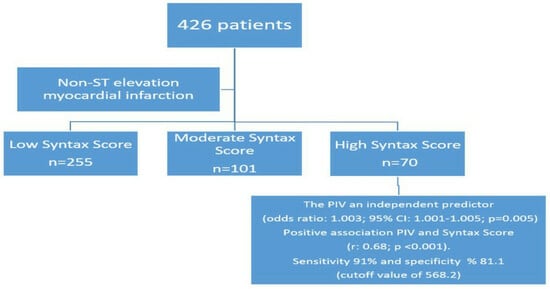
The Role of Pan-Immune-Inflammation Value in Determining the Severity of Coronary Artery Disease in NSTEMI Patients
Abstract
1. Introduction
2. Patients and Methods
3. Laboratory Analysis
4. Transthoracic Echocardiography
5. Evaluation of the Coronary Angiography and SYNTAX Score Calculation
6. Statistical Analysis
7. Results
8. Discussion
9. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baptista, E.A.; Queiroz, B.L. Spatial analysis of cardiovascular mortality and associated factors around the world. BMC Public Health 2022, 22, 1556. [Google Scholar] [CrossRef]
- Hackam, D.G.; Anand, S.S. Emerging risk factors for atherosclerotic vascular disease: A critical review of the evidence. JAMA 2003, 290, 932–940. [Google Scholar] [CrossRef]
- Wilson, P.W. Progressing from risk factors to omics. Circ. Cardiovasc. Genet. 2008, 1, 141–146. [Google Scholar] [CrossRef]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Hedayati, T.; Yadav, N.; Khanagavi, J. Non-ST-Segment Acute Coronary Syndromes. Cardiol. Clin. 2018, 36, 37–52. [Google Scholar] [CrossRef]
- Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef]
- Landesberg, G.; Beattie, W.S.; Mosseri, M.; Jaffe, A.S.; Alpert, J.S. Perioperative myocardial infarction. Circulation 2009, 119, 2936–2944. [Google Scholar] [CrossRef] [PubMed]
- Falk, E.; Shah, P.K.; Fuster, V. Coronary plaque disruption. Circulation 1995, 92, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.; Coetzee, A.R.; Lochner, A. The Pathophysiology of Myocardial Ischemia and Perioperative Myocardial Infarction. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2501–2512. [Google Scholar] [CrossRef]
- Srikanth, S.; Ambrose, J.A. Pathophysiology of coronary thrombus formation and adverse consequences of thrombus during PCI. Curr. Cardiol. Rev. 2012, 8, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.P.; Osakada, G.; Kemper, W.S.; Ross, J., Jr. Cyclical coronary flow reductions in conscious dogs equipped with ameroid constrictors to produce severe coronary narrowing. Basic Res. Cardiol. 1985, 80, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Wei, C.; Liu, Y.; Sun, Q.; Tian, Y.; Wang, X.; Liu, J.; Zhang, Y.; Sun, L. The Prognostic Value of Hematologic Inflammatory Markers in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221146183. