Mechanisms of Cardiovascular Calcification and Experimental Models: Impact of Vitamin K Antagonists
Abstract
:1. Introduction
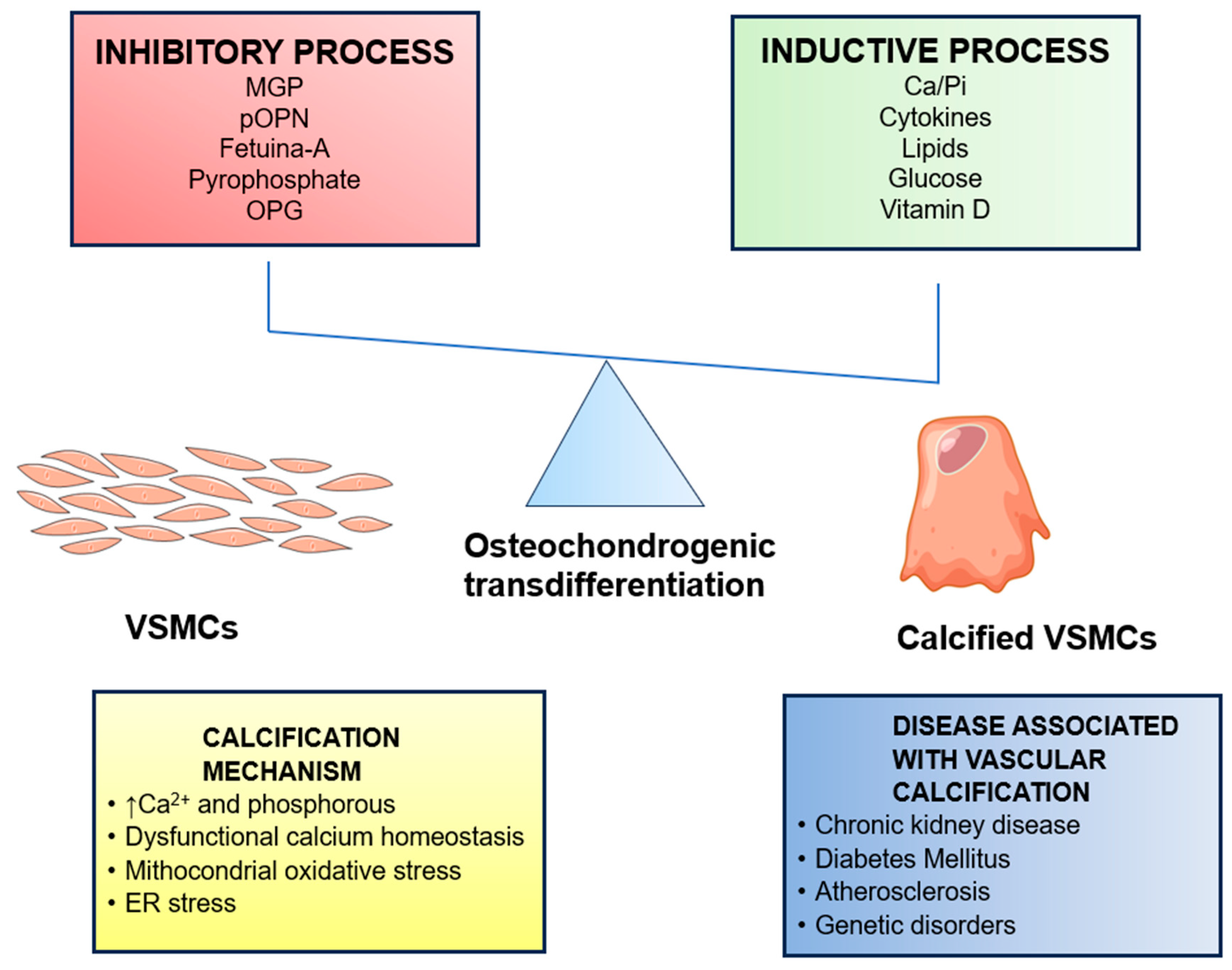
2. Arterial Calcification
3. Medial Artery Calcification
4. Calcification of Cardiac Valves
5. Calcific Uremic Arteriolopathy
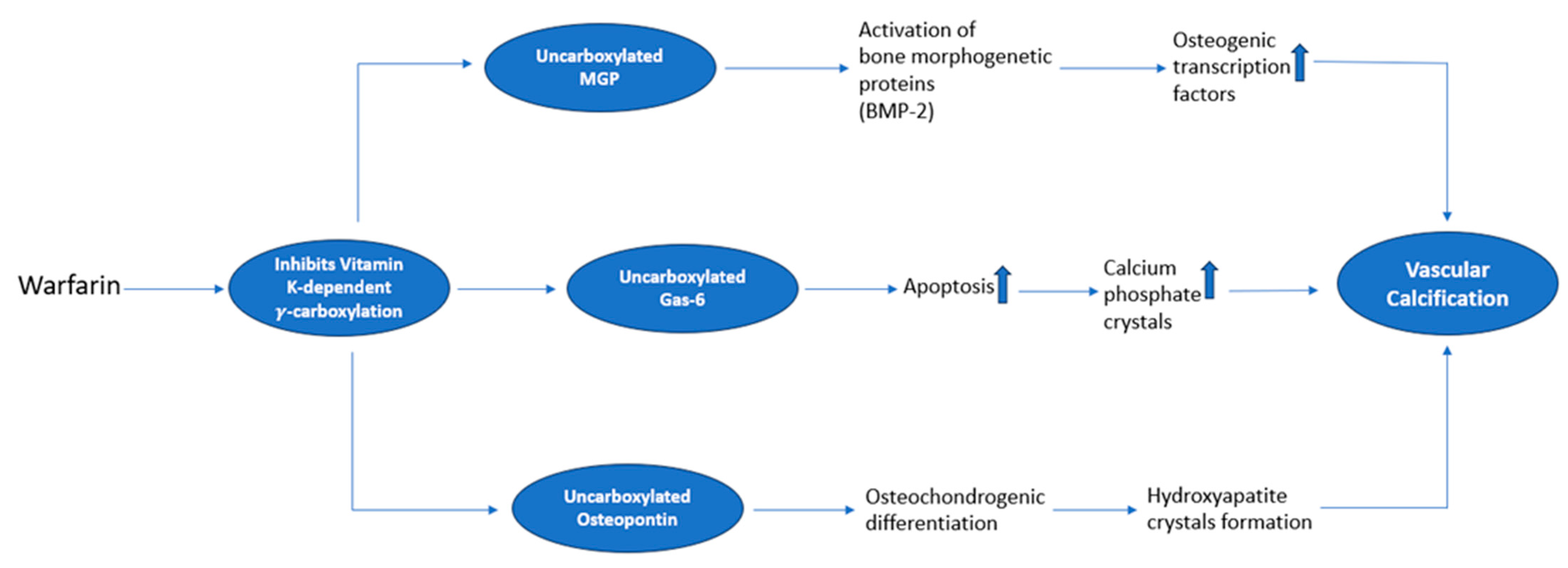
6. Molecular Mechanisms Underlying Vascular Calcifications Associated with VKA
7. miRNA in Cardiovascular Calcification
8. miRNA Transport in Vesicles and Calcification
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rao, Z.; Zheng, Y.; Xu, L.; Wang, Z.; Zhou, Y.; Chen, M.; Dong, N.; Cai, Z.; Li, F. Endoplasmic Reticulum Stress and Pathogenesis of Vascular Calcification. Front. Cardiovasc. Med. 2022, 9, 918056. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, I.-K.; Jeon, J.-H. Vascular Calcification—New Insights into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef]
- Chen, N.-C.; Hsu, C.-Y.; Chen, C.-L. The Strategy to Prevent and Regress the Vascular Calcification in Dialysis Patients. BioMed. Res. Int. 2017, 2017, 9035193. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yu, H.; Zhang, D.; Feng, T.; Miao, M.; Li, J.; Liu, X. Matrix Vesicles as a Therapeutic Target for Vascular Calcification. Front. Cell Dev. Biol. 2022, 10, 825622. [Google Scholar] [CrossRef]
- Ito, T.; Tsuchikane, E.; Nasu, K.; Suzuki, Y.; Kimura, M.; Ehara, M.; Terashima, M.; Kinoshita, Y.; Habara, M.; Suzuki, T.; et al. Impact of lesion morphology on angiographic and clinical outcomes in patients with chronic total occlusion after recanalization with drug-eluting stents: A multislice computed tomography study. Eur. Radiol. 2015, 25, 3084–3092. [Google Scholar] [CrossRef] [PubMed]
- Shreya, D.; Zamora, D.I.; Patel, G.S.; Grossmann, I.; Rodriguez, K.; Soni, M.; Joshi, P.K.; Patel, S.C.; Sange, I. Coronary Artery Calcium Score—A Reliable Indicator of Coronary Artery Disease? Cureus 2021, 13, e20149. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, N.; Erbel, R.; Mahabadi, A.A.; Rauwolf, M.; Möhlenkamp, S.; Moebus, S.; Kälsch, H.; Budde, T.; Schmermund, A.; Stang, A.; et al. Value of Progression of Coronary Artery Calcification for Risk Prediction of Coronary and Cardiovascular Events. Circulation 2018, 137, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Vascular Calcification: An Age-Old Problem of Old Age. Circulation 2013, 127, 2380–2382. [Google Scholar] [CrossRef]
- Andò, G.; Vizzari, G.; Niccoli, G.; Calabrò, P.; Zimarino, M.; Spaccarotella, C.; De Rosa, S.; Piccolo, R.; Gragnano, F.; Mancone, M.; et al. Valutazione e trattamento interventistico delle lesioni coronariche severamente calcifiche. G. Ital. Cardiologia 2021, 22, 480–489. [Google Scholar]
- Tang, L.; Wang, X.; Yang, J.; Wang, Y.; Qu, M.; Li, H. DLFFNet: A new dynamical local feature fusion network for automatic aortic valve calcification recognition using echocardiography. Comput. Methods Programs Biomed. 2024, 243, 107882. [Google Scholar] [CrossRef]
- Lanzer, P.; Boehm, M.; Sorribas, V.; Thiriet, M.; Janzen, J.; Zeller, T.; St Hilaire, C.; Shanahan, C. Medial vascular calcification revisited: Review and perspectives. Eur. Heart J. 2014, 35, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhao, M.; Zhang, C.; Sun, X. IL-1β in atherosclerotic vascular calcification: From bench to bedside. Int. J. Biol. Sci. 2021, 17, 4353–4364. [Google Scholar] [CrossRef] [PubMed]
