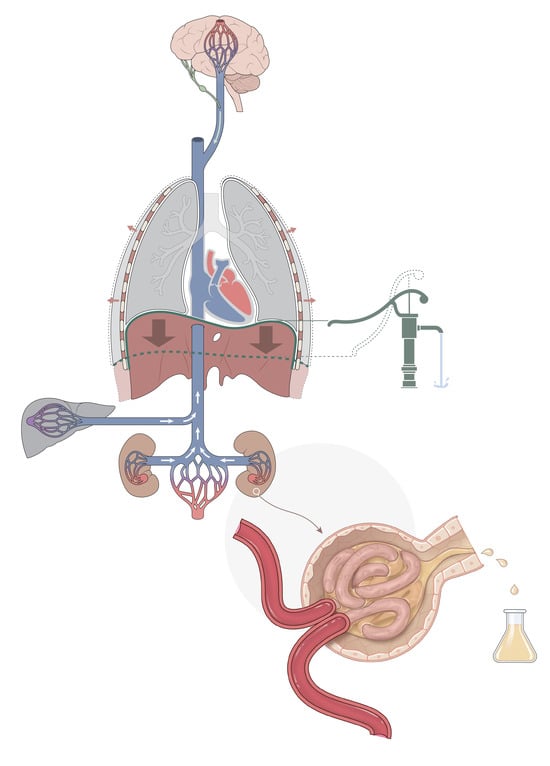
Controlled Mechanical Ventilation in Critically Ill Patients and the Potential Role of Venous Bagging in Acute Kidney Injury
Abstract
:1. Introduction
2. Mechanical Ventilation and AKI
3. Venous Congestion
4. Pros and Cons of PSV
5. Prerequisites for Safe PSV
6. The Brain and Delirium
7. Conclusions and the Future
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreno, A.H.; Burchell, A.R.; Van der Woude, R.; Burke, J.H. Respiratory Regulation of Splanchnic and Systemic Venous Return. Am. J. Physiol. 1967, 213, 455–465. [Google Scholar] [CrossRef]
- van den Akker, J.P.C.; Egal, M.; Groeneveld, A.B.J. Invasive Mechanical Ventilation as a Risk Factor for Acute Kidney Injury in the Critically Ill: A Systematic Review and Meta-Analysis. Crit. Care 2013, 17, R98. [Google Scholar] [CrossRef]
- Hepokoski, M.L.; Malhotra, A.; Singh, P.; Crotty Alexander, L.E. Ventilator-Induced Kidney Injury: Are Novel Biomarkers the Key to Prevention? Nephron 2018, 140, 90–93. [Google Scholar] [CrossRef]
- Ottolina, D.; Zazzeron, L.; Trevisi, L.; Agarossi, A.; Colombo, R.; Fossali, T.; Passeri, M.; Borghi, B.; Ballone, E.; Rech, R.; et al. Acute Kidney Injury (AKI) in Patients with COVID-19 Infection Is Associated with Ventilatory Management with Elevated Positive End-Expiratory Pressure (PEEP). J. Nephrol. 2022, 35, 99–111. [Google Scholar] [CrossRef]
- Seubert, M.E.; Goeijenbier, M. Early Patient-Triggered Pressure Support Breathing in Mechanically Ventilated Patients with COVID-19 May Be Associated with Lower Rates of Acute Kidney Injury. J. Clin. Med. 2023, 12, 1859. [Google Scholar] [CrossRef]
- Aufderheide, T.P.; Alexander, C.; Lick, C.; Myers, B.; Romig, L.; Vartanian, L.; Stothert, J.; McKnite, S.; Matsuura, T.; Yannopoulos, D.; et al. From Laboratory Science to Six Emergency Medical Services Systems: New Understanding of the Physiology of Cardiopulmonary Resuscitation Increases Survival Rates after Cardiac Arrest. Crit. Care Med. 2008, 36, S397–S404. [Google Scholar] [CrossRef]
- Vinje, V.; Ringstad, G.; Lindstrøm, E.K.; Valnes, L.M.; Rognes, M.E.; Eide, P.K.; Mardal, K.-A. Respiratory Influence on Cerebrospinal Fluid Flow—A Computational Study Based on Long-Term Intracranial Pressure Measurements. Sci. Rep. 2019, 9, 9732. [Google Scholar] [CrossRef]
- Kiviniemi, V.; Wang, X.; Korhonen, V.; Keinänen, T.; Tuovinen, T.; Autio, J.; Levan, P.; Keilholz, S.; Zang, Y.F.; Hennig, J.; et al. Ultra-Fast Magnetic Resonance Encephalography of Physiological Brain Activity—Glymphatic Pulsation Mechanisms? J. Cereb. Blood Flow Metab. 2016, 36, 1033–1045. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Giunta, F.; Suter, P.M.; Slutsky, A.S. Mechanical Ventilation as a Mediator of Multisystem Organ Failure in Acute Respiratory Distress Syndrome. JAMA 2000, 284, 43–44. [Google Scholar] [CrossRef]
- Husain-Syed, F.; Slutsky, A.S.; Ronco, C. Lung-Kidney Cross-Talk in the Critically Ill Patient. Am. J. Respir. Crit. Care Med. 2016, 194, 402–414. [Google Scholar] [CrossRef]
- Kuiper, J.W.; Groeneveld, A.B.J.; Slutsky, A.S.; Plötz, F.B. Mechanical Ventilation and Acute Renal Failure. Crit. Care Med. 2005, 33, 1408–1415. [Google Scholar] [CrossRef]
