Peak Slope Ratio of the Recruitment Curves Compared to Muscle Evoked Potentials to Optimize Standing Configurations with Percutaneous Epidural Stimulation after Spinal Cord Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Timeline of the Study
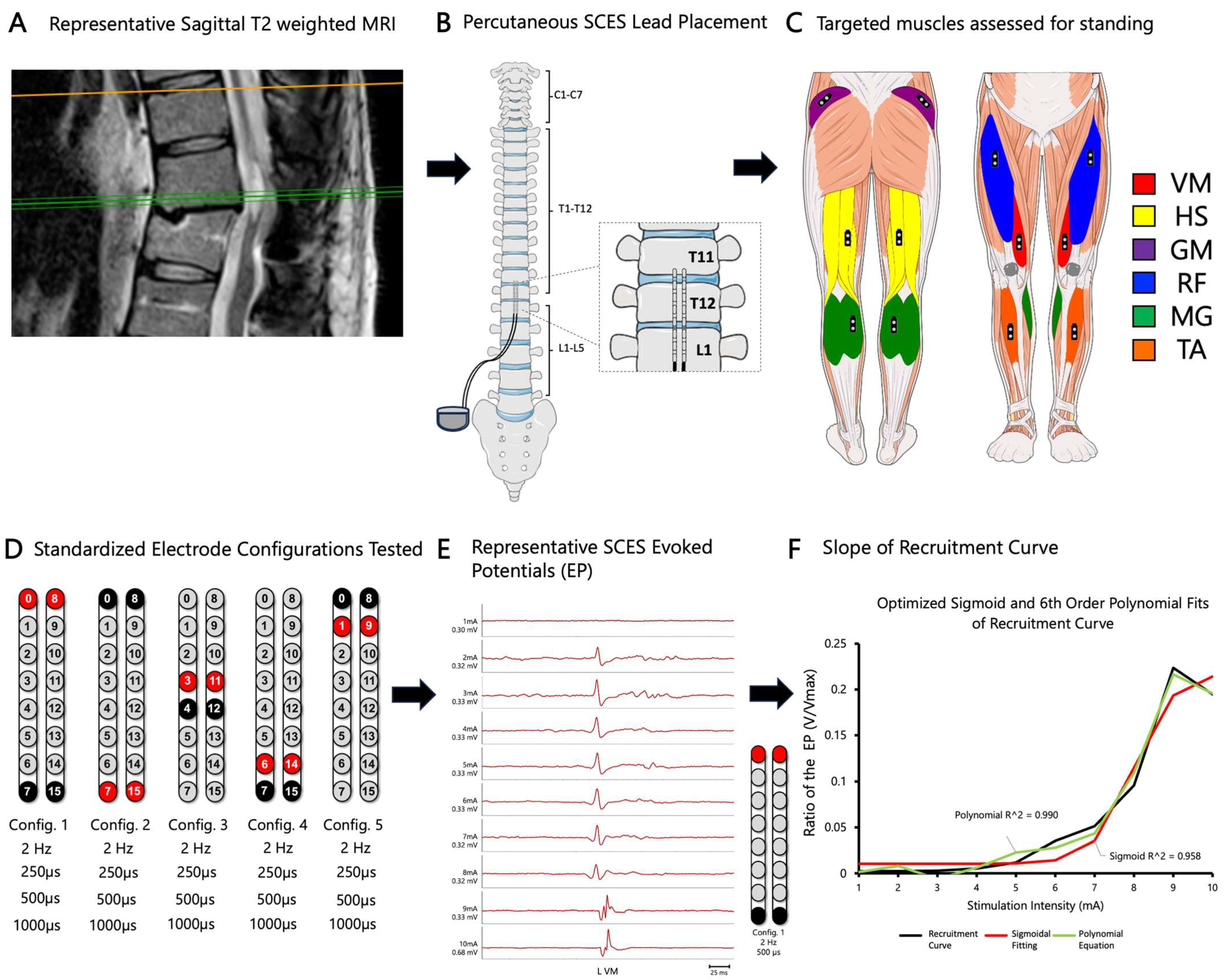
2.3. Magnetic Resonance Imaging
2.4. Implantation of Percutaneous SCES
2.5. Spinal Segmental Mapping
2.6. Developing Recruitment Curves
2.7. EMG Data Analysis
3. Results
3.1. Magnetic Resonance Imaging (MRI) Biomarkers of Spinal Cord Damage
3.2. pSCES-Evoked Supine Recruitment Curves
3.3. Wide versus Narrow-Field Configurations
3.4. Peak Slope Ratio of SCES-Evoked Recruitment Curves
3.5. Standing Performance
4. Discussion
4.1. Rationale of Conducting the Study
4.2. MRI and SCES Configurations
4.3. Supine Mapping and Recruitment Curves
4.4. Significance of Testing SCES Configurations in Supine Position
4.5. Recruitment Curves Yield Different Outcomes
5. Limitations
6. Summary/Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef]
- Wagner, F.B.; Mignardot, J.-B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; Pirondini, E.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.A.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; van Straaten, M.G.; Drubach, D.I.; et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018, 24, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Taccola, G.; Sayenko, D.; Gad, P.; Gerasimenko, Y.; Edgerton, V. And yet it moves: Recovery of volitional control after spinal cord injury. Prog. Neurobiol. 2018, 160, 64–81. [Google Scholar] [CrossRef] [PubMed]
- D’hondt, N.; Marcial, K.M.; Mittal, N.; Costanzi, M.; Hoydonckx, Y.; Kumar, P.; Englesakis, M.F.; Burns, A.; Bhatia, A. A Scoping Review of Epidural Spinal Cord Stimulation for Improving Motor and Voiding Function Following Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2023, 29, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Romanauski, B.; Lam, D.; Lujan, L.; Blanz, S.; Ludwig, K.; Lempka, S.; Shoffstall, A.; Knudson, B.; Nishiyama, Y.; et al. Characterization and applications of evoked responses during epidural electrical stimulation. Bioelectron. Med. 2023, 9, 5. [Google Scholar] [CrossRef]
- Ramadan, A.; König, S.D.; Zhang, M.; Ross, E.K.; Herman, A.; Netoff, T.I.; Darrow, D.P. Methods and system for recording human physiological signals from implantable leads during spinal cord stimulation. Front. Pain Res. 2023, 4, 1072786. [Google Scholar] [CrossRef]
- Calvert, J.S.; Darie, R.; Parker, S.R.; Shaaya, E.; Syed, S.; McLaughlin, B.L.; Fridley, J.S.; Borton, D.A. Spatiotemporal Distribution of Electrically Evoked Spinal Compound Action Potentials During Spinal Cord Stimulation. Neuromodulation Technol. Neural Interface 2023, 26, 961–974. [Google Scholar] [CrossRef]
- Kakulas, B. Neuropathology: The foundation for new treatments in spinal cord injury. Spinal Cord 2004, 42, 549–563. [Google Scholar] [CrossRef]
