Cardio-Hepatic Interaction in Cardiac Amyloidosis
Abstract
:1. Introduction
- (i).
- Non-invasively characterise and model secondary liver affection (dynamic hepatic function and tissue elasticity) in patients with CA;
- (ii).
- Evaluate the diagnostic and prognostic utility of quantitative dynamic liver function tests and vibration-controlled transient elastography (VCTE) regarding mortality.
2. Materials and Methods
2.1. Study Population
2.2. Independent Reference Groups for Microsomal Liver Function
2.3. Statistical Model Development and Analysis
- yit—log stiffness;
- xit—core variables (septum thickness, NT-proBNP, dilatation of liver veins, PDRpeak and FIB-4clean);
- cit—high-dimensional control variables;
- βj—coefficients of the variables of interest;
- δk—coefficients of the high-dimensional control variables;
- uit—disturbance term;
- J, K—number of variables, K can become very large.
3. Results
3.1. Characterisation of the Cardiac Function within the Cohort
3.2. Characterisation of Hepatic Affection in CA
3.3. Effect of Cardiac Congestion on Microsomal Liver Function Expressed by PDRpeak
3.4. Chronic Liver Damage and Fibrosis in Cardiac Amyloidosis
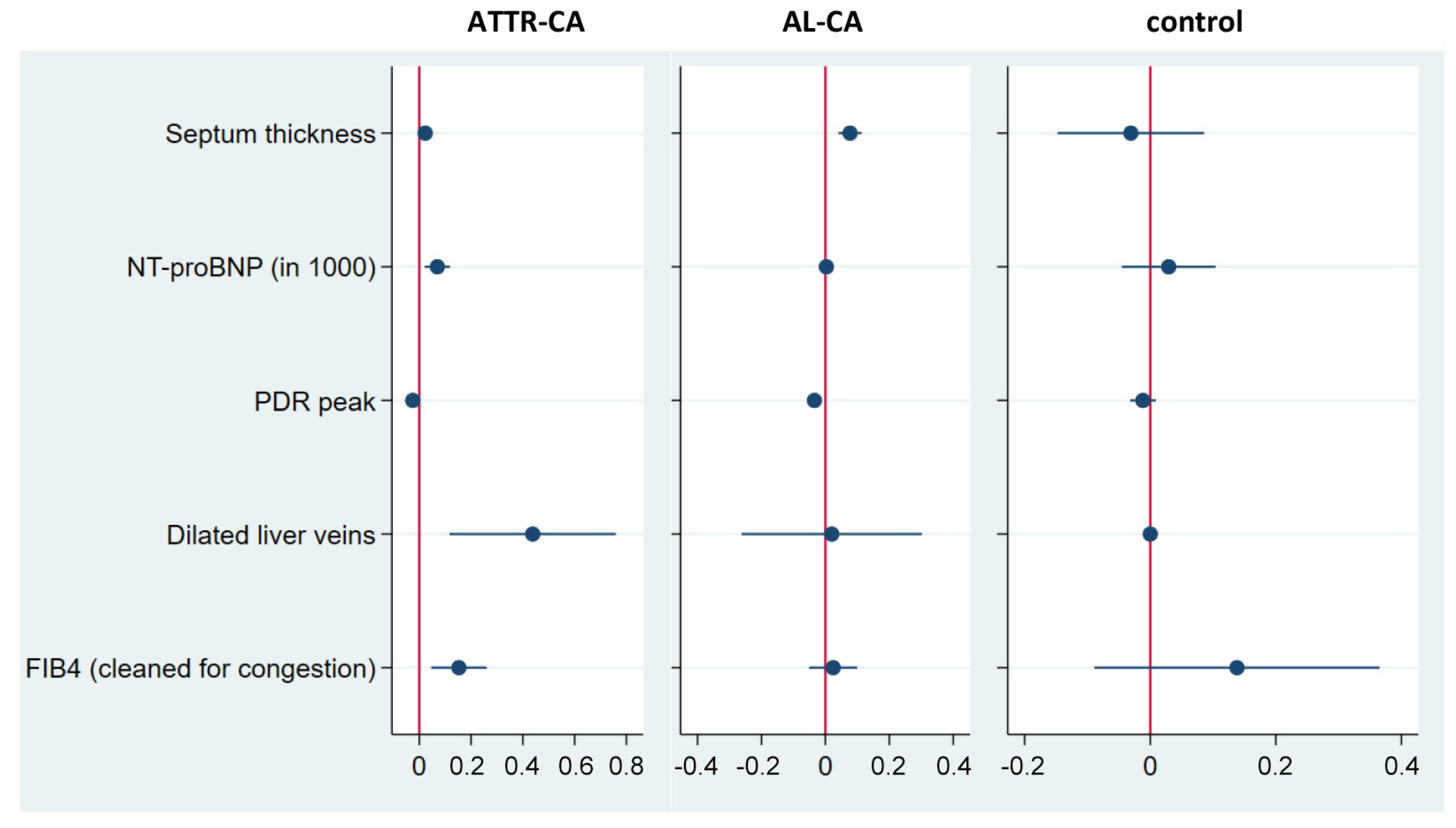
3.5. Modelling Liver Affection in Cardiac Amyloidosis
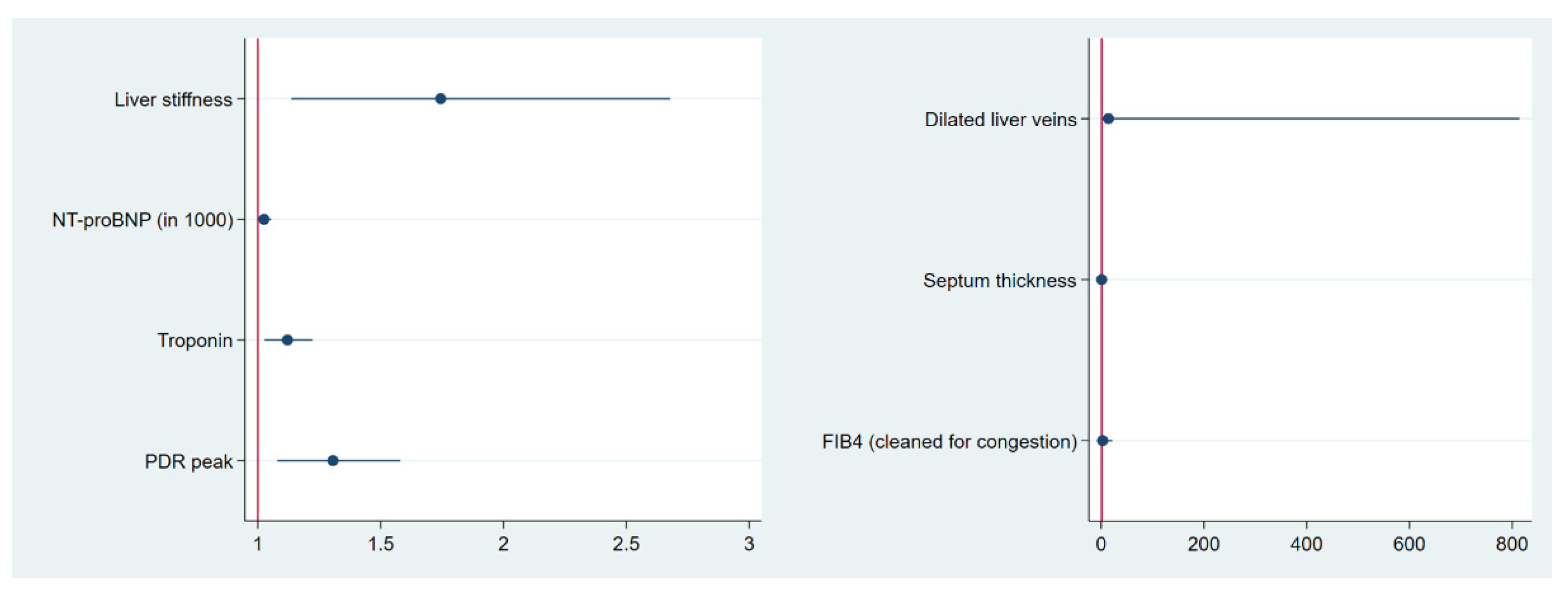
3.6. Predictive Value of the Main Influencing Factors Regarding All-Cause Mortality
4. Discussion
- A significant proportion of ATTR-CA patients (7%) in our sample present with AP elevation and therefore formally fulfil the criteria for liver involvement according to Gertz et al., 2005 [11].
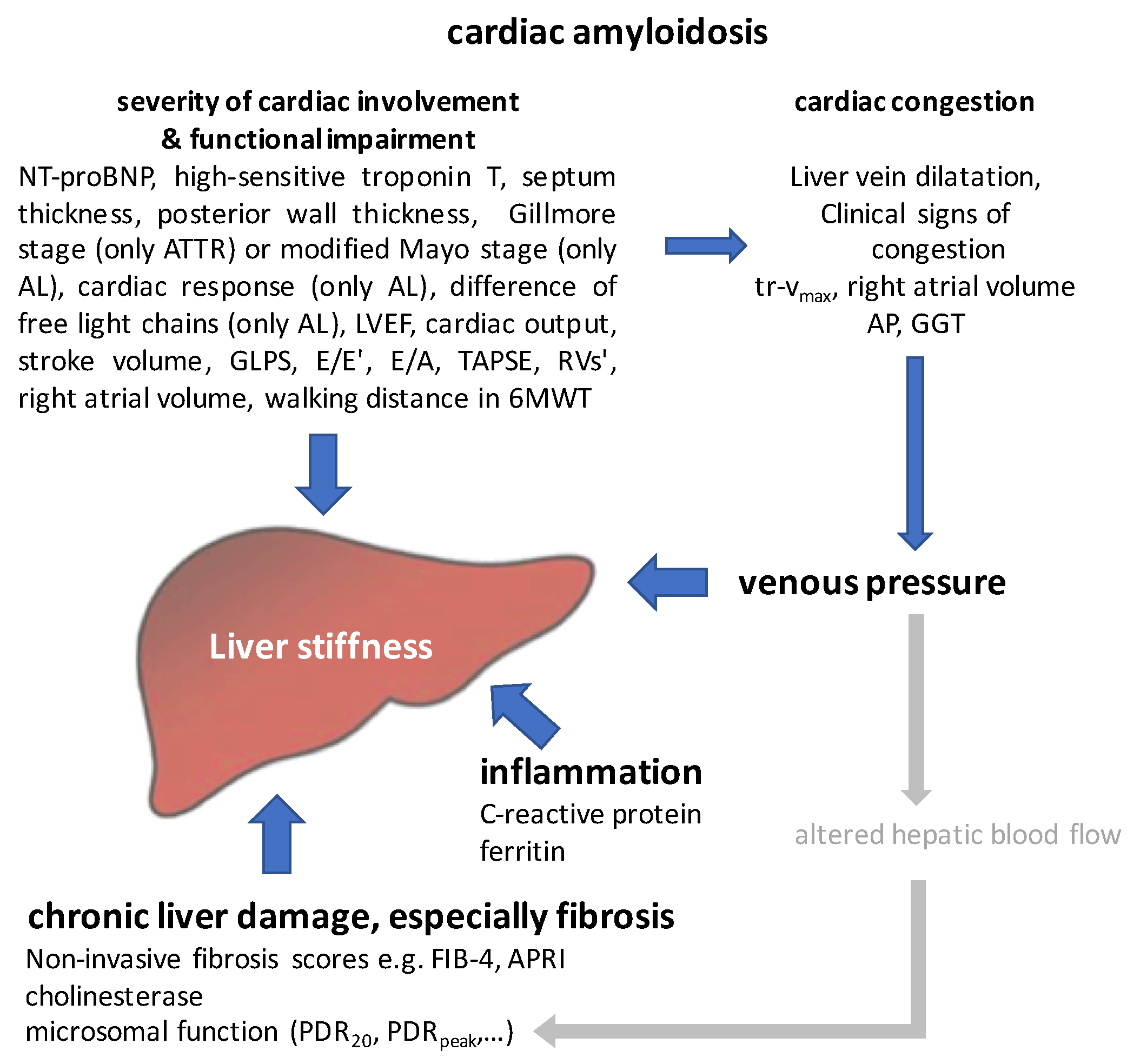
- Secondary liver affection in ATTR-CA results in decreased microsomal liver function related to hepatic congestion.
