the participants were diagnosed with cancer during the study. |
| Bojadzieva [13] | 6 | 9.5 | 3 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | Screening WB-WRI in 6/63 (9.5%) of patients supports the inclusion of WB-MRI and brain MRI in the clinical management of individuals with LFS. |
| Mai [35] | 5 | 4.3 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | Abnormal MRI findings requiring additional follow-up were identified in 32/116 WB-MRIs (27.5%). In total, 27/32 (84.4%) of the abnormal WB-MRIs required follow-up, including two site-specific biopsies, with results showing benign or normal findings. |
| O’Neill [36] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | In total, 89% patients returned for second examinations (95% CI—67–99%). Considered successful feasibility study as upper bound of 95% Confidence Interval of success is ≥90%. |
| Paixiao [25] | 3 | 5 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1st round of WB-MRI—positive rate 11.8%, recall rate 11.8%, invasive investigation rate 3.4%, cancer detection rate 3.4%, execution success 95%. 2nd round of WB-MRI—positive rate 6.7%, recall rate 6.7%, cancer detection rate 1.7%, success rate 100% |
| Omran [12] | 3 | 5 | 4 | 0 | 4 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | In total, 30 new lesions were identified by WB-MRI in 19 individuals (31%) requiring follow up; 9 of the 30 were malignant, in 3 patients. One was recurrence, one was new disease, and one was disseminated disease. All were asymptomatic |
| Kagami [34] | 12 | 10.2 | 5 | 2 | 1 | 2 | 0 | 2 | 0 | 0 | 1 | 0 | The most frequent intervention recommended after initial screening was short-term follow-up imaging (adults, 81%,pediatric, 60%), followed by immediate imaging (adults, 19%, paediatric, 40%). None of the adult cohort and one child in the paediatric cohort were recommended for invasive interventions after the initial screening, while three adults (3/68, 4.4%) and six children (6/50, 12%) were recommended for invasive interventions after subsequent screening. |
| Author | Cancer Location | Initial/Subsequent MRI | ||
|---|---|---|---|---|
| Omran [12] | 1—Pleura, cervical LN 2—Mediastinal LN, pleura, liver, intraabdominal LN 3—Liver, intra-abdominal LN | 1—Initial WBI MRI 2—Initial WB-MRI 3—Initial WB-MRI | ||
| Paixiao [25] | 1—Renal cell carcinoma (right and left kidney) 2—Grade 1 chondrosarcoma (left SI joint) 3—High-grade sarcoma with muscle differentiation (right humerus) | 1—Initial WBI MRI 2—Initial WB-MRI 3—Subsequent WB-MRI | ||
| O’Neill [36] | No new cancers identified | - | ||
| Mai [35] | 1—Lung adenocarcinoma 2—Lung adenocarcinoma 3—Intermediate grade sarcoma in left 4th rib 4—Low-grade spindle cell sarcoma on left chest skin biopsy 5—Brain cancer—astrocytoma frontal lobe | Not specified | ||
| Kagami [34] | Adults 1—RCC 2× primaries 2—Infiltrating ductal carcinoma 3—Lung adenocarcinoma 4—Endocervical adenocarcinoma 5—Leiomyosarcoma | Paediatric 1—Colorectal adenocarcinoma 2—Rhabdomyosarcoma 3—Atypical astrocytoma 4—Colonic adenocarcinoma 5—Pancreatic NET 6—Osteosarcoma 7—High-grade astrocytoma | Adults 1—Initial WB-MRI 2—Subsequent WB-MRI 3—Subsequent WB-MRI 4—Subsequent WB-MRI 5—Initial WB-MRI | Paediatric 1—Subsequent WB-MRI 2—Initial WB-MRI 3—Subsequent WB-MRI 4—Subsequent WB-MRI 5—Subsequent WB-MRI 6—Subsequent WB-MRI 7—Initial WB-MRI |
| Bojadzieva [13] | 1—Recurrent soft tissue sarcoma, new primary abdominal soft tissue sarcoma 2—Sarcoma metastasis 3—Papillary thyroid cancer 4—Gastric cancer 5—Bilateral thyroid cysts not significant on WB-MRI, latera shown to be thyroid cancer 6—Shoulder lesion, excision showed liposarcoma | Not specified | ||
| Saya [33] | 1—Low-grade astrocytoma 2—Low-grade myxosarcoma in abdominal wall 3—Renal cell carcinoma, leiomyosarcoma 4—Chondroblastic osteosarcoma 5—Pericardial cyst on WB-MRI—diagnosed as mediastinal sarcoma 6—B cell acute lymphoma | 1—Initial WB-MRI 2—Initial WB-MRI 3—Initial WB-MRI 4—Initial WB-MRI 5—Initial WB-MRI 6—Initial WB-MRI | ||
| Anupindi [20] | 1—Papillary thyroid carcinoma | 1—Initial WB-MRI | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temperley, H.C.; O’Sullivan, N.J.; Mac Curtain, B.M.; Qian, W.; Temperley, T.S.; Murray, A.; Corr, A.; Brennan, I.; Gallagher, D.; Meaney, J.F.; et al. Whole-Body MRI Screening for Carriers of Germline TP53 Mutations—A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 1223. https://doi.org/10.3390/jcm13051223
Temperley HC, O’Sullivan NJ, Mac Curtain BM, Qian W, Temperley TS, Murray A, Corr A, Brennan I, Gallagher D, Meaney JF, et al. Whole-Body MRI Screening for Carriers of Germline TP53 Mutations—A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(5):1223. https://doi.org/10.3390/jcm13051223
Chicago/Turabian StyleTemperley, Hugo C., Niall J. O’Sullivan, Benjamin M. Mac Curtain, Wanyang Qian, Tatiana S. Temperley, Alannah Murray, Alison Corr, Ian Brennan, David Gallagher, James F. Meaney, and et al. 2024. "Whole-Body MRI Screening for Carriers of Germline TP53 Mutations—A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 5: 1223. https://doi.org/10.3390/jcm13051223