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Tajlil, A.; Separham, A.; Sohrabi, B.; Pourafkari, L.; Roshanravan, N.; Aslanabadi, N.; Najjarian, F.; Mashayekhi, S.; Ghaffari, S. Association of neutrophil to lymphocyte ratio (NLR) with angiographic SYNTAX score in patients with non-ST-Segment elevation acute coronary syndrome (NSTE-ACS). J. Cardiovasc. Thorac. Res. 2021, 13, 216–221. [Google Scholar] [CrossRef]
- Li, H.; Meng, S.; Chen, W.; Lei, X.; Kong, X.; Zhu, H. Comparison of Different Systemic Inflammatory Markers in Predicting Clinical Outcomes with Syntax Score in Patients with Non-ST Segment Elevation Myocardial Infarction: A Retrospective Study. Int. J. Gen. Med. 2023, 16, 2595–2607. [Google Scholar] [CrossRef]
- Lappé, J.M.; Horne, B.D.; Shah, S.H.; May, H.T.; Muhlestein, J.B.; Lappé, D.L.; Kfoury, A.G.; Carlquist, J.F.; Budge, D.; Alharethi, R.; et al. Red cell distribution width, C-reactive protein, the complete blood count, and mortality in patients with coronary disease and a normal comparison population. Clin. Chim. Acta 2011, 412, 2094–2099. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, L.P.; Xie, S.Y.; Huang, H.Y.; Chen, X.Y.; Jiang, T.C.; Guo, L.; Lin, H.X. Pan-Immune-Inflammation Value: A New Prognostic Index in Operative Breast Cancer. Front. Oncol. 2022, 12, 830138. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Nakagawa, S.; Toihata, T.; Harada, K.; Iwatsuki, M.; Hayashi, H.; Miyamoto, Y.; Yoshida, N.; Baba, H. Pan-immune-inflammation Value and Prognosis in Patients with Esophageal Cancer. Ann. Surg. Open 2021, 3, e113. [Google Scholar] [CrossRef]
- Murat, B.; Murat, S.; Ozgeyik, M.; Bilgin, M. Comparison of pan-immune-inflammation value with other inflammation markers of long-term survival after ST-segment elevation myocardial infarction. Eur. J. Clin. Investig. 2023, 53, e13872. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Liu, L.; Cao, S.; Jin, T.; Chen, L.; Wu, G.; Zong, G. Association of Systemic Inflammatory Response Index and Pan-Immune-Inflammation-Value with Long-Term Adverse Cardiovascular Events in ST-Segment Elevation Myocardial Infarction Patients after Primary Percutaneous Coronary Intervention. J. Inflamm. Res. 2023, 16, 3437–3454. [Google Scholar] [CrossRef]