- Angeli, C.A.; Edgerton, V.R.; Gerasimenko, Y.P.; Harkema, S.J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014, 137, 1394–1409. [Google Scholar] [CrossRef] [PubMed]
- Darrow, D.; Balser, D.; Netoff, T.I.; Krassioukov, A.; Phillips, A.; Parr, A.; Samadani, U. Epidural Spinal Cord Stimulation Facilitates Immediate Restoration of Dormant Motor and Autonomic Supraspinal Pathways after Chronic Neurologically Complete Spinal Cord Injury. J. Neurotrauma 2019, 36, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.; Albin, S.R.; O’Dell, D.R.; Berliner, J.C.; Dungan, D.; Sevigny, M.; Draganich, C.; Elliott, J.M.; Weber, K.A., II. Axial MRI biomarkers of spinal cord damage to predict future walking and motor function: A retrospective study. Spinal Cord 2021, 59, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.; Angeli, C.A.; Ugiliweneza, B.; Weber, K.A.; Bert, R.J.; Negahdar, M.; Mesbah, S.; Boakye, M.; Harkema, S.J.; Rejc, E. Spinal cord imaging markers and recovery of standing with epidural stimulation in individuals with clinically motor complete spinal cord injury. Exp. Brain Res. 2022, 240, 279–288. [Google Scholar] [CrossRef]
- Tefertiller, C.; Rozwod, M.; VandeGriend, E.; Bartelt, P.; Sevigny, M.; Smith, A.C. Transcutaneous Electrical Spinal Cord Stimulation to Promote Recovery in Chronic Spinal Cord Injury. Front. Rehabil. Sci. 2022, 2, 740307. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Ali, M.Y.J. Outcome Prediction in Spinal Cord Injury: Myth or Reality. World Neurosurg. 2020, 140, 574–590. [Google Scholar] [CrossRef] [PubMed]
- Berliner, J.C.; O’dell, D.R.; Albin, S.R.; Dungan, D.; Sevigny, M.; Elliott, J.M.; Weber, K.A.; Abdie, D.R.; Anderson, J.S.; Rich, A.A.; et al. The influence of conventional T2 MRI indices in predicting who will walk outside one year after spinal cord injury. J. Spinal Cord Med. 2023, 46, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Kathe, C.; Skinnider, M.A.; Hutson, T.H.; Regazzi, N.; Gautier, M.; Demesmaeker, R.; Komi, S.; Ceto, S.; James, N.D.; Cho, N.; et al. The neurons that restore walking after paralysis. Nature 2022, 611, 540–547. [Google Scholar] [CrossRef]
- Capogrosso, M.; Wagner, F.B.; Gandar, J.; Moraud, E.M.; Wenger, N.; Milekovic, T.; Shkorbatova, P.; Pavlova, N.; Musienko, P.; Bezard, E.; et al. Configuration of electrical spinal cord stimulation through real-time processing of gait kinematics. Nat. Protoc. 2018, 13, 2031–2061. [Google Scholar] [CrossRef]
- Boakye, M.; Ball, T.; Dietz, N.; Sharma, M.; Angeli, C.; Rejc, E.; Kirshblum, S.; Forrest, G.; Arnold, F.W.; Harkema, S. Spinal cord epidural stimulation for motor and autonomic function recovery after chronic spinal cord injury: A case series and technical note. Surg. Neurol. Int. 2023, 14, 87. [Google Scholar] [CrossRef]