- Lanzer, P.; Hannan, F.M.; Lanzer, J.D.; Janzen, J.; Raggi, P.; Furniss, D.; Schuchardt, M.; Thakker, R.; Fok, P.W.; Saez-Rodriguez, J.; et al. Medial Arterial Calcification: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1145–1165. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; June, H.H.; Buckley, J.R.; Williamson, M.K. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arter. Thromb. Vasc. Biol. 2001, 21, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Schmermund, A.; Baumgart, D.; Möhlenkamp, S.; Kriener, P.; Pump, H.; Grönemeyer, D.; Seibel, R.; Erbel, R. Natural history and topographic pattern of progression coronary calcification in symptomatic patients an electron-beam CT study. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 421–426. [Google Scholar] [CrossRef]
- Schmermund, A.; Erbel, R. Unstable coronary plaque and its relation to coronary calcium. Circulation 2001, 104, 1682–1687. [Google Scholar] [CrossRef]
- Qiu, C.; Zheng, H.; Tao, H.; Yu, W.; Jiang, X.; Li, A.; Jin, H.; Lv, A.; Li, H. Vitamin K2 inhibits rat vascular smooth muscle cell calcification by restoring the Gas6/Axl/Akt anti-apoptotic pathway. Mol. Cell. Biochem. 2017, 433, 149–159. [Google Scholar] [CrossRef]
- Danziger, J. Vitamin K-dependent proteins, warfarin, and vascular calcification. Clin. J. Am. Soc. Nephrol. 2008, 3, 1504–1510. [Google Scholar] [CrossRef]
- Neradova, A.; Wasilewski, G.; Prisco, S.; Leenders, P.; Caron, M.; Welting, T.; van Rietbergen, B.; Kramann, R.; Floege, J.; Vervloet, M.G.; et al. Combining phosphate binder therapy with vitamin K2 inhibits vascular calcification in an experimental animal model of kidney failure. Nephrol. Dial. Transplant. 2022, 37, 652–662. [Google Scholar] [CrossRef]
- Bergh, G.V.D.; Branden, A.V.D.; Opdebeeck, B.; Fransen, P.; Neven, E.; De Meyer, G.R.; D’haese, P.C.; Verhulst, A. Endothelial dysfunction aggravates arterial media calcification in warfarin administered rats. FASEB J. 2022, 36, e22315. [Google Scholar] [CrossRef]
- Bergh, G.V.D.; De Moudt, S.; Branden, A.V.D.; Neven, E.; Leysen, H.; Maudsley, S.; De Meyer, G.R.Y.; D’haese, P.; Verhulst, A. Endothelial Contribution to Warfarin-Induced Arterial Media Calcification in Mice. Int. J. Mol. Sci. 2021, 22, 11615. [Google Scholar] [CrossRef]
- Alloza, I.; Goikuria, H.; Idro, J.L.; Triviño, J.C.; Velasco, J.M.F.; Elizagaray, E.; García-Barcina, M.; Montoya-Murillo, G.; Sarasola, E.; Manrique, R.V.; et al. RNAseq based transcriptomics study of SMCs from carotid atherosclerotic plaque: BMP2 and IDs proteins are crucial regulators of plaque stability. Sci. Rep. 2017, 7, 3470. [Google Scholar] [CrossRef]
- Xie, F.; Cui, Q.-K.; Wang, Z.-Y.; Liu, B.; Qiao, W.; Li, N.; Cheng, J.; Hou, Y.-M.; Dong, X.-Y.; Wang, Y.; et al. ILF3 is responsible for hyperlipidemia-induced arteriosclerotic calcification by mediating BMP2 and STAT1 transcription. J. Mol. Cell. Cardiol. 2021, 161, 39–52. [Google Scholar] [CrossRef]
- Balmos, I.A.; Horváth, E.; Brinzaniuc, K.; Muresan, A.V.; Olah, P.; Molnár, G.B.; Nagy, E.E. Inflammation, Microcalcification, and Increased Expression of Osteopontin Are Histological Hallmarks of Plaque Vulnerability in Patients with Advanced Carotid Artery Stenosis. Biomedicines 2023, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Basiak, M.; Hachula, M.; Kosowski, M.; Machnik, G.; Maliglowka, M.; Dziubinska-Basiak, M.; Krysiak, R.; Okopien, B. The Effect of PCSK9 Inhibition on the Stabilization of Atherosclerotic Plaque Determined by Biochemical and Diagnostic Imaging Methods. Molecules 2023, 28, 5928. [Google Scholar] [CrossRef] [PubMed]