- Kern, M.J.; Seto, A.H. Can Automating the SYNTAX Score Move Practice Beyond the Angiogram Alone? JACC Cardiovasc. Interv. 2022, 15, 2487–2489. [Google Scholar] [CrossRef]
- SYNTAX Working Group. SYNTAX Score Calculator. Available online: http://www.SYNTAXscore.com (accessed on 15 January 2011).
- Libby, P. Changing concepts of atherogenesis. J. Intern. Med. 2000, 247, 349–358. [Google Scholar] [CrossRef]
- Liao, J.K. Beyond lipid lowering: The role of statins in vascular protection. Int. J. Cardiol. 2002, 86, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Uchida, T.; Yaguchi, I.; Sakai, Y.; Takayanagi, K.; Morooka, S. Stent-induced expression and activation of the leukocyte integrin Mac-1 is associated with neointimal thickening and restenosis. Circulation 2003, 107, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Zakynthinos, E.; Pappa, N. Inflammatory biomarkers in coronary artery disease. J. Cardiol. 2009, 53, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Blake, G.J.; Ridker, P.M. Inflammatory bio-markers and cardiovascular risk prediction. J. Intern. Med. 2002, 252, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003, 8, 223–246. [Google Scholar]
- Qiao, J.H.; Tripathi, J.; Mishra, N.K.; Cai, Y.; Tripathi, S.; Wang, X.P.; Imes, S.; Fishbein, M.C.; Clinton, S.K.; Libby, P.; et al. Role of macrophage colony-stimulating factor in atherosclerosis: Studies of osteopetrotic mice. Am. J. Pathol. 1997, 150, 1687–1699. [Google Scholar]
- Stary, H.C. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis 1989, 9 (Suppl. 1), I19–I32. [Google Scholar] [PubMed]
- Steinberg, D.; Parthasarathy, S.; Carew, T.E.; Khoo, J.C.; Witztum, J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989, 320, 915–924. [Google Scholar] [CrossRef]
- Ylä-Herttuala, S.; Palinski, W.; Rosenfeld, M.E.; Parthasarathy, S.; Carew, T.E.; Butler, S.; Witztum, J.L.; Steinberg, D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J. Clin. Investig. 1989, 84, 1086–1095. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Libby, P. Molecular bases of the acute coronary syndromes. Circulation 1995, 91, 2844–2850. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.R.; Bernardi, V.H.; López-Cuéllar, J.; Murcia, A.M.; Palacios, I.F.; Gold, H.K.; Mehran, R.; Sharma, S.K.; Nemerson, Y.; Fuster, V.; et al. Macrophages, smooth muscle cells, and tissue factor in unstable angina. Implications for cell-mediated thrombogenicity in acute coronary syndromes. Circulation 1996, 94, 3090–3097. [Google Scholar] [CrossRef] [PubMed]
- Farooq, V.; Girasis, C.; Magro, M.; Onuma, Y.; Morel, M.A.; Heo, J.H.; Garcia-Garcia, H.; Kappetein, A.P.; van den Brand, M.; Holmes, D.R.; et al. The CABG SYNTAX Score—An angiographic tool to grade the complexity of coronary disease following coronary artery bypass graft surgery: From the SYNTAX Left Main Angiographic (SYNTAX-LE MANS) substudy. EuroIntervention 2013, 8, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Aktürk, E.; Aşkın, L.; Taşolar, H.; Türkmen, S.; Kaya, H. Comparison of the Predictive Roles of Risk Scores of In-Hospital Major Adverse Cardiovascular Events in Patients with Non-ST Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Med. Princ. Pract. 2018, 27, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Rigatelli, G.; Picariello, C.; dell’Avvocata, F.; Marcantoni, L.; Pastore, G.; Carraro, M.; Nanjundappa, A.; Faggian, G.; Roncon, L. Correlation and prognostic role of neutrophil to lymphocyte ratio and SYNTAX score in patients with acute myocardial infarction treated with percutaneous coronary intervention: A six-year experience. Cardiovasc. Revascularizat. Med. 2017, 18, 565–571. [Google Scholar] [CrossRef]
- Gur, D.O.; Efe, M.M.; Alpsoy, S.; Akyüz, A.; Uslu, N.; Çelikkol, A.; Gur, O. Systemic Immune-Inflammatory Index as a Determinant of Atherosclerotic Burden and High-Risk Patients with Acute Coronary Syndromes. Arq. Bras. Cardiol. 2022, 119, 382–390. [Google Scholar] [CrossRef]
- Şen, F.; Kurtul, A.; Bekler, Ö. Pan-Immune-Inflammation Value Is Independently Correlated to Impaired Coronary Flow after Primary Percutaneous Coronary Intervention in Patients with ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2023, 211, 153–159. [Google Scholar] [CrossRef]
- Bayramoğlu, A.; Hidayet, Ş. Association between pan-immune-inflammation value and no-reflow in patients with ST elevation myocardial infarction undergoing percutaneous coronary intervention. Scand. J. Clin. Lab. Investig. 2023, 83, 384–389. [Google Scholar] [CrossRef]