- Angeli, C.; Rejc, E.; Boakye, M.; Herrity, A.; Mesbah, S.; Hubscher, C.; Forrest, G.; Harkema, S. Targeted Selection of Stimulation Parameters for Restoration of Motor and Autonomic Function in Individuals with Spinal Cord Injury. Neuromodul. Technol. Neural Interface 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Perret, I.; Bayart, A.; Lackner, P.; Binder, H.; Freundl, B.; Minassian, K. Spinal motor mapping by epidural stimulation of lumbosacral posterior roots in humans. iScience 2021, 24, 101930. [Google Scholar] [CrossRef] [PubMed]
- Rejc, E.; Angeli, C.A. Spinal Cord Epidural Stimulation for Lower Limb Motor Function Recovery in Individuals with Motor Complete Spinal Cord Injury. Phys. Med. Rehabil. Clin. N. Am. 2019, 30, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Rejc, E.; Angeli, C.; Harkema, S. Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PloS ONE 2015, 10, e0133998. [Google Scholar] [CrossRef] [PubMed]
- Pierrot-Deseilligny, E.; Burke, D. The Circuitry of the Human Spinal Cord: Its Role in Motor Control and Movement Disorders; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Atkinson, D.A.; Dy, C.J.; Gurley, K.M.; Smith, V.L.; Angeli, C.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Spinal segment-specific transcutaneous stimulation differentially shapes activation pattern among motor pools in humans. J. Appl. Physiol. 2015, 118, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Trainer, R.; Sutor, T.W.; Goldsmith, J.A.; Alazzam, A.; Goetz, L.L.; Lester, D.; Lavis, T.D. A case study of percutaneous epidural stimulation to enable motor control in two men after spinal cord injury. Nat. Commun. 2023, 14, 2064. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Venigalla, S.; Rehman, M.U.; George, B.; Rejc, E.; Gouda, J.J. Interleaved configurations of percutaneous epidural stimulation enhanced overground stepping in a person with chronic paraplegia. Front. Neurosci. 2023, 17, 1284581. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Gouda, J.J. Single Lead Epidural Spinal Cord Stimulation Targeted Trunk Control and Standing in Complete Paraplegia. J. Clin. Med. 2022, 11, 5120. [Google Scholar] [CrossRef]
- Bonizzato, M.; Hottin, R.G.; Côté, S.L.; Massai, E.; Choinière, L.; Macar, U.; Laferrière, S.; Sirpal, P.; Quessy, S.; Lajoie, G.; et al. Autonomous optimization of neuroprosthetic stimulation parameters that drive the motor cortex and spinal cord outputs in rats and monkeys. Cell Rep. Med. 2023, 4, 101008. [Google Scholar] [CrossRef]
- Govindarajan, L.N.; Calvert, J.S.; Parker, S.R.; Jung, M.; Darie, R.; Miranda, P.; Shaaya, E.; A Borton, D.; Serre, T. Fast inference of spinal neuromodulation for motor control using amortized neural networks. J. Neural Eng. 2022, 19, 056037. [Google Scholar] [CrossRef]