- Perez Nieto, O.R.; Wong, A.; Lopez Fermin, J.; Zamarron Lopez, E.I.; Meade Aguilar, J.A.; Deloya Tomas, E.; Carrion Moya, J.D.; Castillo Gutierrez, G.; Ramos, M.G.O.; García Montes, X.; et al. Aiming for Zero Fluid Accumulation: First, Do No Harm. Anaesthesiol. Intensive Ther. 2021, 53, 162–178. [Google Scholar] [CrossRef]
- Prowle, J.R.; Chua, H.-R.; Bagshaw, S.M.; Bellomo, R. Clinical Review: Volume of Fluid Resuscitation and the Incidence of Acute Kidney Injury—A Systematic Review. Crit. Care 2012, 16, 230. [Google Scholar] [CrossRef]
- Kwiatkowska, E.; Kwiatkowski, S.; Dziedziejko, V.; Tomasiewicz, I.; Domański, L. Renal Microcirculation Injury as the Main Cause of Ischemic Acute Kidney Injury Development. Biology 2023, 12, 327. [Google Scholar] [CrossRef]
- Li, X.; Liu, M.; Bedja, D.; Thoburn, C.; Gabrielson, K.; Racusen, L.; Rabb, H. Acute Renal Venous Obstruction Is More Detrimental to the Kidney than Arterial Occlusion: Implication for Murine Models of Acute Kidney Injury. Am. J. Physiol.-Ren. Physiol. 2012, 302, F519–F525. [Google Scholar] [CrossRef]
- Tamayo-Gutierrez, A.; Ibrahim, H.N. The Kidney in Heart Failure: The Role of Venous Congestion. Methodist DeBakey Cardiovasc. J. 2022, 18, 4–10. [Google Scholar] [CrossRef]
- Scagliola, R.; Brunelli, C. Venous Congestion and Systemic Hypoperfusion in Cardiorenal Syndrome: Two Sides of the Same Coin. Rev. Cardiovasc. Med. 2022, 23, 111. [Google Scholar] [CrossRef]
- Mullens, W.; Nijst, P. Cardiac Output and Renal Dysfunction: Definitely More Than Impaired Flow. J. Am. Coll. Cardiol. 2016, 67, 2209–2212. [Google Scholar] [CrossRef]
- Post, E.H.; Vincent, J.-L. Renal Autoregulation and Blood Pressure Management in Circulatory Shock. Crit. Care 2018, 22, 81. [Google Scholar] [CrossRef]
- Cruces, P.; Salas, C.; Lillo, P.; Salomon, T.; Lillo, F.; Hurtado, D.E. The Renal Compartment: A Hydraulic View. Intensive Care Med. Exp. 2014, 2, 26. [Google Scholar] [CrossRef]
- Vaara, S.T.; Ostermann, M.; Bitker, L.; Schneider, A.; Poli, E.; Hoste, E.; Fierens, J.; Joannidis, M.; Zarbock, A.; van Haren, F.; et al. Restrictive Fluid Management versus Usual Care in Acute Kidney Injury (REVERSE-AKI): A Pilot Randomized Controlled Feasibility Trial. Intensive Care Med. 2021, 47, 665–673. [Google Scholar] [CrossRef]
- Wiedemann, H.P.; Wheeler, A.P.; Bernard, G.R.; Thompson, B.T.; Hayden, D.; deBoisblanc, B.; Connors, A.F.J.; Hite, R.D.; Harabin, A.L. Comparison of Two Fluid-Management Strategies in Acute Lung Injury. N. Engl. J. Med. 2006, 354, 2564–2575. [Google Scholar] [CrossRef]
- Russell, P.S.; Hong, J.; Windsor, J.A.; Itkin, M.; Phillips, A.R.J. Renal Lymphatics: Anatomy, Physiology, and Clinical Implications. Front. Physiol. 2019, 10, 251. [Google Scholar] [CrossRef]
- Mårtensson, J.; Bellomo, R. Are All Fluids Bad for the Kidney? Curr. Opin. Crit. Care 2015, 21, 292–301. [Google Scholar] [CrossRef]
- Gelman, S. Venous Function and Central Venous Pressure: A Physiologic Story. Anesthesiology 2008, 108, 735–748. [Google Scholar] [CrossRef]
- Wolff, C.B.; Collier, D.J.; Shah, M.; Saxena, M.; Brier, T.J.; Kapil, V.; Green, D.; Lobo, M. A Discussion on the Regulation of Blood Flow and Pressure. Adv. Exp. Med. Biol. 2016, 876, 129–135. [Google Scholar] [CrossRef]