- Cetinkaya, Z.; Kelesoglu, S. The Role of Pan-Immune-Inflammation Value in Predicting Contrast-Induced Nephropathy Development in Patients Undergoing Percutaneous Coronary Intervention Due to NSTEMI. Angiology 2023, 33197231211107. [Google Scholar] [CrossRef]
- Núñez, J.; Sanchis, J.; Bodí, V.; Núñez, E.; Mainar, L.; Heatta, A.M.; Husser, O.; Miñana, G.; Merlos, P.; Darmofal, H.; et al. Relationship between low lymphocyte count and major cardiac events in patients with acute chest pain, a non-diagnostic electrocardiogram and normal troponin levels. Atherosclerosis 2009, 206, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Candemir, M.; Kiziltunç, E.; Nurkoç, S.; Şahinarslan, A. Relationship between Systemic Immune-Inflammation Index (SII) and the Severity of Stable Coronary Artery Disease. Angiology 2021, 72, 575–581. [Google Scholar] [CrossRef]
- Balta, S.; Celik, T.; Mikhailidis, D.P.; Ozturk, C.; Demirkol, S.; Aparci, M.; Iyisoy, A. The Relation between Atherosclerosis and the Neutrophil-Lymphocyte Ratio. Clin. Appl. Thromb. Hemost. 2016, 22, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Li, N. Platelet-lymphocyte cross-talk. J. Leukoc. Biol. 2008, 83, 1069–1078. [Google Scholar] [CrossRef]
- Demirtas, S.; Karahan, O.; Yazici, S.; Guclu, O.; Caliskan, A.; Yavuz, C.; Kucuker, A.; Mavitas, B. The relationship between complete blood count parameters and Fontaine’s Stages in patients with peripheral arterial disease. Vascular 2014, 22, 427–431. [Google Scholar] [CrossRef]
- Duran, M.; Gunebakmaz, O.; Uysal, O.K.; Ocak, A.; Yilmaz, Y.; Arinc, H.; Eryol, N.K.; Ergin, A.; Kaya, M.G. Relation between mean platelet volume and coronary collateral vessels in patients with acute coronary syndromes. J. Cardiol. 2013, 61, 295–298. [Google Scholar] [CrossRef][Green Version]
- Pasalic, L.; Wang, S.S.; Chen, V.M. Platelets as Biomarkers of Coronary Artery Disease. Semin. Thromb. Hemost. 2016, 42, 223–233. [Google Scholar] [CrossRef]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in inflammation and atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef]