- Rupp, R.; Biering-Sørensen, F.; Burns, S.P.; Graves, D.E.; Guest, J.; Jones, L.; Read, M.S.; Rodriguez, G.M.; Schuld, C.; Tansey-Md, K.E.; et al. International standards for neurological classification of spinal cord injury: Revised 2019. Top. Spinal Cord Inj. Rehabil. 2021, 27, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.; A Weber, K.; Parrish, T.B.; Hornby, T.G.; Tysseling, V.M.; McPherson, J.G.; Wasielewski, M.; Elliott, J.M. Ambulatory function in motor incomplete spinal cord injury: A magnetic resonance imaging study of spinal cord edema and lower extremity muscle morphometry. Spinal Cord 2017, 55, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Cummins, D.P.; Connor, J.R.; Heller, K.A.; Hubert, J.S.; Kates, M.J.; Wisniewski, K.R.; Berliner, J.C.; O’Dell, D.R.; Elliott, J.M.; Weber, K.A. Establishing the inter-rater reliability of spinal cord damage manual measurement using magnetic resonance imaging. Spinal Cord Ser. Cases 2019, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, B.; Sansur, C.A.; Ibrahimi, D.M.; Simard, J.M.; Hersh, D.S.; Le, E.; Diaz, C.; Massetti, J.; Akhtar-Danesh, N. Intramedullary Lesion Length on Postoperative Magnetic Resonance Imaging is a Strong Predictor of ASIA Impairment Scale Grade Conversion Following Decompressive Surgery in Cervical Spinal Cord Injury. Neurosurgery 2017, 80, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Flanders, A.E.; Spettell, C.M.; Tartaglino, L.M.; Friedman, D.P.; Herbison, G.J. Forecasting motor recovery after cervical spinal cord injury: Value of MR imaging. Radiology 1996, 201, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Flanders, A.E.; Spettell, C.M.; Friedman, D.P.; Marino, R.J.; Herbison, G.J. The Relationship between the Functional Abilities of Patients with Cervical Spinal Cord Injury and the Severity of Damage Revealed by MR Imaging. Am. J. Neuroradiol. 1999, 20, 926–934. [Google Scholar] [PubMed]
- Flanders, A.E.; Schaefer, D.M.; Doan, H.T.; Mishkin, M.M.; Gonzalez, C.F.; Northrup, B.E. Acute cervical spine trauma: Correlation of MR imaging findings with degree of neurologic deficit. Radiology 1990, 177, 25–33. [Google Scholar] [CrossRef]
- Brand, R.v.D.; Heutschi, J.; Barraud, Q.; DiGiovanna, J.; Bartholdi, K.; Huerlimann, M.; Friedli, L.; Vollenweider, I.; Moraud, E.M.; Duis, S.; et al. Restoring Voluntary Control of Locomotion after Paralyzing Spinal Cord Injury. Science 2012, 336, 1182–1185. [Google Scholar] [CrossRef]
- Foldes, S.T.; Weber, D.J.; Collinger, J.L. Altered modulation of sensorimotor rhythms with chronic paralysis. J. Neurophysiol. 2017, 118, 2412–2420. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Angeli, C.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P.; Chu, V.W.T.; Hornby, T.G.; Schmit, B.D.; Gonzalez-Rothi, E.J.; Streeter, K.A.; et al. Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J. Neurophysiol. 2014, 111, 1088–1099. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Farhadi, H.F.; Kukreja, S.; Minnema, A.J.; Vatti, L.; Gopinath, M.; Prevedello, L.; Chen, C.; Xiang, H.; Schwab, J.M. Impact of Admission Imaging Findings on Neurological Outcomes in Acute Cervical Traumatic Spinal Cord Injury. J. Neurotrauma 2018, 35, 1398–1406. [Google Scholar] [CrossRef]