- Trion, A.; van der Laarse, A. Vascular smooth muscle cells and calcification in atherosclerosis. Am. Heart J. 2004, 147, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, D.; Davies, J.D.; Skepper, J.N.; Weissberg, P.L.; Shanahan, C.M. Acetylated low-density lipoprotein stimulates human vascular smooth muscle cell calcification by promoting osteoblastic differentiation and inhibiting phagocytosis. Circulation 2002, 106, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- Demer, L.L.; Tintut, Y. Vascular Calcification: Pathobiology of a Multifaceted Disease. Circulation 2008, 117, 2938. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Faus, S.A.; Williamson, M.K. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arter. Thromb. Vasc. Biol. 1998, 18, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Opdebeeck, B.; Neven, E.; Maudsley, S.; Leysen, H.; Walter, D.; Geryl, H.; D’haese, P.C.; Verhulst, A. A Proteomic Screen to Unravel the Molecular Pathways Associated with Warfarin-Induced or TNAP-Inhibited Arterial Calcification in Rats. Int. J. Mol. Sci. 2023, 24, 3657. [Google Scholar] [CrossRef] [PubMed]
- Uto, K.; Yoshizawa, S.; Aoki, C.; Nishikawa, T.; Oda, H. Inhibition of extracellular matrix integrity attenuates the early phase of aortic medial calcification in a rodent model. Atherosclerosis 2021, 319, 10–20. [Google Scholar] [CrossRef]
- Opdebeeck, B.; Neven, E.; Millán, J.L.; Pinkerton, A.B.; D’Haese, P.C.; Verhulst, A. Pharmacological TNAP inhibition efficiently inhibits arterial media calcification in a warfarin rat model but deserves careful consideration of potential physiological bone formation/mineralization impairment. Bone 2020, 137, 115392. [Google Scholar] [CrossRef]
- Price, P.A.; Faus, S.A.; Williamson, M.K. Warfarin-induced artery calcification is accelerated by growth and vitamin D. Arter. Thromb. Vasc. Biol. 2000, 20, 317–327. [Google Scholar] [CrossRef]
- Byon, C.H.; Sun, Y.; Chen, J.; Yuan, K.; Mao, X.; Heath, J.M.; Anderson, P.G.; Tintut, Y.; Demer, L.L.; Wang, D.; et al. Runx2-upregulated receptor activator of nuclear factor κb ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arter. Thromb. Vasc. Biol. 2011, 31, 1387–1396. [Google Scholar] [CrossRef]
- Wanga, S.; Hibender, S.; Ridwan, Y.; van Roomen, C.; Vos, M.; van der Made, I.; van Vliet, N.; Franken, R.; van Riel, L.A.; Groenink, M.; et al. Aortic microcalcification is associated with elastin fragmentation in Marfan syndrome. J. Pathol. 2017, 243, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Yutzey, K.E.; Demer, L.L.; Body, S.C.; Huggins, G.S.; Towler, D.A.; Giachelli, C.M.; Hofmann-Bowman, M.A.; Mortlock, D.P.; Rogers, M.B.; Sadeghi, M.M.; et al. Calcific aortic valve disease: A consensus summary from the Alliance of Investigators on Calcific Aortic Valve Disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, B.; Lovren, F.; Pan, Y.; Garg, V.; Quan, A.; Tang, G.; Singh, K.K.; Shukla, P.C.; Kalra, N.P.; Peterson, M.D.; et al. miRNA-141 is a novel regulator of BMP-2–mediated calcification in aortic stenosis. J. Thorac. Cardiovasc. Surg. 2012, 144, 256–262.e2. [Google Scholar] [CrossRef] [PubMed]
- Beazley, K.E.; Eghtesad, S.; Nurminskaya, M.V. Quercetin Attenuates Warfarin-induced Vascular Calcification in Vitro Independently from Matrix Gla Protein. J. Biol. Chem. 2013, 288, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.C.; McNair, R.; Figg, N.; Skepper, J.N.; Schurgers, L.; Gupta, A.; Hiorns, M.; Donald, A.E.; Deanfield, J.; Rees, L.; et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 2008, 118, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Baby, D.; Upadhyay, M.; Joseph, M.D.; Asopa, S.J.; Choudhury, B.K.; Rajguru, J.P. Calciphylaxis and its diagnosis: A review. J. Fam. Med. Prim. Care 2019, 8, 2763–2767. [Google Scholar] [CrossRef]