- Kelesoglu, S.; Yilmaz, Y.; Elcık, D.; Kalay, N. Systemic immune inflammation index: A novel predictor for coronary collateral circulation. Perfusion 2022, 37, 605–612. [Google Scholar] [CrossRef]
- Kelesoglu, S.; Yilmaz, Y.; Elcık, D.; Çetınkaya, Z.; Inanc, M.T.; Dogan, A.; Oguzhan, A.; Kalay, N. Systemic Immune Inflammation Index: A Novel Predictor of Contrast-Induced Nephropathy in Patients with Non-ST Segment Elevation Myocardial Infarction. Angiology 2021, 72, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Kelesoglu, S.; Yilmaz, Y.; Elcık, D.; Bireciklioglu, F.; Ozdemir, F.; Balcı, F.; Tuncay, A.; Kalay, N. Increased Serum Systemic Immune-Inflammation Index Is Independently Associated with Severity of Carotid Artery Stenosis. Angiology 2023, 74, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Kelesoglu, S.; Elcik, D.; Ozmen, R.; Kalay, N. Predictive Values of Systemic Immune-Inflammation Index in New-Onset Atrial Fibrillation following Coronary Artery Bypass Grafting. Braz. J. Cardiovasc. Surg. 2023, 38, 96–103. [Google Scholar] [CrossRef]
- Hayıroğlu, M.İ.; Çınar, T.; Çinier, G.; Pay, L.; Yumurtaş, A.Ç.; Tezen, O.; Eren, S.; Kolak, Z.; Çetin, T.; Çiçek, V.; et al. Evaluating systemic immune-inflammation index in patients with implantable cardioverter defibrillator for heart failure with reduced ejection fraction. Pacing Clin. Electrophysiol. 2022, 45, 188–195. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, T.; Chen, L.; Jin, T.; Sheng, Y.; Wu, G.; Zong, G. Systemic immune-inflammation index predicts the severity of coronary stenosis in patients with coronary heart disease. Coron. Artery Dis. 2021, 32, 715–720. [Google Scholar] [CrossRef]
- Liao, L.S.; Bai, Y.P. The dynamics of monocytes in the process of collateralization. Aging Med. (Milton) 2019, 2, 50–55. [Google Scholar] [CrossRef] [PubMed]
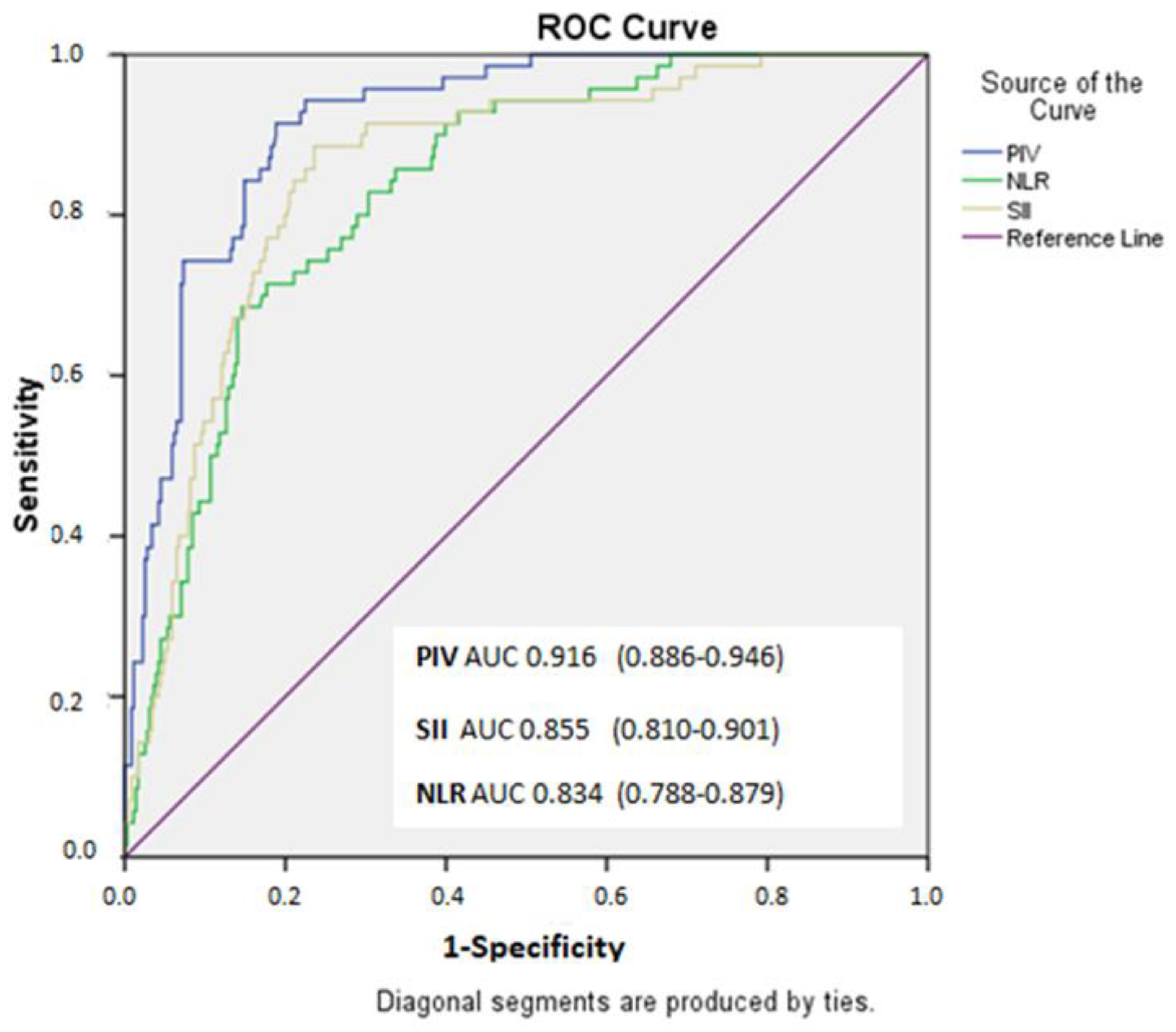
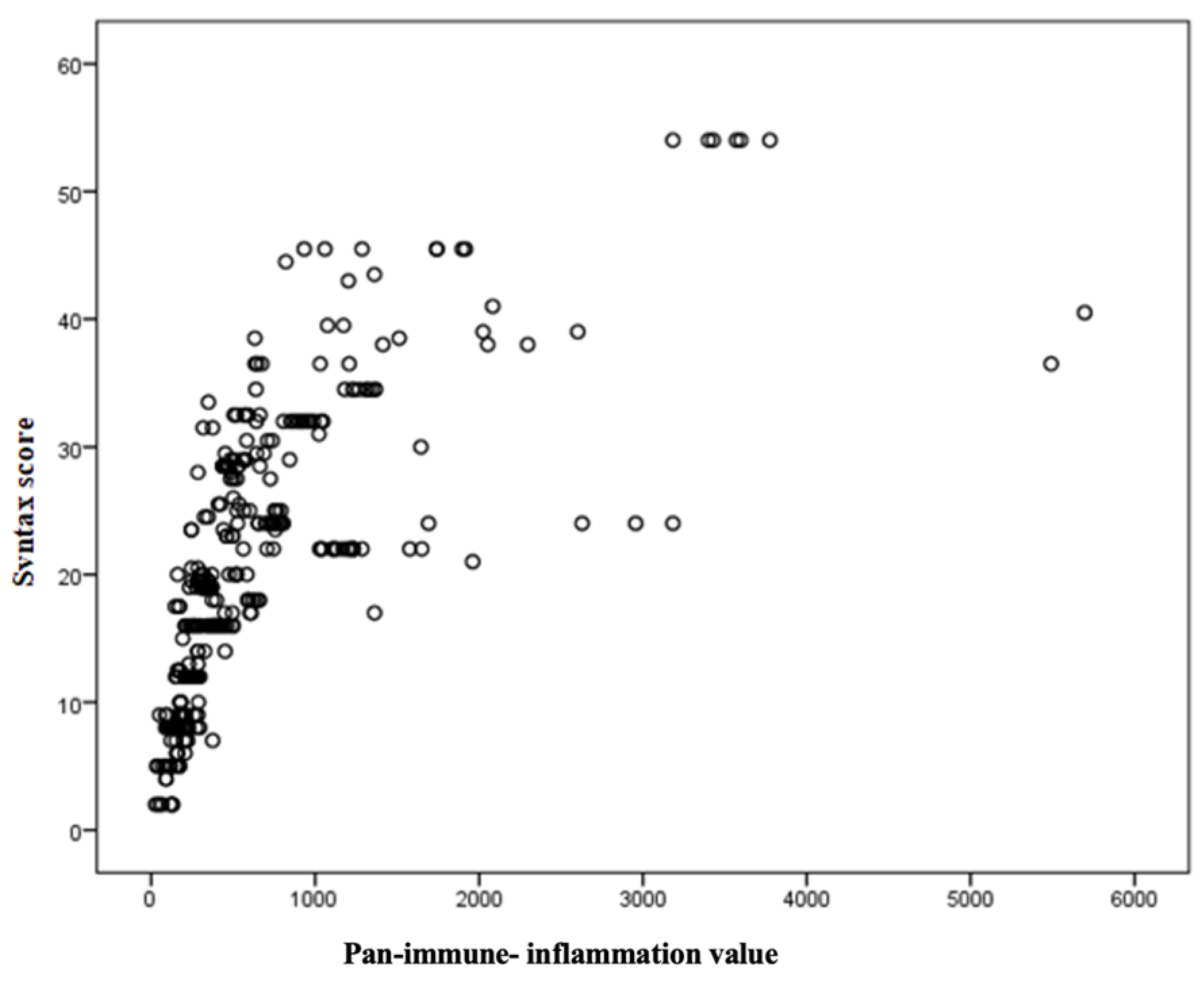
| Variables | Low SYNTAX Score (<22) n:255 | Moderate SYNTAX Score (≤22–32) n:101 | High SYNTAX Score (≥32) n:70 | p |
|---|---|---|---|---|
| Age n (q1–q3) | 66 (57–71) | 65 (55–71) | 66 (58–73) | 0.46 |
| Men, n (%) | 173 (67.8) | 81 (80.2) | 50 (71.4) | 0.07 |
| DM, n (%) | 131 (51.4) | 56 (55.4) | 35 (50) | 0.73 |
| HT, n (%) | 167 (65.5) | 69 (68.3) | 44 (62.9) | 0.75 |