- King, A.J.; Fink, G.D. Chronic Low-Dose Angiotensin II Infusion Increases Venomotor Tone by Neurogenic Mechanisms. Hypertension 2006, 48, 927–933. [Google Scholar] [CrossRef]
- Putensen, C.; Zech, S.; Wrigge, H.; Zinserling, J.; Stüber, F.; Von Spiegel, T.; Mutz, N. Long-Term Effects of Spontaneous Breathing during Ventilatory Support in Patients with Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2001, 164, 43–49. [Google Scholar] [CrossRef]
- Magder, S. Heart-Lung Interaction in Spontaneous Breathing Subjects: The Basics. Ann. Transl. Med. 2018, 6, 348. [Google Scholar] [CrossRef]
- Magder, S.; Guerard, B. Heart-Lung Interactions and Pulmonary Buffering: Lessons from a Computational Modeling Study. Respir. Physiol. Neurobiol. 2012, 182, 60–70. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujino, Y.; Amato, M.B.P.; Kavanagh, B.P. Fifty Years of Research in ARDS. Spontaneous Breathing during Mechanical Ventilation. Risks, Mechanisms, and Management. Am. J. Respir. Crit. Care Med. 2017, 195, 985–992. [Google Scholar] [CrossRef]
- Reddi, B.A.J.; Carpenter, R.H.S. Venous Excess: A New Approach to Cardiovascular Control and Its Teaching. J. Appl. Physiol. 2005, 98, 356–364. [Google Scholar] [CrossRef]
- Tyberg, J.V. Venous Modulation of Ventricular Preload. Am. Heart J. 1992, 123, 1098–1104. [Google Scholar] [CrossRef]
- de Wit, F.; van Vliet, A.L.; de Wilde, R.B.; Jansen, J.R.; Vuyk, J.; Aarts, L.P.; de Jonge, E.; Veelo, D.P.; Geerts, B.F. The Effect of Propofol on Haemodynamics: Cardiac Output, Venous Return, Mean Systemic Filling Pressure, and Vascular Resistances. Br. J. Anaesth. 2016, 116, 784–789. [Google Scholar] [CrossRef]
- Cops, J.; Mullens, W.; Verbrugge, F.H.; Swennen, Q.; De Moor, B.; Reynders, C.; Penders, J.; Achten, R.; Driessen, A.; Dendooven, A.; et al. Selective Abdominal Venous Congestion Induces Adverse Renal and Hepatic Morphological and Functional Alterations despite a Preserved Cardiac Function. Sci. Rep. 2018, 8, 17757. [Google Scholar] [CrossRef]
- Cops, J.; Mullens, W.; Verbrugge, F.H.; Swennen, Q.; Reynders, C.; Penders, J.; Rigo, J.-M.; Hansen, D. Selective Abdominal Venous Congestion to Investigate Cardiorenal Interactions in a Rat Model. PLoS ONE 2018, 13, e0197687. [Google Scholar] [CrossRef]
- Dong, Z.; Gong, K.; Huang, D.; Zhu, W.; Sun, W.; Zhang, Y.; Xin, P.; Shen, Y.; Wu, P.; Li, J.; et al. Myocardial Infarction Accelerates Glomerular Injury and Microalbuminuria in Diabetic Rats via Local Hemodynamics and Immunity. Int. J. Cardiol. 2015, 179, 397–408. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying Systemic Congestion with Point-Of-Care Ultrasound: Development of the Venous Excess Ultrasound Grading System. Ultrasound J. 2020, 12, 16. [Google Scholar] [CrossRef]
- Argaiz, E.R. VExUS Nexus: Bedside Assessment of Venous Congestion. Adv. Chronic Kidney Dis. 2021, 28, 252–261. [Google Scholar] [CrossRef]
- Trpkov, C.; Grant, A.D.M.; Fine, N.M. Intrarenal Doppler Ultrasound Renal Venous Stasis Index Correlates With Acute Cardiorenal Syndrome in Patients with Acute Decompensated Heart Failure. CJC Open 2021, 3, 1444–1452. [Google Scholar] [CrossRef]