- Nigwekar, S.U.; Thadhani, R.; Brandenburg, V.M. Calciphylaxis. N. Engl. J. Med. 2018, 378, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Turek, M.; Stępniewska, J.; Różański, J. The Multifactorial Pathogenesis of Calciphylaxis: A Case Report. Am. J. Case Rep. 2021, 22, e930026-1–e930026-5. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Aebert, H.; Vermeer, C.; Bültmann, B.; Janzen, J. Oral anticoagulant treatment: Friend or foe in cardiovascular disease? Blood 2004, 104, 3231–3232. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Liu, K.; Li, X.; Wang, Y.; Li, W.; Li, B. AntagomiR-29b inhibits vascular and valvular calcification and improves heart function in rats. J. Cell. Mol. Med. 2020, 24, 11546–11557. [Google Scholar] [CrossRef]
- De Maré, A.; Opdebeeck, B.; Neven, E.; D’Haese, P.C.; Verhulst, A. Sclerostin Protects Against Vascular Calcification Development in Mice. J. Bone Miner. Res. 2022, 37, 687–699. [Google Scholar] [CrossRef] [PubMed]
- De Maré, A.; Maudsley, S.; Azmi, A.; Hendrickx, J.O.; Opdebeeck, B.; Neven, E.; D’haese, P.C.; Verhulst, A. Sclerostin as Regulatory Molecule in Vascular Media Calcification and the Bone–Vascular Axis. Toxins 2019, 11, 428. [Google Scholar] [CrossRef]
- Leszczynska, A.; Murphy, J.M. Vascular Calcification: Is it rather a Stem/Progenitor Cells Driven Phenomenon? Front. Bioeng. Biotechnol. 2018, 6, 10. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Martín-Núñez, E.; Hernández-Carballo, C.; Ferri, C.; Tagua, V.G.; Delgado-Molinos, A.; López-Castillo, Á.; Rodríguez-Ramos, S.; Cerro-López, P.; López-Tarruella, V.C.; et al. Fibroblast growth factor 23 expression in human calcified vascular tissues. Aging 2019, 11, 7899–7913. [Google Scholar] [CrossRef]
- Nigam, V.; Sievers, H.H.; Jensen, B.C.; Sier, H.A.; Simpson, P.C.; Srivastava, D.; Mohamed, S.A. Altered Micrornas in Bicuspid Aortic Valve: A Comparison between Stenotic and Insufficient Valves. J. Heart Valve Dis. 2010, 19, 459–465. [Google Scholar]
- Opdebeeck, B.; D’haese, P.C.; Verhulst, A. Molecular and Cellular Mechanisms that Induce Arterial Calcification by Indoxyl Sulfate and P-Cresyl Sulfate. Toxins 2020, 12, 58. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.J.; Robertson, G.R.; Lee, V.W. Vitamin D in Vascular Calcification: A Double-Edged Sword? Nutrients 2018, 10, 652. [Google Scholar] [CrossRef] [PubMed]
- Petsophonsakul, P.; Burgmaier, M.; Willems, B.; Heeneman, S.; Stadler, N.; Gremse, F.; Reith, S.; Burgmaier, K.; Kahles, F.; Marx, N.; et al. Nicotine promotes vascular calcification via intracellular Ca2+-mediated, Nox5-induced oxidative stress, and extracellular vesicle release in vascular smooth muscle cells. Cardiovasc. Res. 2022, 118, 2196–2210. [Google Scholar] [CrossRef] [PubMed]
- Krüger, T.; Oelenberg, S.; Kaesler, N.; Schurgers, L.J.; van de Sandt, A.M.; Boor, P.; Schlieper, G.; Brandenburg, V.M.; Fekete, B.C.; Veulemans, V.; et al. Warfarin induces cardiovascular damage in mice. Arter. Thromb. Vasc. Biol. 2013, 33, 2618–2624. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Faus, S.A.; Williamson, M.K. Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arter. Thromb. Vasc. Biol. 2001, 21, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Chan, W.S.; Jolson, D.M.; Williamson, M.K. The elastic lamellae of devitalized arteries calcify when incubated in serum: Evidence for a serum calcification factor. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Price, P.; Roublick, A.; Williamson, M. Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int. 2006, 70, 1577–1583. [Google Scholar] [CrossRef]
- Essalihi, R.; Dao, H.H.; Gilbert, L.A.; Bouvet, C.; Semerjian, Y.; McKee, M.D.; Moreau, P. Regression of medial elastocalcinosis in rat aorta: A new vascular function for carbonic anhydrase. Circulation 2005, 112, 1628–1635. [Google Scholar] [CrossRef]