| Smoker n (%) | 154 (60.4) | 74 (73.3) | 47 (67.1) | 0.07 |
| Hypercholesterolemia (n, %) | 78 (30.6) | 25 (24.8) | 19 (27.1) | 0.52 |
| Left ventricular ejection fraction (%) | 55.5 ± 5.4 a | 53.6 ± 6.7 b | 54.2 ± 5.3 b | 0.011 |
| Systolic blood pressure (mm Hg) | 120 (110–130) a | 100 (100–110) b | 95 (90–100) c | <0.001 |
| Diastolic blood pressure (mm Hg) | 80 (70–80) a | 60 (60–70) b | 60 (60–65) c | <0.001 |
| Heart rate (beats/min) | 75 (65–90) a | 90 (70–103) b | 110 (89–116) c | <0.001 |
| Killip Class | ||||
| Class I | 220 (86.6) | 45 (45.5) | 8 (11.4) | <0.001 |
| Class II | 31 (12.2) | 37 (37.4) | 21 (30) | |
| Class III | 1 (0.4) | 17 (17.2) | 36 (51.4) | |
| Class IV | 0 | 0 | 5 (7.1) | |
| Grace score | 118 (96–128) a | 153 (133–157) b | 170 (157–179) c | <0.001 |
| Glucose (mg/dL) | 154.5 ± 76.7 | 173.8 ± 90.2 | 150.3 ± 60.4 | 0.07 |
| Creatinine (mg/dL) | 0.87 ± 0.2 a | 0.95 ± 0.32 b | 1.01 ± 0.38 b | <0.001 |
| GFR (mL/min/1.73 m2) | 86.8 ± 20.4 a | 81.6 ± 26.9 ab | 76.7 ± 27.1 b | 0.006 |
| Total cholesterol (mg/dL) | 168.4 ± 48.3 | 163.9 ± 39.9 | 170.1 ± 33.9 | 0.85 |
| AST | 23 (18.5–33) a | 28 (21–43.2) a | 34 (20–72.5) b | <0.001 |
| ALT | 19 (13–26.2) | 20 (15–27) | 20 (14.7–33.2) | 0.28 |
| LDL-C (mg/dL) | 125.9 ± 35.7 | 120.2 ± 35.3 | 127.5 ± 45.5 | 0.52 |
| HDL-C (mg/dL) | 42.7 ± 9.6 | 41.3 ± 9.4 | 45.2 ± 10.4 | 0.26 |
| Troponin | 562 (63–1832) a | 542.5 (129.7–2243.5) ab | 2133 (230.5–9603) b | 0.014 |
| CRP | 4.87 (2.07–10) | 7.6 (4.7–19.5) | 8.5 (4.5–21.5) | <0.001 |
| Neutrophil count (103/µL) | 4.37 ± 1.7 | 14.2 ± 16.1 | 10.8 ± 3.1 | 0.06 |
| Lymphocyte count (103/µL) | 2.06 ± 0.67 | 2.1 ± 1 | 1.57 ± 0.8 | 0.67 |
| Monocyte (103/µL) | 0.6 ± 0.19 a | 0.75 ± 0.25 b | 1.03 ± 1.1 c | <0.001 |
| Platelet count (103/µL) | 227.7 ± 67.7 a | 245.5 ± 62.2 b | 262.1 ± 76 b | <0.001 |
| NLR | 1.8 (1.4–2.5) a | 3.5 (2.6–5.8) b | 4.8 (3.2–7.5) c | <0.001 |
| SII | 418 (310.4–543.2) a | 896.1 (627.3–1327.6) b | 1286.2 (890.3–1757.9) c | <0.001 |
| PIV | 249.8 (171.7–349.1) a | 582.1 (464.7–800.8) b | 1054.4 (670.1–1569.4) c | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p | |
| Left ventricular ejection fraction (%) | 0.979 | 0.939–1.02 | 0.308 | 1.01 | 0.918–1.126 | 0.754 |