- Martínez-Pérez, R.; Cepeda, S.; Paredes, I.; Alen, J.F.; Lagares, A. MRI Prognostication Factors in the Setting of Cervical Spinal Cord Injury Secondary to Trauma. World Neurosurg. 2017, 101, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.; Verma, H.; Narasimhaiah, S.; Chopra, S. Predicting the role of preoperative intramedullary lesion length and early decompressive surgery in ASIA impairment scale grade improvement following subaxial traumatic cervical spinal cord injury. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2022, 84, 144–156. [Google Scholar] [CrossRef]
- Rejc, E.; Angeli, C.A.; Bryant, N.; Harkema, S.J. Effects of Stand and Step Training with Epidural Stimulation on Motor Function for Standing in Chronic Complete Paraplegics. J. Neurotrauma 2017, 34, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Solinsky, R.; Specker-Sullivan, L.; Wexler, A. Current barriers and ethical considerations for clinical implementation of epidural stimulation for functional improvement after spinal cord injury. J. Spinal Cord Med. 2020, 43, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Mesbah, S.; Herrity, A.; Ugiliweneza, B.; Angeli, C.; Gerasimenko, Y.; Boakye, M.; Harkema, S. Neuroanatomical mapping of the lumbosacral spinal cord in individuals with chronic spinal cord injury. Brain Commun. 2023, 5, fcac330. [Google Scholar] [CrossRef]








| ID | Sex | Age (yrs.) | Weight (kg) | Height (cm) | BMI | TSI (yrs.) | NLI | AIS | Classification |
|---|---|---|---|---|---|---|---|---|---|
| 0881 | M | 25 | 48.6 | 174.3 | 16.0 | 6 | C8 | A | Tetraplegia |
| 0882 | M | 36 | 99.4 | 182.2 | 29.9 | 9 | T11 | B | Paraplegia |
| 0883 | M | 38 | 99.0 | 180.5 | 30.4 | 12 | T6 | A | Paraplegia |
| 0884 | M | 54 | 93.4 | 182.8 | 28.0 | 24 | T4 | A | Paraplegia |
| ID | Lesion Length (mm) | Lesion Volume (mm3) | Axial Damage Ratio |
|---|---|---|---|
| 0881 | 32.0 | 1932.3 mm3 | 0.92 |
| 0882 | 23.8 | 1881.2 mm3 | 0.81 |
| 0883 | 88.1 | Unable to Assess | Unable to Assess |
| 0884 | 15.5 | Unable to Assess | 0.8 |
| Configuration 1 Wide field configuration  | Configuration 2 Wide field configuration  | Configuration 3 Central narrow field configuration  | Configuration 4 Caudal narrow field configuration  | Configuration 5 rostral narrow field configuration  | |||||||||||||||||||||||||||
| Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | ||||||||||||||||||||||
| Pulse Duration (µs) | 250 | 500 | 1000 | 250 | 500 | 1000 | 250 | 500 | 1000 | 250 | 500 | 1000 | 250 | 500 | 1000 | 250 | 500 | 1000 | 250 | 500 | 1000 | 250 | 500 | 1000 | 250 | 500 | 1000 | 250 | 500 | 1000 | |
| 0881 | VM/HS | 0.38 | 1.14 +# | 0.33 | 0.17 | 0.77 # | 0.72 | 0.46 | 0.39 | 0.32 | 0.24 | 0.19 | 0.16 | 0.52 | 1.31 +# | 0.83 # | 0.17 | 0.76 # | 0.34 # | 0.26 | 0.38 | 0.39 | 0.24 | 0.23 | 0.30 | 0.90 | 0.48 | 0.90 | 1.91 + | 0.52 | 0.79 |