- Beazley, K.E.; Deasey, S.; Lima, F.; Nurminskaya, M.V. Transglutaminase 2-mediated activation of β-catenin signaling has a critical role in warfarin-induced vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 123–130. [Google Scholar] [CrossRef]
- Beazley, K.E.; Banyard, D.; Lima, F.; Deasey, S.C.; Nurminsky, D.I.; Konoplyannikov, M.; Nurminskaya, M.V. Transglutaminase inhibitors attenuate vascular calcification in a preclinical model. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 43–51. [Google Scholar] [CrossRef]
- Huang, Z.; Li, J.; Jiang, Z.; Qi, Y.; Tang, C.; Du, J. Effects of Adrenomedullin, C-type natriuretic peptide, and parathyroid hormone-related peptide on calcification in cultured rat vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2003, 42, 89–97. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Proudfoot, D.; Farzaneh-Far, A.; Weissberg, P.L. The role of gla proteins in vascular calcification. Crit. Rev. Eukaryot. Gene Expr. 1998, 8, 357–375. [Google Scholar] [CrossRef]
- Wu, S.Y.; Zhang, B.H.; Pan, C.S.; Jiang, H.F.; Pang, Y.Z.; Tang, C.S.; Qi, Y.F. Endothelin-1 is a potent regulator in vivo in vascular calcification and in vitro in calcification of vascular smooth muscle cells. Peptides 2003, 24, 1149–1156. [Google Scholar] [CrossRef]
- Liu, Y.; Drozdov, I.; Shroff, R.; Beltran, L.E.; Shanahan, C.M. Prelamin A Accelerates vascular calcification via activation of the DNA damage response and senescence-associated secretory phenotype in vascular smooth muscle cells. Circ. Res. 2013, 112, e99–e109. [Google Scholar] [CrossRef]
- Son, B.-K.; Akishita, M.; Iijima, K.; Ogawa, S.; Arai, T.; Ishii, H.; Maemura, K.; Aburatani, H.; Eto, M.; Ouchi, Y. Thrombomodulin, a novel molecule regulating inorganic phosphate-induced vascular smooth muscle cell calcification. J. Mol. Cell. Cardiol. 2013, 56, 72–80. [Google Scholar] [CrossRef]
- Rangrez, A.Y.; M’Baya-Moutoula, E.; Metzinger-Le Meuth, V.; Hénaut, L.; Djelouat, M.S.E.I.; Benchitrit, J.; Massy, Z.A.; Metzinger, L. Inorganic Phosphate Accelerates the Migration of Vascular Smooth Muscle Cells: Evidence for the Involvement of miR-223. PLoS ONE 2012, 7, e47807. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.; Zhang, S.-Y.; Guan, S.-M.; Zhou, S.-Q.; Fang, X. Role of Wnt/β-Catenin Pathway in the Arterial Medial Calcification and Its Effect on the OPG/RANKL System. Curr. Med. Sci. 2019, 39, 28–36. [Google Scholar] [CrossRef]
- Seime, T.; Akbulut, A.C.; Liljeqvist, M.L.; Siika, A.; Jin, H.; Winski, G.; van Gorp, R.H.; Karlöf, E.; Lengquist, M.; Buckler, A.J.; et al. Proteoglycan 4 Modulates Osteogenic Smooth Muscle Cell Differentiation during Vascular Remodeling and Intimal Calcification. Cells 2021, 10, 1276. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Qian, C.; Abela, G.; Sun, W.; Kong, X. Ginkgo Biloba Extract EGB761 Alleviates Warfarin-induced Aortic Valve Calcification Through the BMP2/Smad1/5/Runx2 Signaling Pathway. J. Cardiovasc. Pharmacol. 2021, 78, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Lu, L.; Zhang, H.; Gao, M.; Ghosh, S.; Liu, Z.; Qi, J.; Wang, J.; Chen, J.; Huang, H. Warfarin Accelerates Aortic Calcification by Upregulating Senescence-Associated Secretory Phenotype Maker Expression. Oxidative Med. Cell. Longev. 2020, 2020, 2043762. [Google Scholar] [CrossRef] [PubMed]
- Demer, L.L.; Boström, K.I. Conflicting Forces of Warfarin and Matrix Gla Protein in the Artery Wall. Arter. Thromb. Vasc. Biol. 2015, 35, 9–10. [Google Scholar] [CrossRef]
- Proudfoot, D. Calcium Signaling and Tissue Calcification. Cold Spring Harb. Perspect. Biol. 2019, 11, a035303. [Google Scholar] [CrossRef]