- Reduced PDRpeak in AL-CA may result from altered pharmacokinetics due to changed hepatic blood flow.
- Liver stiffness may act as a summatory surrogate for liver affection going beyond fibrosis and also reflects impaired cardiac function in CA, hypervolemia and congestion. Based on this, we were able to model the interaction between liver and heart in ATTR-CA and AL-CA: septum thickness, NT-proBNP and PDRpeak have been identified as predictors of liver stiffness for both entities. The dilatation of liver veins and FIB-4clean are significant predictors only in ATTR-CA.
- Liver stiffness, high-sensitive troponin, NT-proBNP and PDRpeak are predictors of all-cause mortality, suggesting them as promising factors for risk stratification in cardiac amyloidosis.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AL amyloidosis | systemic light chain amyloidosis |
| AL-CA | cardiac light chain amyloidosis |
| ALF | acute liver failure |
| ALT | alanine aminotransferase (=GPT) |
| AP | alkaline phosphatase |
| APRI | AST to platelet ratio index |
| AST | aspartate aminotransferase (=GOT) |
| ATTR amyloidosis | transthyretin amyloidosis |
| ATTR-CA | cardiac transthyretin amyloidosis |
| AUC | area under the curve |
| CA | cardiac amyloidosis |
| COX survival regression | Cox proportional hazard regression |
| CYP1A2 | cytochrome P450 1A2 |
| ECOG Performance Status | Eastern Cooperative Oncology Group Performance Status |
| eGFR | estimated glomerular filtration rate |
| FIB-4 (score) | fibrosis-4 (score) |
| FIB-4clean | fibrosis-4 (score) cleaned for its statistical component related to congestion by regressing the score on NT-proBNP and liver vein congestion |
| GGT | gamma-glutamyl transferase |
| GLDH | glutamate dehydrogenase |
| GLPS | global longitudinal strain |
| GOT | aspartate aminotransferase (AST) |
| GPT | alanine aminotransferase (ALT) |
| HCV | hepatitis C virus |
| HFpEF | heart failure with preserved ejection fraction |
| IQR | interquartile range |
| IQR med | IQR/median |
| kPA | kilopascal |
| LASSO | least absolute shrinkage and selection operator regression |
| LVEF | left ventricular ejection fraction |
| LSS | Ludwig’s staging system |
| MAPK pathway | mitogen-activated protein kinase pathway |
| MBT | 13C-methacetin breath test |
| ML | machine learn |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| NYHA | New York Heart Association |
| PBC | primary biliary cholangitis |
| PDRpeak | maximal percentage of dose rate |
| PDR20 | percentage of dose rate at 20 min |
| PK model | pharmacokinetic model |
| posterior wall | left ventricular posterior wall |
| septum | interventricular septum |
| TAPSE | tricuspid annular plane systolic excursion |
| tr-vmax | maximal tricuspid regurgitation velocity |
| ULN | upper limit of normal |
| VTCE | vibration-controlled transient elastography |
| 6MWT | 6 min walk test |
References
- Ihne, S.; Morbach, C.; Obici, L.; Palladini, G.; Stork, S. Amyloidosis in Heart Failure. Curr. Heart Fail. Rep. 2019, 16, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Ihne, S.; Morbach, C.; Sommer, C.; Geier, A.; Knop, S.; Störk, S. Amyloidosis—The diagnosis and treatment of an underdiagnosed disease. Dtsch. Arztebl. Int. 2020, 117, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Sachchithanantham, S.; Milani, P.; Gillmore, J.; Foli, A.; Lachmann, H.; Basset, M.; Hawkins, P.; Merlini, G.; Wechalekar, A.D. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood 2015, 126, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Grogan, M.; Dispenzieri, A. Natural history and therapy of AL cardiac amyloidosis. Heart Fail. Rev. 2015, 20, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Grogan, M.; Scott, C.G.; Kyle, R.A.; Zeldenrust, S.R.; Gertz, M.A.; Lin, G.; Klarich, K.W.; Miller, W.L.; Maleszewski, J.J.; Dispenzieri, A. Natural History of Wild-Type Transthyretin Cardiac Amyloidosis and Risk Stratification Using a Novel Staging System. J. Am. Coll. Cardiol. 2016, 68, 1014–1020. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Damy, T.; Fontana, M.; Hutchinson, M.; Lachmann, H.J.; Martinez-Naharro, A.; Quarta, C.C.; Rezk, T.; Whelan, C.J.; Gonzalez-Lopez, E.; et al. A new staging system for cardiac transthyretin amyloidosis. Eur. Heart J. 2017, 39, 2799–2806. [Google Scholar] [CrossRef]