- Mauri, T.; Cambiaghi, B.; Spinelli, E.; Langer, T.; Grasselli, G. Spontaneous Breathing: A Double-Edged Sword to Handle with Care. Ann. Transl. Med. 2017, 5, 292. [Google Scholar] [CrossRef]
- Putensen, C.; Muders, T.; Varelmann, D.; Wrigge, H. The Impact of Spontaneous Breathing during Mechanical Ventilation. Curr. Opin. Crit. Care 2006, 12, 13–18. [Google Scholar] [CrossRef]
- Hering, R.; Peters, D.; Zinserling, J.; Wrigge, H.; von Spiegel, T.; Putensen, C. Effects of Spontaneous Breathing during Airway Pressure Release Ventilation on Renal Perfusion and Function in Patients with Acute Lung Injury. Intensive Care Med. 2002, 28, 1426–1433. [Google Scholar] [CrossRef]
- Arold, S.P.; Bartolák-Suki, E.; Suki, B. Variable Stretch Pattern Enhances Surfactant Secretion in Alveolar Type II Cells in Culture. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 296, L574–L581. [Google Scholar] [CrossRef]
- Hubmayr, R.D.; Rodarte, J.R.; Walters, B.J.; Tonelli, F.M. Regional Ventilation during Spontaneous Breathing and Mechanical Ventilation in Dogs. J. Appl. Physiol. 1987, 63, 2467–2475. [Google Scholar] [CrossRef]
- Reber, A.; Nylund, U.; Hedenstierna, G. Position and Shape of the Diaphragm: Implications for Atelectasis Formation. Anaesthesia 1998, 53, 1054–1061. [Google Scholar] [CrossRef]
- Al-Bassam, W.; Dade, F.; Bailey, M.; Eastwood, G.; Osawa, E.; Eyeington, C.; Anstey, J.; Yi, G.; Ralph, J.; Kakho, N.; et al. “Likely Overassistance” during Invasive Pressure Support Ventilation in Patients in the Intensive Care Unit: A Multicentre Prospective Observational Study. Crit. Care Resusc. 2019, 21, 18–24. [Google Scholar]
- Metnitz, P.G.H.; Metnitz, B.; Moreno, R.P.; Bauer, P.; Del Sorbo, L.; Hoermann, C.; de Carvalho, S.A.; Ranieri, V.M. Epidemiology of Mechanical Ventilation: Analysis of the SAPS 3 Database. Intensive Care Med. 2009, 35, 816–825. [Google Scholar] [CrossRef]
- Mascheroni, D.; Kolobow, T.; Fumagalli, R.; Moretti, M.P.; Chen, V.; Buckhold, D. Acute Respiratory Failure Following Pharmacologically Induced Hyperventilation: An Experimental Animal Study. Intensive Care Med. 1988, 15, 8–14. [Google Scholar] [CrossRef]
- Telias, I.; Damiani, F.; Brochard, L. The Airway Occlusion Pressure (P(0.1)) to Monitor Respiratory Drive during Mechanical Ventilation: Increasing Awareness of a Not-so-New Problem. Intensive Care Med. 2018, 44, 1532–1535. [Google Scholar] [CrossRef]
- Decavèle, M.; Similowski, T.; Demoule, A. Detection and Management of Dyspnea in Mechanically Ventilated Patients. Curr. Opin. Crit. Care 2019, 25, 86–94. [Google Scholar] [CrossRef]
- Schmidt, M.; Banzett, R.B.; Raux, M.; Morélot-Panzini, C.; Dangers, L.; Similowski, T.; Demoule, A. Unrecognized Suffering in the ICU: Addressing Dyspnea in Mechanically Ventilated Patients. Intensive Care Med. 2014, 40, 1–10. [Google Scholar] [CrossRef]
- Demoule, A.; Hajage, D.; Messika, J.; Jaber, S.; Diallo, H.; Coutrot, M.; Kouatchet, A.; Azoulay, E.; Fartoukh, M.; Hraiech, S.; et al. Prevalence, Intensity, and Clinical Impact of Dyspnea in Critically Ill Patients Receiving Invasive Ventilation. Am. J. Respir. Crit. Care Med. 2022, 205, 917–926. [Google Scholar] [CrossRef]