- Elango, K.; Javaid, A.; Khetarpal, B.K.; Ramalingam, S.; Kolandaivel, K.P.; Gunasekaran, K.; Ahsan, C. The Effects of Warfarin and Direct Oral Anticoagulants on Systemic Vascular Calcification: A Review. Cells 2021, 10, 773. [Google Scholar] [CrossRef]
- Goettsch, C.; Rauner, M.; Pacyna, N.; Hempel, U.; Bornstein, S.R.; Hofbauer, L.C. miR-125b regulates calcification of vascular smooth muscle cells. Am. J. Pathol. 2011, 179, 1594–1600. [Google Scholar] [CrossRef]
- Albinsson, S.; Suarez, Y.; Skoura, A.; Offermanns, S.; Miano, J.M.; Sessa, W.C. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1118–1126. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Xing, L.; Di Chen, D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells 2010, 28, 357–364. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, R.-L.; Croce, C.M.; Stein, J.L.; Lian, J.B.; van Wijnen, A.J.; Stein, G.S. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc. Natl. Acad. Sci. USA 2011, 108, 9863–9868. [Google Scholar] [CrossRef]
- Leopold, J.A. MicroRNAs Regulate Vascular Medial Calcification. Cells 2014, 3, 963–980. [Google Scholar] [CrossRef]
- Elia, L.; Quintavalle, M.; Zhang, J.; Contu, R.; Cossu, L.; Latronico, M.V.G.; Peterson, K.L.; Indolfi, C.; Catalucci, D.; Chen, J.; et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: Correlates with human disease. Cell Death Differ. 2009, 16, 1590–1598. [Google Scholar] [CrossRef]
- Santovito, D.; Mezzetti, A.; Cipollone, F. MicroRNAs and atherosclerosis: New actors for an old movie. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 937–943. [Google Scholar] [CrossRef]
- Raitoharju, E.; Lyytikäinen, L.-P.; Levula, M.; Oksala, N.; Mennander, A.; Tarkka, M.; Klopp, N.; Illig, T.; Kähönen, M.; Karhunen, P.J.; et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis 2011, 219, 211–217. [Google Scholar] [CrossRef]
- Goettsch, C.; Hutcheson, J.D.; Aikawa, E. MicroRNA in cardiovascular calcification: Focus on targets and extracellular vesicle delivery mechanisms. Circ. Res. 2013, 112, 1073–1084. [Google Scholar] [CrossRef]
- Shao, M.; Yu, M.; Zhao, J.; Mei, J.; Pan, Y.; Zhang, J.; Wu, H.; Yu, M.; Liu, F.; Chen, G. miR-21-3p regulates AGE/RAGE signalling and improves diabetic atherosclerosis. Cell Biochem. Funct. 2020, 38, 965–975. [Google Scholar] [CrossRef]
- Armstrong, Z.B.; Boughner, D.R.; Drangova, M.; Rogers, K.A. Angiotensin II type 1 receptor blocker inhibits arterial calcification in a pre-clinical model. Cardiovasc. Res. 2011, 90, 165–170. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Maldonado, N.; Aikawa, E. Small entities with large impact: Microcalcifications and atherosclerotic plaque vulnerability. Curr. Opin. Lipidol. 2014, 25, 327–332. [Google Scholar] [CrossRef]
- Kapustin, A.N.; Shanahan, C.M. Emerging roles for vascular smooth muscle cell exosomes in calcification and coagulation. J. Physiol. 2016, 594, 2905–2914. [Google Scholar] [CrossRef]
- Liberman, M.; Marti, L. Vascular Calcification Regulation by Exosomes in the Vascular Wall. Adv. Exp. Med. Biol. 2017, 998, 151–160. [Google Scholar]
- Zhang, C.; Zhang, K.; Huang, F.; Feng, W.; Chen, J.; Zhang, H.; Wang, J.; Luo, P.; Huang, H. Exosomes, the message transporters in vascular calcification. J. Cell. Mol. Med. 2018, 22, 4024–4033. [Google Scholar] [CrossRef]
- Gui, T.; Zhou, G.; Sun, Y.; Shimokado, A.; Itoh, S.; Oikawa, K.; Muragaki, Y. MicroRNAs that target Ca2+ transporters are involved in vascular smooth muscle cell calcification. Mod. Pathol. 2012, 92, 1250–1259. [Google Scholar] [CrossRef]