- Mishra, S.; Guan, J.; Plovie, E.; Seldin, D.C.; Connors, L.H.; Merlini, G.; Falk, R.H.; MacRae, C.A.; Liao, R. Human amyloidogenic light chain proteins result in cardiac dysfunction, cell death, and early mortality in zebrafish. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H95–H103. [Google Scholar] [CrossRef]
- Mishra, S.; Joshi, S.; Ward, J.E.; Buys, E.P.; Mishra, D.; Mishra, D.; Morgado, I.; Fisch, S.; Lavatelli, F.; Merlini, G.; et al. Zebrafish model of amyloid light chain cardiotoxicity: Regeneration versus degeneration. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1158–H1166. [Google Scholar] [CrossRef]
- Shi, J.; Guan, J.; Jiang, B.; Brenner, D.A.; Del Monte, F.; Ward, J.E.; Connors, L.H.; Sawyer, D.B.; Semigran, M.J.; Macgillivray, T.E.; et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 4188–4193. [Google Scholar] [CrossRef]
- Milani, P.; Merlini, G.; Palladini, G. Light Chain Amyloidosis. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018022. [Google Scholar] [CrossRef]
- Gertz, M.A.; Comenzo, R.; Falk, R.H.; Fermand, J.P.; Hazenberg, B.P.; Hawkins, P.N.; Merlini, G.; Moreau, P.; Ronco, P.; Sanchorawala, V.; et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis. Am. J. Hematol. 2005, 79, 319–328. [Google Scholar] [CrossRef]
- Kaswala, D.H.; Lai, M.; Afdhal, N.H. Fibrosis Assessment in Nonalcoholic Fatty Liver Disease (NAFLD) in 2016. Dig. Dis. Sci. 2016, 61, 1356–1364. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- National Guideline Centre (UK). Non-Alcoholic Fatty Liver Disease: Assessment and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2016. [Google Scholar]
- Holzhutter, H.G.; Lock, J.F.; Taheri, P.; Bulik, S.; Goede, A.; Stockmann, M. Assessment of hepatic detoxification activity: Proposal of an improved variant of the (13)c-methacetin breath test. PLoS ONE 2013, 8, e70780. [Google Scholar] [CrossRef]
- Keller, J.; Hammer, H.F.; Afolabi, P.R.; Benninga, M.; Borrelli, O.; Dominguez-Munoz, E.; Dumitrascu, D.; Goetze, O.; Haas, S.L.; Hauser, B.; et al. European guideline on indications, performance and clinical impact of (13) C-breath tests in adult and pediatric patients: An EAGEN, ESNM, and ESPGHAN consensus, supported by EPC. United Eur. Gastroenterol. J. 2021, 9, 598–625. [Google Scholar] [CrossRef] [PubMed]
- Ozercan, A.M.; Ozkan, H. Vibration-controlled Transient Elastography in NAFLD: Review Study. Eur. J. Hepatogastroenterol. 2022, 12 (Suppl. S1), S41–S45. [Google Scholar] [CrossRef]
- Millonig, G.; Friedrich, S.; Adolf, S.; Fonouni, H.; Golriz, M.; Mehrabi, A.; Stiefel, P.; Poschl, G.; Buchler, M.W.; Seitz, H.K.; et al. Liver stiffness is directly influenced by central venous pressure. J. Hepatol. 2010, 52, 206–210. [Google Scholar] [CrossRef]
- Millonig, G.; Reimann, F.M.; Friedrich, S.; Fonouni, H.; Mehrabi, A.; Buchler, M.W.; Seitz, H.K.; Mueller, S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology 2008, 48, 1718–1723. [Google Scholar] [CrossRef]
- Berzigotti, A.; De Gottardi, A.; Vukotic, R.; Siramolpiwat, S.; Abraldes, J.G.; Garcia-Pagan, J.C.; Bosch, J. Effect of meal ingestion on liver stiffness in patients with cirrhosis and portal hypertension. PLoS ONE 2013, 8, e58742. [Google Scholar] [CrossRef]