| GM/RF | 1.59 +# | 1.05 +# | 1.67 +# | 1.76 +# | 1.01 +# | 1.91 +# | 1.55 +# | 3.44 +# | 8.64 +# | 1.86 +# | 3.90 +# | 2.24 +# | 0.96 # | 1.17 +# | 1.19 +# | 1.63 +# | 1.13 +# | 1.08 +# | 8.75 +# | 2.32 +# | 1.48 +# | 0.81 # | 2.43 +# | 1.18 +# | 0.68 | 0.42 | 2.12 + | 0.67 | 0.20 | 0.43 | |
| MG/TA | 1.05 +# | 0.94 | 1.11 +# | 1.30 +# | 0.89 | 1.15 +# | 1.77 +# | 1.76 +# | 1.04 +# | 1.40 +# | 1.16 +# | 1.25 +# | 1.02 + | 0.91 | 0.71 | 0.48 # | 0.93 | 0.86 | 1.15 +# | 0.93 # | 1.06 +# | 1.89 +# | 1.02 +# | 1.06 +# | 4.55 + | 0.42 | 0.02 | 9.04 + | 1.83 + | 0.88 | |
| 0882 | VM/HS | 0.06 | 0.47 | 0.26 | 0.54 | 0.72 | 0.41 | 1.80 +# | 1.39 +# | 0.86 # | 1.49 +# | 1.23 +# | 1.07 +# | 0.13 | 0.62 | 0.13 | 0.13 | 1.34 + | 0.54 | 0.14 | 0.41 | 0.39 | 0.22 | 0.11 | 0.39 | 0.18 | 3.50 +# | 0.87 | 0.29 | 0.55 | 0.40 |
| GM/RF | 2.31 +# | 1.62 +# | 1.82 +# | 12.74 +# | 3.97 +# | 2.92 +# | 0.43 | 0.49 | 0.64 # | 1.46 +# | 1.21 +# | 1.28 +# | 2.51 +# | 1.62 +# | 4.93 +# | 12.69 +# | 1.81 +# | 2.80 +# | 1.50 +# | 0.90 # | 1.22 +# | 2.57 + | 12.54 +# | 4.85 +# | 0.76 | 0.32 | 0.25 | 1.08 +# | 0.84 # | 1.89 +# | |
| MG/TA | 4.08 +# | 1.39 +# | 0.46 # | 1.09 + | 1.51 +# | 0.46 # | 0.76 | 1.19 +# | 2.71 +# | 0.10 | 0.26 | 0.35 | 3.73 + | 1.95 +# | 2.84 +# | 0.49 | 0.53 | 0.44 | 9.64 +# | 2.93 +# | 9.34 +# | 0.07 | 0.17 | 0.18 | 0.73 # | 0.83 | 1.09 +# | 0.84 | 0.37 # | 0.37 # | |
| 0883 | VM/HS | 3.95 +# | 1.82 +# | 0.79 | 0.55 # | 0.71 # | 0.44 | 9.24 + | 0.94 | 0.82 # | 0.13 | 1.53 + | 0.81 # | 0.35 | 1.69 + | 6.95 +# | 1.05 + | 0.68 | 0.66 | 0.14 | 0.50 | 2.81 + | 5.12 + | 0.97 # | 0.83 # | 23.62 + | 1.14 + | 0.67 | 0.44 | 0.64 | 0.12 |
| GM/RF | Malfunction of GM senor | ||||||||||||||||||||||||||||||
| MG/TA | 0.10 | 17.50 + | 4.47 + | 1.73 + | 2.85 + | 1.86 + | 0.03 | 0.33 | 0.80 | 0.85 | 3.43 + | 0.75 | 0.30 | 0.25 | 0.08 | 0.67 | 0.53 | 0.59 | 0.08 | 0.01 | 0.58 | 1.64 + | 2.61 + | 2.06 + | 0.14 | 0.12 | 0.04 | 0.25 | 0.76 | 0.38 | |
| 0884 | VM/HS | 0.45 | 0.40 | 0.86 # | 0.27 | 0.54 | 0.53 # | 0.56 | * | 1.11 + | 8.11 + | * | 0.04 | 2.57 + | * | * | 4.68 + | * | * | 0.04 | 0.19 | 0.20 | 0.08 | 0.09 | 0.11 | Activating Abdominal Muscles | |||||
| GM/RF | Malfunction of GM senor | ||||||||||||||||||||||||||||||
| MG/TA | 1.55 +# | 1.23 +# | 2.03 +# | 1.03 +# | 0.80 # | 0.88 # | 4.77 + | * | 0.02 | 0.08 | * | 4.06 + | 0.17 | * | * | 0.33 | * | * | 3.24 + | 2.47 +# | 0.81 # | 1.55 +# | 0.68 # | 0.90 # | Activating Abdominal Muscles | ||||||
| Configuration 1 Caudal wide field Configuration | Configuration 2 Rostral wide field Configuration | Configuration 3 Central narrow field configuration | Configuration 4 Caudal narrow field configuration | Configuration 5 Rostral narrow field configuration | ||
 |  |  |  |  | ||
| Subject ID | Selected Configurations Based on the Evoked Potentials | Recommended Configurations Based on the Slope Ratio of the Recruitment Curves | Agreement | Potential Recommendations | Standing Performance | |
| 0881 | VM/HS |  15 Hz & 700–750 µs | Config. 1 and 3 @ 500 µs | Config. 1 and 3 | Perfect Agreement | - Trunk control with support at both knees |