- Xia, Z.Y.; Hu, Y.; Xie, P.L.; Tang, S.Y.; Luo, X.H.; Liao, E.Y.; Chen, F.; Xie, H. Runx2/miR-3960/miR-2861 Positive Feedback Loop Is Responsible for Osteogenic Transdifferentiation of Vascular Smooth Muscle Cells. Biomed. Res. Int. 2015, 2015, 624037. [Google Scholar] [CrossRef]
- Pan, W.; Liang, J.; Tang, H.; Fang, X.; Wang, F.; Ding, Y.; Huang, H.; Zhang, H. Differentially expressed microRNA profiles in exosomes from vascular smooth muscle cells associated with coronary artery calcification. Int. J. Biochem. Cell Biol. 2020, 118, 105645. [Google Scholar] [CrossRef] [PubMed]

| Authors | Outcome Animal Models In Vivo (Rats) |
|---|---|
| Price P.A. et al., 1998 [29] | This study explores the role of MGP in preventing calcification of arteries and heart valves following treatment with warfarin and vitamin K. |
| Price P.A. et al., 2000 [33] | Growth and vitamin D treatment enhance the extent of artery calcification in rats given sufficient doses of Warfarin to inhibit γ-carboxylation of MGP. |
| Price P.A. et al., 2001 [14] | Osteoprotegerin can potently inhibit the calcification of arteries induced by warfarin and vitamin D. |
| Price P.A. et al., 2001 [54] | Bisphosphonates inhibit the calcification of arteries and heart valves at doses comparable to the doses that inhibit bone resorption. |
| Price P.A. et al., 2006 [55] | The elastic lamellae of devitalized aortas calcify rapidly in serum. |
| Price P.A. et al., 2006 [56] | Medial artery calcification in uremic rats correlates with increased serum bone Gla protein (BGP; osteocalcin). |
| Essalihi et al., 2005 [57] | Vascular mineral loss induced by the blockade of endothelin receptors seems dependent on the activation of membrane-bound CA IV. |
| Neradova et al., 2022 [19] | Treatment of phosphate chelators with a high vitamin K2 content prevents vitamin K deficiency and attenuates the development of VC. |
| Van den Bergh et al., 2021 [21] | Endothelial cells could potentially function as an additional source of osteogenic progenitor cells in arterial calcification. |
| De Maré et al., 2022 [45] | Sclerostin induces a protective effect during vascular calcification in murine models of VC induced by renal failure or inhibition of MGP. |
| Van den Bergh et al., 2022 [20] | Endothelial dysfunction can contribute to the early stages of development of medial artery calcification. |
| Opdebeeck et al., 2023 [30] | Inhibition of TNAP SBI-425 attenuated medial artery calcification in a rat model induced by warfarin. |
| Uto et al., 2021 [31] | The inhibition of lysyl oxidase (LOX) by β-aminopropionitrile (BAPN) attenuated medial artery calcification in rat models fed a diet containing warfarin and vitamin K1. |
| Fang et al., 2020 [44] | The mir-29b/TGF-β3 axis could play a regulatory role in the pathogenesis of VC. |
| Opdebeeck et al., 2020 [32] | TNAP SBI425 inhibitor significantly reduced aortic and arterial calcification in warfarin-induced VC models. |
| De Maré et al., 2019 [46] | The production of sclerostin in serum, aorta, and bone was investigated in rats with warfarin-induced VC. |
| Authors | Outcome Models In Vitro (Cell Culture) |
|---|---|
| Beazley K.E. et al., 2012 [58] | Inhibition of canonical β-catenin pathway or TG2 activity prevents warfarin-regulated calcification. |
| Beazley K.E. et al., 2013 [38] | New β-catenin-targeting strategies prevent VC induced by warfarin and identify quercetin as a potential therapeutic. |
| Beazley K.E. et al., 2013 [59] | Inhibition of the TG2/β-catenin signaling axis seems to prevent warfarin-induced elastocalcinosis and to control isolated systolic hypertension. |
| Zhiyu H. et al., 2003 [60] | Adrenomedullin and PTHrP inhibited VSMC calcification partially through the cAMP/PKA pathway, whereas CNP inhibited VSMC calcification through the cGMP/PKG pathway. |