- Potthoff, A.; Attia, D.; Pischke, S.; Kirschner, J.; Mederacke, I.; Wedemeyer, H.; Manns, M.P.; Gebel, M.J.; Rifai, K. Influence of different frequencies and insertion depths on the diagnostic accuracy of liver elastography by acoustic radiation force impulse imaging (ARFI). Eur. J. Radiol. 2013, 82, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Cales, P. Clinical interpretation of Fibroscan(R) results: A real challenge. Liver Int. 2010, 30, 1400–1402. [Google Scholar] [CrossRef]
- Goetze, O.; Breuer, M.; Geier, A.; Fried, M.; Weber, A.; Jochum, W.; Ilan, Y.; Mullhaupt, B. The 13C-methactin breath test is non-inferior to liver biopsy in predicting liver-related death and transplantation: A 7-year prospective follow-up study in 132 patients with chronic hepatitis C infection. GastroHep 2020, 2, 344–350. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Candelli, M.; Di Francesco, D.; Ciocca, F.; Taglieri, G.; Armuzzi, A.; Gasbarrini, G.; Gasbarrini, A. Study of liver function in healthy elderly subjects using the 13C-methacetin breath test. Aliment. Pharmacol. Ther. 2003, 17, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Pfaffenbach, B.; Gotze, O.; Szymanski, C.; Hagemann, D.; Adamek, R.J. The 13C-methacetin breath test for quantitative noninvasive liver function analysis with an isotope-specific nondispersive infrared spectrometer in liver cirrhosis. Dtsch. Med. Wochenschr. 1998, 123, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Lalazar, G.; Pappo, O.; Hershcovici, T.; Hadjaj, T.; Shubi, M.; Ohana, H.; Hemed, N.; Ilan, Y. A continuous 13C methacetin breath test for noninvasive assessment of intrahepatic inflammation and fibrosis in patients with chronic HCV infection and normal ALT. J. Viral. Hepat. 2008, 15, 716–728. [Google Scholar] [CrossRef]
- Kochel-Jankowska, A.; Hartleb, M.; Jonderko, K.; Kaminska, M.; Kasicka-Jonderko, A. 13C-methacetin breath test correlates with clinical indices of liver disease severity in patients with primary biliary cirrhosis. J. Physiol. Pharmacol. 2013, 64, 27–33. [Google Scholar]
- Fontana, R.J.; Stravitz, R.T.; Durkalski, V.; Hanje, J.; Hameed, B.; Koch, D.; Reuben, A.; Ganger, D.; Olson, J.; Liou, I.; et al. Prognostic Value of the (13) C-Methacetin Breath Test in Adults with Acute Liver Failure and Non-acetaminophen Acute Liver Injury. Hepatology 2021, 74, 961–972. [Google Scholar] [CrossRef]
- Vranova, J.; Hendrichova, M.; Kolarova, H.; Kratka, K.; Rosina, J.; Horak, J. (1)(3)C-methacetin breath test in the evaluation of disease severity in patients with liver cirrhosis. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2013, 157, 392–400. [Google Scholar] [CrossRef]
- Mueller, S.; Sandrin, L. Liver stiffness: A novel parameter for the diagnosis of liver disease. Hepat. Med. 2010, 2, 49–67. [Google Scholar] [CrossRef]
- Belloni, A.; Chernozhukov, V.; Hansen, C. Inference in High-Dimensional Panel Models With an Application to Gun Control. J. Bus. Econ. Stat. 2016, 34, 590–605. [Google Scholar] [CrossRef]
- Lane, E.A.; Parashos, I. Drug pharmacokinetics and the carbon dioxide breath test. J. Pharmacokinet. Biopharm. 1986, 14, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Brunger, A.F.; van Rheenen, R.; Gans, R.O.B.; Hazenberg, B.P.C.; Nienhuis, H.L.A. How well does liver span as part of the consensus criteria for liver involvement in AL amyloidosis perform? Amyloid 2023, 30, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Malek, F.; Hendrichova, M.; Kratka, K.; Sedlakova, M.; Vranova, J.; Horak, J. Correlation of the functional liver mass with left ventricular ejection fraction and left atrial diameter in patients with congestive heart failure. Int. J. Cardiol. 2008, 127, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Hendrichova, M.; Malek, F.; Koprivova, H.; Vranova, J.; Ostadal, P.; Kratka, K.; Sedlakova, M.; Horak, J. Correlation of NT-proBNP with metabolic liver function as assessed with (13)C-methacetin breath test in patients with acute decompensated heart failure. Int. J. Cardiol. 2010, 144, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Law, S.; Petrie, A.; Chacko, L.; Cohen, O.C.; Ravichandran, S.; Gilbertson, J.A.; Rowczenio, D.; Wechalekar, A.; Martinez-Naharro, A.; Lachmann, H.J.; et al. Disease progression in cardiac transthyretin amyloidosis is indicated by serial calculation of National Amyloidosis Centre transthyretin amyloidosis stage. ESC Heart Fail. 2020, 7, 3942–3949. [Google Scholar] [CrossRef]