| GM/RF | Config. 1, 2, 3, 4 @ 250, 500, 1000 µs | Config. 1 and 3 | Potentially Test config. 2 or 4 | |||
| MG/TA | Config. 1, 2, 4 @ 250, 500, 1000 µs | Config. 1 | Potentially Test config. 2 or 4 | |||
| 0882 | VM/HS |  15 Hz & 500 µs | Config. 2,5 @ 500 µs | Config. 2 and 5 | Perfect Agreement | - Independent standing between the parallel bars or with a roller walker. - The config. 2 and 5 were common across the agonist-antagonist muscle groups |
| GM/RF | Config. 1, 2, 3, 4, 5 @ 250, 500, 1000 µs | Config. 2 and 5 | Potentially Test config. 1, 3 or 4 | |||
| MG/TA | Config. 1, 2, 3, 4, 5 @ 250, 500, 1000 µs | Config. 2 and 5 | Potentially Test config. 1, 3 or 4 | |||
| 0883 | VM/HS |  10–15 Hz & 370–760 µs | Config. 1, 3 @ 250–1000 µs | Config. 1 | Potentially Test config. 3 | - Maximum assistance at the hips and knee joints to achieve standing - Potentially testing config. 3 or 4 |
| GM/RF | Malfunction of the GM sensor | |||||
| MG/TA | Config. 1, 4 @ 250–1000 µs | Config. 1 | Potentially Test config. 4 | |||
| 0884 | VM/HS |  15 Hz & 700 µs | Config. 1, 2 and 3 @ 250–1000 µs | Config. 1 | Potentially Test config. 2 and 3 | - Independent standing with spot or min ass. to support the knee joints - Potentially test config. 2. |
| GM/RF | Malfunction of the GM sensor | |||||
| MG/TA | Config. 1, 2 and 4 @ 250–1000 µs | Config. 1 | Potentially Test config. 2 and 4 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alazzam, A.M.; Ballance, W.B.; Smith, A.C.; Rejc, E.; Weber, K.A., II; Trainer, R.; Gorgey, A.S. Peak Slope Ratio of the Recruitment Curves Compared to Muscle Evoked Potentials to Optimize Standing Configurations with Percutaneous Epidural Stimulation after Spinal Cord Injury. J. Clin. Med. 2024, 13, 1344. https://doi.org/10.3390/jcm13051344
Alazzam AM, Ballance WB, Smith AC, Rejc E, Weber KA II, Trainer R, Gorgey AS. Peak Slope Ratio of the Recruitment Curves Compared to Muscle Evoked Potentials to Optimize Standing Configurations with Percutaneous Epidural Stimulation after Spinal Cord Injury. Journal of Clinical Medicine. 2024; 13(5):1344. https://doi.org/10.3390/jcm13051344
Chicago/Turabian StyleAlazzam, Ahmad M., William B. Ballance, Andrew C. Smith, Enrico Rejc, Kenneth A. Weber, II, Robert Trainer, and Ashraf S. Gorgey. 2024. "Peak Slope Ratio of the Recruitment Curves Compared to Muscle Evoked Potentials to Optimize Standing Configurations with Percutaneous Epidural Stimulation after Spinal Cord Injury" Journal of Clinical Medicine 13, no. 5: 1344. https://doi.org/10.3390/jcm13051344
APA StyleAlazzam, A. M., Ballance, W. B., Smith, A. C., Rejc, E., Weber, K. A., II, Trainer, R., & Gorgey, A. S. (2024). Peak Slope Ratio of the Recruitment Curves Compared to Muscle Evoked Potentials to Optimize Standing Configurations with Percutaneous Epidural Stimulation after Spinal Cord Injury. Journal of Clinical Medicine, 13(5), 1344. https://doi.org/10.3390/jcm13051344