| PIV a | 1.003 | 1.002–1.003 | <0.001 | 1.003 | 1.001–1.005 | 0.005 |
| SII a | 1.001 | 1.001–1.002 | <0.001 | 1.001 | 1–1.001 | 0.042 |
| NLR a | 1.226 | 1.138–1.321 | <0.001 | 1.181 | 1.022–1.364 | 0.024 |
| Systolic blood pressure (mm Hg) | 0.901 | 0.876–0.927 | <0.001 | 0.88 | 0.783–0.989 | 0.033 |
| Diastolic blood pressure (mm Hg) | 0.858 | 0.823–0.896 | <0.001 | 1.208 | 1.027–1.421 | 0.022 |
| Creatinine (mg/dL) | 0.614 | 0.1–2921.2 | 0.91 | 2.28 | 0.321–16.1 | 0.41 |
| CRP | 1.005 | 0.99–1.01 | 0.263 | 0.989 | 0.964–1.015 | 0.416 |
| AST | 1.003 | 1–1.006 | 0.046 | 1.002 | 0.99–1.005 | 0.268 |
| Troponin | 1 | 1–1.001 | 0.004 | 1 | 1.000–1.001 | 0.564 |
| Heart rate (beats/min) | 1.063 | 1.046–1.080 | <0.001 | 1.017 | 0.981–1.055 | 0.347 |
| r | p | |
|---|---|---|
| PIV | 0.68 | <0.001 |
| SII | 0.53 | <0.001 |
| NLR | 0.49 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cetinkaya, Z.; Kelesoglu, S.; Tuncay, A.; Yilmaz, Y.; Karaca, Y.; Karasu, M.; Secen, O.; Cinar, A.; Harman, M.; Sahin, S.; et al. The Role of Pan-Immune-Inflammation Value in Determining the Severity of Coronary Artery Disease in NSTEMI Patients. J. Clin. Med. 2024, 13, 1295. https://doi.org/10.3390/jcm13051295
Cetinkaya Z, Kelesoglu S, Tuncay A, Yilmaz Y, Karaca Y, Karasu M, Secen O, Cinar A, Harman M, Sahin S, et al. The Role of Pan-Immune-Inflammation Value in Determining the Severity of Coronary Artery Disease in NSTEMI Patients. Journal of Clinical Medicine. 2024; 13(5):1295. https://doi.org/10.3390/jcm13051295
Chicago/Turabian StyleCetinkaya, Zeki, Saban Kelesoglu, Aydin Tuncay, Yucel Yilmaz, Yucel Karaca, Mehdi Karasu, Ozlem Secen, Ahmet Cinar, Murat Harman, Seyda Sahin, and et al. 2024. "The Role of Pan-Immune-Inflammation Value in Determining the Severity of Coronary Artery Disease in NSTEMI Patients" Journal of Clinical Medicine 13, no. 5: 1295. https://doi.org/10.3390/jcm13051295
APA StyleCetinkaya, Z., Kelesoglu, S., Tuncay, A., Yilmaz, Y., Karaca, Y., Karasu, M., Secen, O., Cinar, A., Harman, M., Sahin, S., Akin, Y., & Yavcin, O. (2024). The Role of Pan-Immune-Inflammation Value in Determining the Severity of Coronary Artery Disease in NSTEMI Patients. Journal of Clinical Medicine, 13(5), 1295. https://doi.org/10.3390/jcm13051295