- Palladini, G.; Lavatelli, F.; Russo, P.; Perlini, S.; Perfetti, V.; Bosoni, T.; Obici, L.; Bradwell, A.R.; D’Eril, G.M.; Fogari, R.; et al. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood 2006, 107, 3854–3858. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.K.; Levy, W.C.; Vasbinder, A.; Teruya, S.; De Los Santos, J.; Leedy, D.; Maurer, M.S. Diuretic Dose and NYHA Functional Class Are Independent Predictors of Mortality in Patients With Transthyretin Cardiac Amyloidosis. JACC CardioOncol 2020, 2, 414–424. [Google Scholar] [CrossRef]
- Brons, M.; Muller, S.A.; Rutten, F.H.; van der Meer, M.G.; Vrancken, A.; Minnema, M.C.; Baas, A.F.; Asselbergs, F.W.; Oerlemans, M. Evaluation of the cardiac amyloidosis clinical pathway implementation: A real-world experience. Eur. Heart J. Open 2022, 2, oeac011. [Google Scholar] [CrossRef]
- Tini, G.; Milani, P.; Zampieri, M.; Caponetti, A.G.; Fabris, F.; Foli, A.; Argiro, A.; Mazzoni, C.; Gagliardi, C.; Longhi, S.; et al. Diagnostic pathways to wild-type transthyretin amyloid cardiomyopathy: A multicentre network study. Eur. J. Heart Fail. 2023, 25, 845–853. [Google Scholar] [CrossRef]

| ATTR-CA | AL-CA | Control | ATTR-CA vs. Control | AL-CA vs. Control | ATTR-CA vs. AL-CA | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n patients | 26 | 17 | 31 | ||||||||||||||||
| n observations | 60 | 52 | 65 | ||||||||||||||||
| n | mean | SD | n | mean | SD | n | mean | SD | z | p value | z | p value | z | p value | |||||
| age [years] | 26 | 74.9 | ± | 7.2 | 17 | 64.0 | ± | 8.1 | 31 | 63.1 | ± | 11.6 | 4.6781 | *** | 0.3162 | n.s. | 4.500 | *** | |
| sex | male | 26 | 84.6% | 17 | 41.2% | 31 | 54.8% | ||||||||||||
| female | 26 | 15.4% | 17 | 58.8% | 31 | 45.2% | |||||||||||||
| ECOG | 26 | 0.5 | ± | 0.5 | 16 | 0.5 | ± | 0.7 | 30 | 0.2 | ± | 0.5 | 2.0418 | * | 1.5588 | n.s. | −0.061 | n.s. | |
| cardiac comorbidity | 26 | 76.9% | ± | 43.0% | 17 | 52.9% | ± | 51.4% | 31 | 45.2% | ± | 50.6% | 2.5631 | * | 0.5040 | n.s. | 1.593 | n.s. | |
| hepatic comorbidity | 26 | 19.2% | ± | 40.2% | 16 | 12.5% | ± | 34.2% | 31 | 22.6% | ± | 42.5% | −0.3053 | n.s. | −0.8801 | n.s. | 0.579 | n.s. | |
| number of involved organs by amyloidosis | 26 | 1.6 | ± | 0.5 | 17 | 2.0 | ± | 1.3 | 31 | 1.2 | ± | 0.5 | 2.7619 | ** | 2.4877 | * | −1.303 | n.s. | |
| severity of cardiac amyloidosis # | |||||||||||||||||||
| stage I | 15 | 58% | 1 | 6% | |||||||||||||||
| stage II | 8 | 31% | 5 | 29% | |||||||||||||||
| stage III | 3 | 12% | 11 | 65% | |||||||||||||||
| NYHA | I | 6 | 23.1% | 6 | 28.6% | 18 | 58.1% | ||||||||||||
| II | 8 | 30.8% | 8 | 38.1% | 9 | 29.0% | |||||||||||||
| III | 12 | 46.2% | 6 | 28.6% | 4 | 12.9% | |||||||||||||
| IV | 0 | 0.0% | 1 | 4.8% | 0 | 0.0% | |||||||||||||
| rhythm | sinus rhythm | 26 | 53.8% | ± | 50.8% | 17 | 82.4% | ± | 39.3% | 31 | 90.3% | ± | 30.1% | −3.2173 | ** | −0.7276 | n.s. | −2.067 | * |
| atrial fibrillation | 26 | 38.5% | ± | 49.6% | 17 | 17.6% | ± | 39.3% | 31 | 9.7% | ± | 30.1% | 2.5869 | ** | 0.7276 | n.s. | 1.528 | n.s. | |
| pacemaker rhythm | 26 | 7.7% | ± | 27.2% | 17 | 0.0% | ± | 0.0% | 31 | 0.0% | ± | 0.0% | 1.4434 | n.s. | N/A | n.s. | 1.443 | n.s. | |
| NT-proBNP [pg/mL] | 26 | 2782.6 | ± | 2290.8 | 17 | 9601.3 | ± | 13,988.5 | 31 | 320.8 | ± | 552.4 | 5.3507 | *** | 2.7343 | ** | −1.992 | * | |