- Hooper, M.H.; Girard, T.D. Sedation and Weaning from Mechanical Ventilation: Linking Spontaneous Awakening Trials and Spontaneous Breathing Trials to Improve Patient Outcomes. Anesthesiol. Clin. 2011, 29, 651–661. [Google Scholar] [CrossRef]
- Blanch, L.; Villagra, A.; Sales, B.; Montanya, J.; Lucangelo, U.; Luján, M.; García-Esquirol, O.; Chacón, E.; Estruga, A.; Oliva, J.C.; et al. Asynchronies during Mechanical Ventilation Are Associated with Mortality. Intensive Care Med. 2015, 41, 633–641. [Google Scholar] [CrossRef]
- De Oliveira, B.; Aljaberi, N.; Taha, A.; Abduljawad, B.; Hamed, F.; Rahman, N.; Mallat, J. Patient-Ventilator Dyssynchrony in Critically Ill Patients. J. Clin. Med. 2021, 10, 4550. [Google Scholar] [CrossRef]
- Yoshida, T.; Uchiyama, A.; Matsuura, N.; Mashimo, T.; Fujino, Y. Spontaneous Breathing during Lung-Protective Ventilation in an Experimental Acute Lung Injury Model: High Transpulmonary Pressure Associated with Strong Spontaneous Breathing Effort May Worsen Lung Injury. Crit. Care Med. 2012, 40, 1578–1585. [Google Scholar] [CrossRef]
- Bertoni, M.; Telias, I.; Urner, M.; Long, M.; Del Sorbo, L.; Fan, E.; Sinderby, C.; Beck, J.; Liu, L.; Qiu, H.; et al. A Novel Non-Invasive Method to Detect Excessively High Respiratory Effort and Dynamic Transpulmonary Driving Pressure during Mechanical Ventilation. Crit. Care 2019, 23, 346. [Google Scholar] [CrossRef]
- Pesenti, A.; Rossi, N.; Calori, A.; Foti, G.; Rossi, G.P. Effects of Short-Term Oxygenation Changes on Acute Lung Injury Patients Undergoing Pressure Support Ventilation. Chest 1993, 103, 1185–1189. [Google Scholar] [CrossRef]
- González, C.; Almaraz, L.; Obeso, A.; Rigual, R. Oxygen and Acid Chemoreception in the Carotid Body Chemoreceptors. Trends Neurosci. 1992, 15, 146–153. [Google Scholar] [CrossRef]
- Spinelli, E.; Pesenti, A.; Slobod, D.; Fornari, C.; Fumagalli, R.; Grasselli, G.; Volta, C.A.; Foti, G.; Navalesi, P.; Knafelj, R.; et al. Clinical Risk Factors for Increased Respiratory Drive in Intubated Hypoxemic Patients. Crit. Care 2023, 27, 138. [Google Scholar] [CrossRef]
- Dianti, J.; Fard, S.; Wong, J.; Chan, T.C.Y.; Del Sorbo, L.; Fan, E.; Amato, M.B.P.; Granton, J.; Burry, L.; Reid, W.D.; et al. Strategies for Lung- and Diaphragm-Protective Ventilation in Acute Hypoxemic Respiratory Failure: A Physiological Trial. Crit. Care 2022, 26, 259. [Google Scholar] [CrossRef]
- Mailhot, T.; Cossette, S.; Lambert, J.; Beaubien-Souligny, W.; Cournoyer, A.; O’Meara, E.; Maheu-Cadotte, M.-A.; Fontaine, G.; Bouchard, J.; Lamarche, Y.; et al. Delirium After Cardiac Surgery and Cumulative Fluid Balance: A Case-Control Cohort Study. J. Cardiothorac. Vasc. Anesth. 2019, 33, 93–101. [Google Scholar] [CrossRef]
- Mikkelsen, M.E.; Christie, J.D.; Lanken, P.N.; Biester, R.C.; Thompson, B.T.; Bellamy, S.L.; Localio, A.R.; Demissie, E.; Hopkins, R.O.; Angus, D.C. The Adult Respiratory Distress Syndrome Cognitive Outcomes Study: Long-Term Neuropsychological Function in Survivors of Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2012, 185, 1307–1315. [Google Scholar] [CrossRef]