| Shanahan C Met. et al., 1998 [61] | Several other Gla-containing proteins with the potential to regulate or perhaps contribute to VC are present in the human vasculature. |
| Sheng Ying Wu et al., 2003 [62] | Endothelin might be involved in the pathogenesis of vascular calcification. |
| Trion A. et al., 2004 [26] | VSMCs contribute to the development of an atherosclerotic lesion by migration, proliferation, and secretion of matrix components. |
| Liu Y. et al., 2013 [63] | Prelamin A promotes VSMC calcification and aging by inducing persistent DNA damage signaling, which acts upstream of VSMC osteogenic differentiation and the senescence-associated secretory phenotype. |
| Son B.K. et al., 2012 [64] | TM is a novel molecule that promotes apoptosis and vascular calcification by regulation of Gas6, presumably via EGF receptors/ERK axes. |
| Rangrez AY et al., 2012 [65] | Results suggest that (i) high levels of Pi increase VSMC migration and calcification, (ii) altered expression levels of miR-223 could play a part in this process, and (iii) miR-223 is a potential new biomarker of VSMC damage. |
| Nie et al., 2019 [66] | Activation of the Wnt/β-catenin pathway regulates arterial calcification by activation of the OPG/RANKL system. |
| Seime et al., 2021 [67] | Proteoglycan 4 (PRG4) modulates the function of SMC and the osteogenic phenotype during vascular remodeling and intimal calcification. |
| Liu et al., 2021 [68] | EGB761 inhibited vascular calcification and osteogenic differentiation by suppressing the BMP2/Smad1/5/Runx2 signaling pathway. |
| Wei et al., 2020 [69] | Warfarin can induce senescence of vascular cells and contribute to the spread of vascular inflammation and oxidative stress via SASP. |
| Coronary Artery Disease | Source | Finding |
|---|---|---|
| miR-133a, miR-208a, miR-146a/b, miR34a, miR-221, miR-222, miR-122, miR-370, miR-624 | Serum, peripheral blood mononuclear cells, plasma, and platelets | Level of expression: increased |
| miR-17, miR-21, miR-20a, miR92a, miR-27a, miR-22a, miR-126, miR-145, miR-155, miR221, miR-130a, miR208b, let-7d, miR-135a, miR147, let-7i, miR-140, miR-182, miR-181a | Serum, endothelial progenitor cell, peripheral blood mononuclear cell, monocytes | Level of expression: decreased |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siracusa, C.; Carino, A.; Carabetta, N.; Manica, M.; Sabatino, J.; Cianflone, E.; Leo, I.; Strangio, A.; Torella, D.; De Rosa, S. Mechanisms of Cardiovascular Calcification and Experimental Models: Impact of Vitamin K Antagonists. J. Clin. Med. 2024, 13, 1405. https://doi.org/10.3390/jcm13051405
Siracusa C, Carino A, Carabetta N, Manica M, Sabatino J, Cianflone E, Leo I, Strangio A, Torella D, De Rosa S. Mechanisms of Cardiovascular Calcification and Experimental Models: Impact of Vitamin K Antagonists. Journal of Clinical Medicine. 2024; 13(5):1405. https://doi.org/10.3390/jcm13051405
Chicago/Turabian StyleSiracusa, Chiara, Annarita Carino, Nicole Carabetta, Marzia Manica, Jolanda Sabatino, Eleonora Cianflone, Isabella Leo, Antonio Strangio, Daniele Torella, and Salvatore De Rosa. 2024. "Mechanisms of Cardiovascular Calcification and Experimental Models: Impact of Vitamin K Antagonists" Journal of Clinical Medicine 13, no. 5: 1405. https://doi.org/10.3390/jcm13051405
APA StyleSiracusa, C., Carino, A., Carabetta, N., Manica, M., Sabatino, J., Cianflone, E., Leo, I., Strangio, A., Torella, D., & De Rosa, S. (2024). Mechanisms of Cardiovascular Calcification and Experimental Models: Impact of Vitamin K Antagonists. Journal of Clinical Medicine, 13(5), 1405. https://doi.org/10.3390/jcm13051405