| troponin [pg/mL] | 26 | 49.1 | ± | 24.9 | 17 | 99.9 | ± | 88.9 | 31 | 11.5 | ± | 7.8 | 7.3875 | *** | 4.0893 | *** | −2.299 | * | |
| eGFRMDRD [mL/min] | 26 | 64.8 | ± | 18.9 | 17 | 58.9 | ± | 29.8 | 31 | 75.8 | ± | 19.3 | −2.1583 | * | −2.1125 | * | 0.735 | n.s. | |
| septum [mm] | 26 | 16.7 | ± | 3.7 | 17 | 13.5 | ± | 2.6 | 31 | 10.0 | ± | 1.4 | 8.6865 | *** | 5.0339 | *** | 3.310 | *** | |
| posterior wall [mm] | 26 | 13.9 | ± | 2.6 | 17 | 11.8 | ± | 2.1 | 31 | 9.2 | ± | 1.5 | 8.2203 | *** | 4.4663 | *** | 2.956 | ** | |
| LVEF [%] | 26 | 54.3 | ± | 12.4 | 17 | 62.1 | ± | 11.4 | 31 | 63.5 | ± | 5.5 | −3.5077 | *** | −0.4557 | n.s. | −2.139 | * | |
| cardiac output [L/min] | 23 | 4.3 | ± | 1.5 | 13 | 6.2 | ± | 2.7 | 29 | 5.4 | ± | 1.2 | −2.9021 | *** | 1.0297 | −2.338 | ** | ||
| stroke volume [mL] | 23 | 66.1 | ± | 21.5 | 15 | 78.6 | ± | 38.0 | 30 | 82.8 | ± | 22.4 | −2.7577 | ** | −0.3982 | n.s. | −1.158 | n.s. | |
| GLPS [%] | 24 | −11.3 | ± | 3.3 | 16 | −13.4 | ± | 4.1 | 30 | −18.3 | ± | 2.3 | 8.7357 | *** | 4.5080 | *** | 1.641 | n.s. | |
| apical sparing | 26 | 100.0% | ± | 100.0% | 17 | 88.2% | ± | 66.8% | 31 | 45.2% | ± | 49.4% | 2.5475 | * | 2.3320 | n.s. | 0.463 | n.s. | |
| E/A | 13 | 1.9 | ± | 1.4 | 13 | 2.2 | ± | 1.0 | 27 | 1.0 | ± | 0.3 | 2.2190 | * | 4.0794 | *** | −0.677 | n.s. | |
| E/E’ | 22 | 15.5 | ± | 6.5 | 16 | 15.9 | ± | 7.5 | 30 | 8.6 | ± | 2.1 | 4.8143 | *** | 3.8022 | *** | −0.156 | n.s. | |
| tr-vmax [m/s] | 18 | 3.0 | ± | 0.4 | 15 | 2.8 | ± | 0.3 | 19 | 2.4 | ± | 0.3 | 3.9130 | *** | 3.4562 | *** | 0.916 | n.s. | |
| % normal | 8 | 44% | 5 | 33% | 17 | 89% | |||||||||||||
| % pathologic (>2.8) | 10 | 56% | 10 | 67% | 2 | 11% | |||||||||||||
| diastolic dysfunction | 26 | 73.1% | ± | 45.2% | 17 | 76.5% | ± | 43.7% | 31 | 12.9% | ± | 34.1% | 5.5832 | *** | 5.1917 | *** | −0.245 | n.s. | |
| acute heart failure according to ESC criteria | 26 | 69.2% | ± | 47.1% | 17 | 76.5% | ± | 43.7% | 31 | 67.7% | ± | 47.5% | 0.1184 | n.s. | 0.6412 | n.s. | −0.515 | n.s. | |
| walking distance in 6 min walking test [m] | 23 | 367.3 | ± | 84.9 | 14 | 374.3 | ± | 112.7 | 23 | 402.6 | ± | 111.1 | −1.2095 | n.s. | −0.7453 | n.s. | −0.199 | ||
| total bilirubin [mg/dL] | 26 | 0.8 | ± | 0.3 | 17 | 0.6 | ± | 0.3 | 31 | 0.5 | ± | 0.2 | 5.3905 | *** | 1.8334 | p < 0.1 | 1.906 | p < 0.1 | |
| AP [U/L] | 26 | 85.8 | ± | 41.0 | 17 | 74.8 | ± | 22.7 | 31 | 69.9 | ± | 17.3 | 1.8448 | p < 0.1 | 0.7626 | n.s. | 1.136 | n.s. | |
| GGT [U/L] | 26 | 94.8 | ± | 92.4 | 17 | 47.6 | ± | 44.3 | 31 | 30.6 | ± | 14.8 | 3.5049 | *** | 1.5370 | n.s. | 2.238 | * | |
| AST [U/L] | 26 | 34.9 | ± | 11.7 | 17 | 29.8 | ± | 15.2 | 31 | 25.0 | ± | 7.8 | 3.6853 | *** | 1.2144 | n.s. | 1.179 | n.s. | |
| ALT [U/L] | 26 | 30.3 | ± | 13.8 | 17 | 38.4 | ± | 57.7 | 31 | 26.4 | ± | 13.8 | 1.0577 | n.s. | 0.8492 | n.s. | −0.575 | n.s. | |
| GLDH [U/L] | 25 | 4.8 | ± | 3.5 | 17 | 5.1 | ± | 6.1 | 31 | 4.1 | ± | 7.1 | 0.4327 | n.s. | 0.4931 | n.s. | −0.204 | n.s. | |
| cholinesterase [U/L] | 25 | 6944.7 | ± | 1615.3 | 17 | 6287.8 | ± | 2098.8 | 31 | 8352.9 | ± | 1850.7 | −3.0381 | ** | −3.3968 | *** | 1.090 | n.s. | |
| serum albumin [g/dL] | 26 | 4.5 | ± | 0.2 | 17 | 3.9 | ± | 0.7 | 31 | 4.3 | ± | 0.8 | 1.2566 | n.s. | −1.6510 | p < 0.1 | 3.209 | ** | |
| liver vein dilatation (%) | 26 | 11.5% | 15 | 6.6% | 30 | 0.0% | 5.0990 | * | 0.9904 | n.s. | 0.696 | n.s. | |||||||
| FIB−4 | 26 | 2.77 | ± | 0.96 | 17 | 2.34 | ± | 1.84 | 31 | 1.37 | ± | 0.60 | 6.4743 | *** | 2.1131 | ** | 0.896 | n.s. | |
| PDRpeak [%] | 22 | 25.9 | ± | 7.1 | 13 | 24.5 | ± | 5.6 | 27 | 31.3 | ± | 15.2 | −1.6334 | n.s. | −2.0640 | * | 0.677 | n.s. | |
| stiffness [kPa] | 20 | 7.9 | ± | 4.5 | 11 | 5.0 | ± | 2.0 | 24 | 5.5 | ± | 3.3 | 1.9779 | * | −0.6534 | n.s. | 2.533 | * | |