- Ouchi, A.; Sakuramoto, H.; Hoshino, H.; Matsuishi, Y.; Sakaguchi, T.; Enomoto, Y.; Hoshino, T.; Shimojo, N.; Inoue, Y. Association between Fluid Overload and Delirium/Coma in Mechanically Ventilated Patients. Acute Med. Surg. 2020, 7, e508. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Huyghens, L.; Parra, J.; Schiettecatte, J.; Smitz, J.; Vincent, J.-L. Hypotension and a Positive Fluid Balance Are Associated with Delirium in Patients with Shock. PLoS ONE 2018, 13, e0200495. [Google Scholar] [CrossRef]
- Hogan, B.M.; Bower, N.I. Lymphatics and the Brain: It’s Time to Go Fishing. Circ. Res. 2021, 128, 59–61. [Google Scholar] [CrossRef]
- Da Mesquita, S. Charting the Meningeal Lymphatic Network. J. Exp. Med. 2022, 219, e20220891. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and Functional Features of Central Nervous System Lymphatic Vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Lee, E.; Biko, D.M.; Sherk, W.; Masch, W.R.; Ladino-Torres, M.; Agarwal, P.P. Understanding Lymphatic Anatomy and Abnormalities at Imaging. Radiographics 2022, 42, 487–505. [Google Scholar] [CrossRef]

| Prerenal | Examples |
|---|---|
| Shock: | |
| - Diminished cardiac output | adrenal insufficiency, heart failure |
| - Hypovolemia | |
| - Vasoplegia | sepsis, sedation/opioids |
| Vascular obstruction | vasculitis |
| Hypoxia | asphyxia |
| Renal | |
| Renal interstitial edema | |
| Ischemia | |
| Toxic injury | medication, rhabdomyolysis, sepsis |
| Vascular | vasculitis, HUS/TTP, sickle cell crisis, SLE |
| Nephritis | radiation |
| Infection | |
| Hemolysis | |
| Osmotic | |
| Infiltrative | amyloidosis, lymphomatous |
| Postrenal urinary | |
| prostatic hyperplasia, kidney stones, retroperitoneal fibrosis, bladder dysfunction | |
| Postrenal venous | |
| Abdominal compartment syndrome | renal interstitial edema |
| Venous bagging | renal interstitial edema |
| - Exacerbation by high PEEP | |
| - Predominant controlled mechanical ventilation lacking decreased pleural pressures | |
| Miscellaneous | |
| Cytokines, proinflammatory mediators | VILI/VIKI, sepsis |
| Hypercapnia | |
| Permissive hypoxemia | |
| Genetic/metabolic | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seubert, M.E.; Goeijenbier, M. Controlled Mechanical Ventilation in Critically Ill Patients and the Potential Role of Venous Bagging in Acute Kidney Injury. J. Clin. Med. 2024, 13, 1504. https://doi.org/10.3390/jcm13051504
Seubert ME, Goeijenbier M. Controlled Mechanical Ventilation in Critically Ill Patients and the Potential Role of Venous Bagging in Acute Kidney Injury. Journal of Clinical Medicine. 2024; 13(5):1504. https://doi.org/10.3390/jcm13051504
Chicago/Turabian StyleSeubert, Mark E., and Marco Goeijenbier. 2024. "Controlled Mechanical Ventilation in Critically Ill Patients and the Potential Role of Venous Bagging in Acute Kidney Injury" Journal of Clinical Medicine 13, no. 5: 1504. https://doi.org/10.3390/jcm13051504
APA StyleSeubert, M. E., & Goeijenbier, M. (2024). Controlled Mechanical Ventilation in Critically Ill Patients and the Potential Role of Venous Bagging in Acute Kidney Injury. Journal of Clinical Medicine, 13(5), 1504. https://doi.org/10.3390/jcm13051504