| IQR med [%] | 20 | 24.6 | ± | 14.0 | 10 | 16.3 | ± | 3.8 | 21 | 22.4 | ± | 8.9 | 0.5752 | n.s. | −2.6804 | ** | 2.453 | * | |
| NT-proBNP as congestion surrogate | |||
|---|---|---|---|
| PDRpeak HR (95% CI) | FIB-4 HR (95% CI) | ||
| n observations | 148 | 177 | |
| constant | 29.805 *** [25.646; 33.964] | 1.451 *** [1.218; 1.684] | |
| cardiac manifestation | ATTR-CA | −0.737 [−6.593; 5.118] | 1.352 *** [0.520; 2.185] |
| AL-CA | −5.849 ** [−10.460; −1.238] | 0.600 * [−0.053; 1.253] | |
| NT-proBNP # | overall | −1.726 ** [−3.445; −0.006] | 0.036 [−0.034; 0.107] |
| ATTR-CA | 0.787 [−1.216; 2.789] | −0.039 [−0.189; 0.112] | |
| AL-CA | 1.652 * [−0.070; 3.374] | −0.020 [−0.096; 0.057] | |
| liver vein dilation as congestion surrogate | |||
| n observations | 143 | 172 | |
| constant | 28.921 *** [25.182; 32.660] | 1.478 *** [1.255; 1.700] | |
| cardiac manifestation | ATTR-CA | −1.429 [−6.311; 3.453] | 1.326 *** [0.774; 1.878] |
| AL-CA | −5.130 ** [−9.533; −0.726] | 0.700 ** [0.034; 1.367] | |
| dilated liver veins | overall | −2.240 * [−4.738; 0.258] | −0.168 [−1.242; 0.907] |
| ATTR-CA | −6.021 ** [−11.390; −0.653] | 0.304 [−0.893; 1.501] | |
| AL-CA | ## | ## | |
| Model 1 | Model 2 | ||
|---|---|---|---|
| n observations | 76 | 101 | |
| n patients | 35 | 52 | |
| Core Variables | Log Liver Stiffness | Log Liver Stiffness | |
| septum thickness | control | −0.136 ** [−0.255; −0.016] | −0.032 [−0.149; 0.085] |
| ATTR-CA | 0.021 ** [0.002; 0.040] | 0.023 ** [0.002; 0.044] | |
| AL-CA | 0.055 *** [0.016; 0.094] | 0.077 *** [0.041; 0.113] | |
| NT-proBNP | control | 1.722 ** [0.165; 3.279] | 0.036 [−0.032; 0.105] |
| ATTR-CA | 0.069 *** [0.028; 0.111] | 0.070 *** [0.021; 0.118] | |
| AL-CA | 0.002 [−0.002; 0.007] | 0.003 [−0.001; 0.008] | |
| PDRpeak | control | −0.003 [−0.018; 0.012] | −0.014 [−0.033; 0.004] |
| ATTR-CA | −0.026 *** [−0.041; −0.011] | −0.025 *** [−0.040; −0.010] | |
| AL-CA | −0.031 *** [−0.048; −0.015] | −0.034 *** [−0.051; −0.017] | |
| dilated liver veins | control | # | # |
| ATTR-CA | 0.376 ** [0.064; 0.688] | 0.438 *** [0.117; 0.759] | |
| AL-CA | 0.066 [−0.229; 0.362] | 0.020 [−0.262; 0.302] | |
| FIB−4clean | control | 0.132 [−0.230; 0.494] | 0.179 [−0.205; 0.564] |
| ATTR-CA | 0.144 *** [0.043; 0.245] | 0.153 *** [0.046; 0.260] | |
| AL-CA | 0.011 [−0.064; 0.087] | 0.024 [−0.051; 0.099] | |
| LASSO-selected controls | AL-CA, ATTR-CA, AP, TAPSE | AL-CA, ATTR-CA, AP | |
| constant | 3.215 *** [1.544; 4.885] | 2.359 *** [0.994; 3.725] | |
| COX Analysis | 1 | 2 |
|---|---|---|
| HR [95% CI] | HR [95% CI] | |
| liver stiffness | 1.744 ** [1.136; 2.678] | |
| NT-proBNP (in 1000) | 1.025 * [0.997; 1.054] | |
| hs-TNT | 1.120 ** [1.027; 1.222] | |
| PDRpeak | 1.305 *** [1.079; 1.579] | |
| dilated liver veins | 14.101 [0.244; 813.970] | |
| septum thickness | 1.062 [0.876; 1.288] | |
| FIB-4clean | 2.798 [0.365; 21.425] | |
| n observations | 105 | 105 |
| Pseudo R2 | 0.642 | 0.272 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ihne-Schubert, S.M.; Goetze, O.; Gerstendörfer, F.; Sahiti, F.; Schade, I.; Papagianni, A.; Morbach, C.; Frantz, S.; Einsele, H.; Knop, S.; et al. Cardio-Hepatic Interaction in Cardiac Amyloidosis. J. Clin. Med. 2024, 13, 1440. https://doi.org/10.3390/jcm13051440
Ihne-Schubert SM, Goetze O, Gerstendörfer F, Sahiti F, Schade I, Papagianni A, Morbach C, Frantz S, Einsele H, Knop S, et al. Cardio-Hepatic Interaction in Cardiac Amyloidosis. Journal of Clinical Medicine. 2024; 13(5):1440. https://doi.org/10.3390/jcm13051440
Chicago/Turabian StyleIhne-Schubert, Sandra Michaela, Oliver Goetze, Felix Gerstendörfer, Floran Sahiti, Ina Schade, Aikaterini Papagianni, Caroline Morbach, Stefan Frantz, Hermann Einsele, Stefan Knop, and et al. 2024. "Cardio-Hepatic Interaction in Cardiac Amyloidosis" Journal of Clinical Medicine 13, no. 5: 1440. https://doi.org/10.3390/jcm13051440







